Abstract
Pregnancy increases plasma cystatin C, but levels are much higher in preeclampsia. Previous studies have not quantified preeclampsia risk with varying cystatin C concentrations or adjusted for confounders. We performed a case-control study of 100 preeclampsia cases and 100 random pregnancies uncomplicated by hypertension (controls). All women were free of pre-existing hypertension, diabetes, and renal disease, and gave birth to singletons. Plasma cystatin C was measured at delivery. Adjusted odds ratios (OR) and 95% confidence intervals (CI) of preeclampsia by quartiles (based on control distribution) of maternal plasma cystatin C were estimated using multivariable logistic regression models. Mean cystatin C levels were elevated in preeclampsia cases compared with controls (1.38 ± 0.04 vs. 1.22 ± 0.03 mg/L, p < 0.01). Compared to the first quartile, the estimated risk of preeclampsia was increased by approximately 12-fold for the fourth quartile, after adjusting for maternal age, body mass index, physical inactivity, smoking, and gestational age. Increased plasma levels of cystatin C were independently associated with preeclampsia. Further studies are required to assess the role of cystatin C levels in preeclampsia severity and maternal and fetal outcomes.
INTRODUCTION
Preeclampsia affects 5–7% of all pregnancies and is a leading cause of maternal and perinatal morbidity and mortality.Citation[1],Citation[2] Preeclampsia is associated with increased risk of ischemic strokeCitation[3] and cardiovascular disease.Citation[4],Citation[5] Epidemiologic studies have identified subgroups of women at high risk of developing this pregnancy complication, such as those with chronic hypertension, older age, black race, and increased body mass.Citation[1],Citation[2],Citation[6] Although maternal kidney disease predisposes to preeclampsia and other pregnancy complications,Citation[7] kidney injury in preeclampsia is usually an acute event, likely secondary to the widespread endothelial vascular dysfunction.
Preeclampsia manifests by proteinuria, hypertension, and impaired renal function.Citation[8] Serum creatinine is an unreliable measure of kidney function in pregnancy.Citation[9] Cystatin C has been proposed as a measure of kidney function in population studiesCitation[10] and in pregnancy.Citation[9] Cystatin C is an endogenous low molecular weight protein (13 kDa) synthesized by all nucleated cells and produced at a constant rate. It is freely filtered in the kidneys and then completely metabolized by the proximal tubular cells. Increased cystatin C concentrations is associated with cardiovascular outcomes in population studies.Citation[11]]
Prior studies have shown increased cystatin C levels in preeclampsia.Citation[12] However, these studies have not quantified preeclampsia risk with varying cystatin C levels, nor have they adjusted for potential confounders. In our study, we evaluated the relationship between plasma cystatin C and preeclampsia in a case-control study of pregnant women without pre-pregnancy comorbidities.
MATERIALS AND METHODS
Study Population
This case-control study was conducted from April 1998 to June 2002 at Swedish Medical Center and Tacoma General Hospital in Seattle and Tacoma, Washington, respectively. This study has been described in detail elsewhere.Citation[13] Preeclampsia cases were identified by daily surveillance of labor and delivery logs and confirmed by medical records. Women were recruited during their postpartum hospital stay. Using the then-current American College of Obstetricians and Gynecologists (ACOG) guidelines,Citation[14] preeclampsia was defined as sustained pregnancy-induced hypertension with proteinuria. Hypertension was defined as sustained blood pressure readings of ≥140/90 mmHg (with readings taking place ≥ 6 hours apart) and/or a sustained 15 mm Hg diastolic rise or a 30 mm Hg systolic blood pressure above first-trimester values. Proteinuria was defined as urine protein concentration ≥30 mg/dL (or 1+ on a urine dipstick) on ≥2 random specimens collected ≥ 4 hours apart.
Controls were women with pregnancies uncomplicated by pregnancy-induced hypertension. Each day during the enrollment period, controls were numbered in the order in which they were admitted and delivered within two hours of a case, and were approached in the order in which they were identified by research personnel.
During the study period, we enrolled 310 preeclampsia cases and 502 control subjects. Those with pre-existing diabetes, chronic hypertension, and renal disease were excluded from this analysis. From this study population, we randomly selected 100 preeclampsia cases and 100 controls for inclusion in this study of maternal plasma cystatin C concentrations.
The procedures used in this study were in agreement with the protocols approved by the respective Institutional Review Boards of Swedish Medical Center and Tacoma General Hospital. The study was conducted in accordance with the principles of the Declaration of Helsinki. All participants provided written informed consent.
