Abstract
Creatol (CTL) is a hydroxyl radical adduct of creatinine (Cr). The serum methylguanidine (MG) level and the MG/Cr molar ratio are reported to be biomarkers for oxidative stress. The aim of this study was to examine whether urinary excretion of CTL, another oxidative stress-related marker, is increased in patients with chronic renal failure (CRF). One hundred twenty-four non-dialyzed patients with chronic renal failure (serum Cr level, 1.3–10.0 mg/dL) were recruited from our hospitals. Urine and serum levels of CTL and MG were determined by high-performance liquid chromatography with the use of 9, 10- phenanthrenequinone as a fluorogenic reagent. The CTL/Cr and (CTL+MG)/Cr molar ratios in spot urine samples were also compared with those in 24-h urine samples. The urinary CTL/Cr and (CTL+MG)/Cr molar ratios increased with decreases in Cr clearance in patients with CRF. Correlations between serum and spot urine (CTL+MG)/Cr and between serum and spot urine CTL/Cr were quite similar to those in 24-h urine samples. CTL/Cr and (CTL+MG)/Cr molar ratios in both 24-h urine and spot urine samples appear to be useful indices of the severity of CRF.
INTRODUCTION
Because hydroxyl radical is so reactive that its activity cannot be monitored in vivo, an indirect biomarker of the hydroxyl radical would be useful in patients with various diseases. Although 8-hydroxyguanine and 8-hydroxydeoxyguanosine, hydroxyl radical adducts of guanine, and deoxyguanosine have been used as biomarkers for hydroxyl radicals, their use is limited because they show only the amounts of hydroxyl radical inside nuclei and mitochondria.Citation[1–5] Creatol (CTL, 5-hydroxy creatinine) is another useful in vivo biomarker of hydroxyl radical adduct (see )Citation[6–9]. Because its precursor creatinine (Cr) is distributed not only in the nuclei and mitochondria but also in the cytosol and outside the cell, CTL can be used as a hydroxyl radical-related biomarker in patients with various diseases.
Figure 1. Non-enzymatic oxidation of creatinine (Cr) into creatol (CTL) and its related metabolites in humans. The endogenous compounds shown in the squares are detectable in human serum and urine.
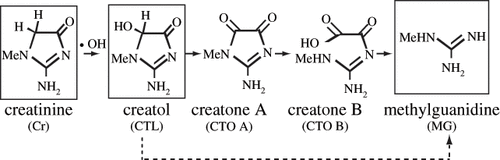
CTL is partially converted to methylguanidine (MG) in vivo (see ).Citation[9],Citation[10] CTL and MG are detectable in the urine but not the serum of healthy individuals. Serum MG is undetectable in patients with early stage renal failure; it is detected only in patients with end-stage renal failure. However, serum CTL is detectable in all patients with chronic renal failure because the serum concentration of CTL is ten times higher than that of MG. Thus, the serum CTL level and the CTL/Cr molar ratio are also reported to be good biomarkers of hydroxyl radicals.Citation[10–13] Theoretically, the molar sum, CTL+MG, and its ratio to Cr, (CTL+MG)/Cr, may be the best indices for the precise hydroxyl radical level in vivo.
The present study examined whether the urine CTL/Cr and (CTL+MG)/Cr molar ratios correlate with the severity of chronic renal failure. In addition, this study examined whether a simple spot urine analysis of the CTL/Cr and (CTL+MG)/Cr molar ratios can be used for monitoring renal injury in non-dialyzed patients with chronic renal failure.
SUBJECTS AND METHODS
One hundred twenty-four non-dialyzed patients with chronic renal failure were recruited from the authors' hospitals. None of the patients took antioxidants, including vitamin C, statins, or other medications that might affect systemic oxidative stress. Concentrations of Cr, CTL, and MG were measured in serum and urine specimens collected from these patients. Of the 124 patients, 47 had diabetes (38 men, mean age: 62.6 ± 18.9 years, and 9 women, mean age: 59.7 ± 14.8 years; serum Cr level: 1.3–8.0 mg/dL), and 77 did not have diabetes (37 men, mean age: 48.7 ± 12.5 years, and 40 women, mean age: 50.9 ± 14.8 years; serum Cr level: 1.4–10.0 mg/dL).