Data and Blood Sample Collection Procedures
A structured interview questionnaire, administered during participants' postpartum hospital stay, was used to collect information on maternal sociodemographic, medical, reproductive, and lifestyle characteristics during in-person interviews.
Non-fasting blood samples were collected in 10 ml tripotassium EDTA Vacutainer tubes during the intrapartum period. These were protected from ultraviolet light, kept on wet ice, and processed within 30 minutes of phlebotomy. The median time between participants' last meal and phlebotomy was two hours both for cases and controls. Plasma was decanted into cryovials and kept frozen at -70°C or below until analysis.
Laboratory Analytical Procedures
Cystatin C was determined in plasma using enzyme-linked immunosorbent assay (human cystatin C ELISA assay, Alpco Diagnostics, Windham, New Hampshire, USA) and a “vmax” kinetic microplate reader (Molecular Devices, Sunnyvale, California, USA). The intra-assay coefficients of variation for the assay standards were less than 10%. All samples were assayed by the UNC Bioanalytical Core Labs ELISA facility without knowledge of pregnancy outcome. Results were expressed as mg/L.
Statistical Analysis
We examined the frequency distributions of maternal socio-demographic characteristics and medical and reproductive histories according to case-control status. We also used Student's t-test or nonparametric rank sum test for comparison of the difference of continuous variables between cases and controls. For categorical variables, we used the chi-square test or the Fisher's exact test where appropriate.
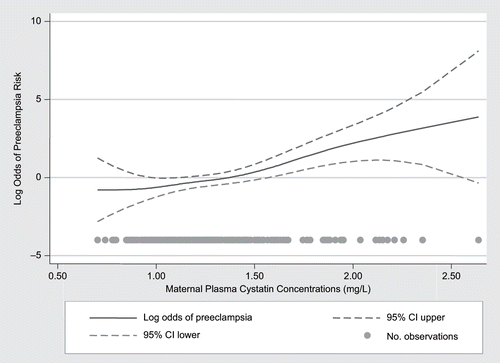
We initially evaluated the linearity in the log odds of the outcome for continuous cystatin C levels using generalizing additive models (see ). To estimate the relative association between preeclampsia and levels of maternal plasma cystatin C, we categorized each subject according to quartiles determined by the distribution among controls. We used the lowest quartile as the referent group, and estimated odds ratios (OR) and 95% confidence intervals (CI) for each of the remaining three quartiles. To assess confounding, we entered covariates into a logistic regression model one at a time, then compared the adjusted and unadjusted odds ratios.Citation[15] Final logistic regression models included covariates that altered unadjusted odds ratios by at least 10%, as well as those covariates of a priori interest (e.g., maternal age and pre-pregnancy body mass index, BMI). We considered the following covariates as possible confounders in this analysis: physical inactivity during pregnancy, maternal smoking status, and gestational age at delivery. Because the ORs changed dramatically when gestational age at delivery was incorporated in the model, we did a sub-analysis restricted to cases with gestational age ≥35 weeks versus controls with gestational age ≥35 weeks (this turned out to be all the controls). We regarded this as optimally controlling for confounding by gestational age differences between cases and controls.
Figure 1. The log odds of preeclampsia risk by maternal plasma cystatin C after adjusting for all the other confounders, including gestational age at delivery (using a generalizing additive model). Straight line represents the estimates, and dotted lines the 95% percentiles. Dots represent the density of the measures by level of cystatin C.
A post hoc analysis was conducted to examine the influence of steroid therapy during pregnancy on the hypotheses. Steroid treatment in this study population was related to promoting fetal lung maturation in those women predisposed to preterm delivery. Forty preeclampsia cases and eight control subjects had received the steroid treatment. We evaluated the confounder effect as previously described and effect modification by stratified analyses. All analyses were performed using Stata 9.0 statistical software (Stata, College Station, Texas, USA). All continuous variables are presented as mean and standard error (SE). All reported CI were calculated at the 95% level.
RESULTS
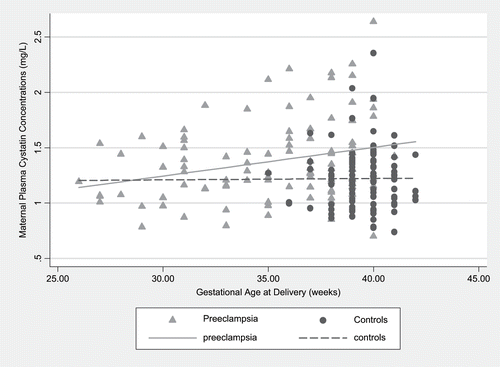
The descriptive characteristics of women developing preeclampsia and controls are summarized in . Overall, most women were Caucasian, well-educated, and nulliparous. Women developing preeclampsia were slightly younger, with a mean age of 29.8 ± 0.7 years compared to 31.8 ± 0.5 years in controls (p < 0.01). In addition, cases had higher pre-pregnancy BMI than controls (27.1 ± 0.7 versus 23.0 ± 0.4 kg/m2, respectively), and more often cases were physically inactive and current smokers. Cases also had lower mean gestational age at delivery (see ).
Table 1 Characteristics of preeclampsia cases and controls according to selected characteristics, Seattle and Tacoma, Washington, 1998–2002
Cystatin C plasma concentrations, measured at delivery, were elevated in cases compared with controls (mean levels 1.38 ± 0.04 versus 1.22 ± 0.03 mg/L, p < 0.01; see ). High cystatin C levels were associated with increased risk of preeclampsia (see and ). Compared to the first quartile, the estimated risk of preeclampsia was increased by approximately eight-fold in the second quartile, 3.6-fold for the third quartile, and 12-fold for the fourth quartile after adjusting for maternal age, BMI, physical inactivity, smoking, and gestational age. A significant p value for trend was observed across quartiles of cystatin C (see ). Forty-eight women received corticosteroids at delivery, of whom 40 were preeclampsia cases and 8 were control subjects. Adjusting for corticosteroid exposure did not significantly change the estimated risk of preeclampsia.
Table 2 Odds ratios and 95% confidence intervals of preeclampsia according to the quartile of cystatin C concentrations in control subjects, Seattle and Tacoma, Washington, 1998–2002
Cystatin C levels increased with gestational age, but cases had a wider range of cystatin C levels than controls (see ). Only one control delivered preterm. Therefore, we performed further analyses to compare cases and controls delivering at or after 35 weeks of gestation. Compared to the first quartile, the adjusted odds ratio for preeclampsia was 7.7 (95% CI, 1.4, 42) for the second quartile of cystatin C levels, 3.6 (95% CI, 0.7, 20) for the third quartile and 12.3 (95% CI, 2.4, 63) for the fourth quartile.
DISCUSSION
Our study shows an increased risk of preeclampsia associated with high cystatin C plasma levels among women without preexisting kidney disease, diabetes, and hypertension. The increased risk was independent of maternal age, pre-pregnancy BMI, and physical inactivity, and remains after adjustments for gestational age, maternal smoking, and corticosteroid exposure, which have all been associated with increased cystatin C levels.Citation[16],Citation[17]]
The pathogenesis of preeclampsia involves abnormal placenta development, release of placenta angiogenic factors,Citation[18] systemic inflammatory response, and endothelium dysfunction.Citation[19] Kidney dysfunction may occur as result of hemodynamic changes, structural glomerular lesions such as glomerular endotheliosis,Citation[8] and possibly podocyte injury and depletion.Citation[20] Glomerular endotheliosis is characterized by swelling and vacuolization of endothelial cells with occlusion of the capillary lumen, leading to impairment of glomerular ultrafiltration.Citation[21] Recently, urinary podocyte loss was described in women with preeclampsia but not in those with uncomplicated pregnancies.Citation[20]]
Cystatin C is considered an endogenous marker of kidney function. In pregnancy, cystatin C has been previously shown to correlate with measures of kidney function such as plasma clearance of iohexolCitation[9],Citation[22] but not with creatinine clearance.Citation[23] In biopsy studies, cystatin C correlates with the degree of glomerular endotheliosis, the typical glomerular lesion of preeclampsia.Citation[22] Therefore, high cystatin C levels in pregnancy likely reflect renal dysfunction in preeclampsia.
We did not find a dose-response relationship between quartiles of cystatin C and the risk of preeclampsia, but did find a significant linear trend for preeclampsia on continuous levels of cystatin C. Our estimates were imprecise due to the small number of mothers in each quartile, which also prevented additional subgroup analyses. Therefore, our findings need to be confirmed in large studies. In particular, future studies should address if cystatin C levels vary with the severity of preeclampsia and degree of organ damage.