Preparation of serum and urine samples and sample storage were performed as reported elsewhere.Citation[9–11] Serum and urine Cr, CTL, and MG levels were determined by means of high-performance liquid chromatography with 9, 10-phenanthrenequinone used as a fluorogenic reagent.Citation[9–11] Intra- and inter-assay coefficients of variation (CVs) for the determination of CTL were 1.05% and 1.84%, respectively. CVs for peak area and retention time were 1.05% and 0.39%, respectively.
Data are expressed as mean values ± SD. Regression lines were obtained by the least squares method. Coefficients of determination (r2) were calculated. Pearson's correlation coefficient was used to examine correlation between each of the variables. A p value of < 0.05 was considered statistically significant.
RESULTS
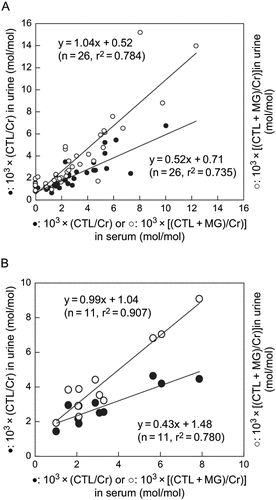
Serum CTL/Cr and (CTL+MG)/Cr increased in proportion to decreases in creatinine clearance (CLCr) in both groups of patients (see ). Urine CTL/Cr and (CTL+ MG)/Cr also increased in relation to decreases in CLCr (see ). However, there was a significant difference between urine CTL/Cr and urine (CTL+MG)/Cr (p < 0.05), suggesting that urinary MG levels could not be ignored. In both the diabetes and non-diabetes patients with chronic renal failure, there was a positive correlation between serum (CTL+MG)/Cr and urine (CTL+MG)/Cr. The correlation coefficient was close to 1.0, whereas the correlation coefficient for the relation between the serum (CTL+MG)/Cr and urine CTL/Cr was approximately 0.4 (see ). The serum CTL/Cr value was significantly larger (p < 0.05) than the urine CTL/Cr value in both diabetes and non-diabetes patients. Correlations between serum and spot urine CTL/Cr and between serum and spot urine (CTL+MG)/Cr were nearly the same as correlations between serum CTL/Cr and (CTL+MG)/Cr and corresponding 24-h urine values (see ). Furthermore, there was a positive correlation between spot urine CTL/Cr and 24-h urine CTL/Cr and between spot urine (CTL+MG)/Cr and 24-h urine (CTL+MG)/Cr, and the correlation coefficients were quite similar (see ). There were no significant differences in urine CTL/Cr and (CTL+MG)/Cr values between diabetes and non-diabetes patients. The suggested endogenous intermediates such as creatones A and B (see ) were not detected in the serum or urine of the study patients.
Figure 2. Correlation between CLCr and serum CTL/Cr, serum (CTL+MG)/ Cr, urine CTL/Cr, and urine (CTL+MG)/Cr in chronic renal failure patients with and without diabetes. Abbreviations: CLCr = creatinine clearance, CTL = creatol, Cr = creatinine, MG = methylguanidine. Closed circles: diabetes patients; open circles: non-diabetes patients.
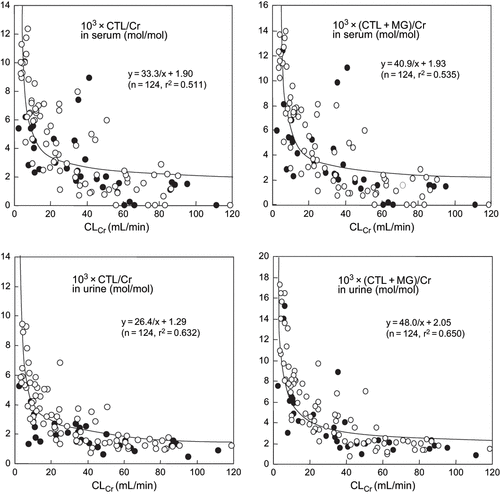
Figure 3. Correlation between serum (CTL+MG)/Cr and urine (CTL+MG)/Cr, serum CTL/Cr, and urine CTL/Cr in chronic renal failure patients (A) with, and (B) without diabetes. Abbreviations: CTL = creatol, MG = methylguanidine, Cr = creatinine.
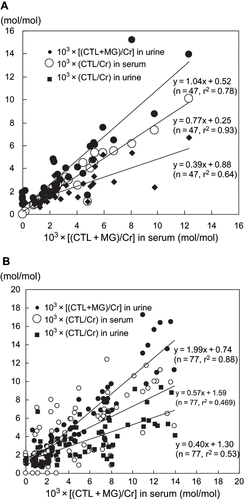
Figure 4. Correlation between serum CTL/Cr and (CTL+MG)/Cr ratios and corresponding ratios in (A) 24-h urine, and (B) spot urine samples from chronic renal failure patients with diabetes. Abbreviations: CTL = creatol, MG = methylguanidine, Cr = creatinine. Closed circles: CTL/Cr; open circles: (CTL+MG)/Cr.
DISCUSSION
In vitro analysis has shown that Cr is oxidized by hydroxyl radicals to CTL, which is subsequently converted to MG directly or via creatones A and B (see ).Citation[8] Approximately 0.2% of Cr is oxidized by hydroxyl radicals in healthy subjects.Citation[7],Citation[10] Because oxidized metabolites are excreted into the urine, the concentrations of CTL and MG in the urine can be determined.
Serum levels of MG have been investigated since Giovanetti's group reported that MG accumulates in the serum of uremic patients as a uremic toxin.Citation[14] Ozasa et al.Citation[15] reported that MG and CTL are present at similar levels in the serum of uremic rats. In humans, however, conversion of CTL to MG is poor, and MG can be detected as a minor Cr metabolite derived by reactive oxygen species. This may be because of a low contribution of MG synthases.Citation[16],Citation[17] The serum CTL/Cr ratio was shown to be higher than the urine CTL/Cr ratio. This difference may indicate that enzymatic and/or non-enzymatic conversion of CTL to MG occurs during the excretion of CTL into the urine via the kidney. The values obtained from 24-h urine and spot urine samples were quite similar, suggesting that very little decomposition occurred during storage.
The urinary (CTL+MG)/Cr ratio may be a useful in vivo biomarker of oxidative stress derived from hydroxyl radicals. The ratio reflects the total conversion of Cr to its metabolites oxidized by hydroxyl radicals. Healthy individuals excrete sufficient CTL and MG into the urine for detection, whereas CTL and MG are not detectable in the serum. The serum (CTL+MG)/Cr ratio is negligible, whereas the urinary ratio shows a certain range of values. In the present study, the urinary (CTL+MG)/Cr ratio increased steadily in proportion to the progression of chronic renal failure, correlating significantly with the decrease in CLCr.
Because serum MG can be detected only when chronic renal failure progresses to end-stage kidney disease, the urinary (CTL+MG)/Cr ratio is more useful than the serum (CTL+MG)/Cr ratio for the evaluation of chronic renal failure. Twenty-four-hour urine collection may not be necessary for the calculation of the urinary (CTL+MG)/Cr ratio; spot urine can be used satisfactorily. It is likely that the urinary (CTL+MG)/Cr ratio increases in patients with other diseases, which would cause an increase in hydroxyl radical production in vivo, whereas the serum (CTL+MG)/Cr may not necessarily increase insofar as patients have adequate renal function. The synchronized increase in the serum and urine (CTL+MG)/Cr values may suggest an increase in hydroxyl radical production in the presence of severe renal failure.
The present results showed that the measurement of CTL and MG together with Cr in serum and urine can provide information concerning hydroxyl radical production in patients with chronic renal failure that cannot be obtained by a single measurement of Cr. The CTL/Cr and (CTL+MG)/Cr molar ratios, especially the latter, obtained from both 24-h urine and spot urine samples appear to be useful indices of the degree of renal failure as well as of in vivo oxidative stress in patients with chronic renal failure.
REFERENCES
- Kasai H, Nishimura S. Hydroxylation of deoxy guanosine at the C8-position by polyphenols and aminophenols in the presence of hydrogen peroxide and ferric ion. Jpn J Cancer Res. 1984; 75: 565–566
- Helbock HJ, Beckman KB, Ames BN. 8-Hydroxydeoxyguanosine and 8-hydroxy-guanine as biomarkers of oxidative DNA damage. Methods Enzymol. 1999; 300: 156–166
- Richter C. Oxidative damage to mitochondrial DNA and its relationship to ageing. Int J Biochem Cell Biol. 1995; 27: 647–653
- Yokozawa T, Fujioka K, Oura H. Increase in kidney 8-hydroxydeoxyguanosine level with the progression of renal failure. Nephron. 1992; 61: 236–237
- Ha H, Kim KH. Pathogenesis of diabetic nephropathy: the role of oxidative stress and protein kinase C. Diabetes Res Clin Pract. 1999; 45: 147–151
- Nakamura K, Ienaga K. Creatol (5-hydroxycreatinine), a new toxin candidate in uremic patients. Experientia. 1990; 46: 470–472
- Ienaga K, Nakamura Y, Yamanaka M, et al. The use of 13 C‐labeling to prove that creatinine is oxidized by mammals into creatol and 5-hydroxy-1–methylhydantoin. J Chem Soc Chem Commun. 1991; 509–510
- Nakamura K, Ienaga K, Yokozawa T, Fujitsuka N, Oura H. Production of methylguanidine from creatinine via creatol by active oxygen species: analyses of the catabolism in vitro. Nephron. 1991; 58: 42–46
- Fujitsuka N, Yokozawa T, Oura H, Nakamura K, Ienaga K. Major role of hydroxyl radical in the conversion of creatinine to creatol. Nephron. 1994; 68: 280–281
- Yokozawa T, Fujitsuka N, Oura H, Ienaga K, Nakamura K. Comparison of methylguanidine production from creatinine and creatol in vivo. Nephron. 1991; 58: 125–126
- Nakamura K, Ienaga K, Nakano K, et al. Diabetic renal failure and serum accumulation of the creatinine oxidative metabolites, creatol and methylguanidine. Nephron. 1996; 73: 520–525
- Ienaga K, Nakamura K, Fukunaga Y, Nakano K, Kanatsuna T. Creatol and chronic renal failure. Kidney Int. 1994; 46(Suppl. 47)S22–S24
- Nakamura K, Ienaga K, Nakano K, et al. Creatol, a creatinine metabolite, as a useful determinant of renal function. Nephron. 1994; 66: 140–146
- Giovannetti S, Balestri PL, Barsotti G. Methylguanidine in uremia. Arch Intern Med. 1973; 131: 709–713
- Ozasa H, Watanabe T, Nakamura K, Fukunaga Y, Ienaga K, Hagiwara K. Changes in serum levels of creatol and methylguanidine in renal injury induced by lipid peroxide produced by vitamin E deficiency and GSH depletion in rats. Nephron. 1997; 75: 224–229
- Fujitsuka N, Yokozawa T, Oura H, et al. L-gulono- γ-lactone oxidase is the enzyme responsible for production of methylguanidine in the rat liver. Nephron. 1993; 63: 445–451
- Yokozawa T, Fujitsuka N, Oura H, et al. Purification of methylguanidine synthase from the rat kidney. Nephron. 1993; 63: 452–457