Our study is limited by its cross-sectional design, and therefore we are unable to establish whether increased concentrations of cystatin C levels precede the clinical diagnosis of preeclampsia. We used structured questionnaires to collect information pertaining to maternal pre-pregnancy status and documented clinical history. Therefore, the accuracy of data did not depend on participants' memory. Serum creatinine measures were unavailable in this study due to the lack of sampling during routine pregnancy care.
In conclusion, we have shown an association among high plasma cystatin C levels and preeclampsia, which likely reflects the acute kidney dysfunction associated with preeclampsia. Prospective cohort studies are needed to clarify the temporal relation between alterations in cystatin C concentrations and the occurrence of preeclampsia, and to determine whether elevated cystatin C concentrations in early pregnancy are predictive of poor maternal and fetal outcomes in preeclampsia.
FUNDING SOURCE
This research was supported by awards from the National Institutes of Health (HD/HL R01–34888 and HD/HL R01–32562) and by the AHA#0675001N grant.
REFERENCES
- Roberts JM, Gammill HS. Preeclampsia: Recent insights. Hypertension. Dec, 2005; 46(6)1243–1249
- Zhang J, Meikle S, Trumble A. Severe maternal morbidity associated with hypertensive disorders in pregnancy in the United States. Hypertens Pregnancy. 2003; 22(2)203–212
- Wilson BJ, Watson MS, Prescott GJ, et al. Hypertensive diseases of pregnancy and risk of hypertension and stroke in later life: results from cohort study. BMJ. Apr 19, 2003; 326(7394)845
- Smith GC, Pell JP, Walsh D. Pregnancy complications and maternal risk of ischaemic heart disease: A retrospective cohort study of 129,290 births. Lancet. Jun 23, 2001; 357(9273)2002–2006
- Arnadottir GA, Geirsson RT, Arngrimsson R, Jonsdottir LS, Olafsson O. Cardiovascular death in women who had hypertension in pregnancy: A case-control study. BJOG. Mar, 2005; 112(3)286–292
- Bodnar LM, Ness RB, Markovic N, Roberts JM. The risk of preeclampsia rises with increasing prepregnancy body mass index. Ann Epidemiol. Aug, 2005; 15(7)475–482
- Hou S. Pregnancy in chronic renal insufficiency and end-stage renal disease. Am J Kidney Dis. Feb, 1999; 33(2)235–252
- Lafayette RA, Druzin M, Sibley R, et al. Nature of glomerular dysfunction in pre-eclampsia. Kidney Int. Oct, 1998; 54(4)1240–1249
- Strevens H, Wide-Swensson D, Grubb A. Serum cystatin C is a better marker for preeclampsia than serum creatinine or serum urate. Scand J Clin Lab Invest. 2001; 61(7)575–580
- Levin A. Cystatin C, serum creatinine, and estimates of kidney function: Searching for better measures of kidney function and cardiovascular risk. Ann Intern Med. Apr 5, 2005; 142(7)586–588
- Shlipak MG, Sarnak MJ, Katz R, et al. Cystatin C and the risk of death and cardiovascular events among elderly persons. N Engl J Med. May 19, 2005; 352(20)2049–2060
- Strevens H, Wide-Swensson D, Torffvit O, Grubb A. Serum cystatin C for assessment of glomerular filtration rate in pregnant and non-pregnant women. Indications of altered filtration process in pregnancy. Scand J Clin Lab Invest. 2002; 62(2)141–147
- Zhang C, Williams MA, King IB, et al. Vitamin C and the risk of preeclampsia—results from dietary questionnaire and plasma assay. Epidemiology. Jul, 2002; 13(4)409–416
- ACOG technical bulletin. Hypertension in pregnancy. Int J Gynaecol Obstet. May, 1996; 53(2)175–183
- Rothman KJ, Greenland S. Modern Epidemiology 2nd. Lippincott-Raven Publishers, Philadelphia, Pa 1998
- Risch L, Herklotz R, Blumberg A, Huber AR. Effects of glucocorticoid immunosuppression on serum cystatin C concentrations in renal transplant patients. Clin Chem. Nov, 2001; 47(11)2055–2059
- Knight EL, Verhave JC, Spiegelman D, et al. Factors influencing serum cystatin C levels other than renal function and the impact on renal function measurement. Kidney Int. Apr, 2004; 65(4)1416–1421
- Levine RJ, Maynard SE, Qian C, et al. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med. Feb 12, 2004; 350(7)672–683
- Shah DM. Preeclampsia: New insights. Curr Opin Nephrol Hypertens. May, 2007; 16(3)213–220
- Garovic VD, Wagner SJ, Turner ST, et al. Urinary podocyte excretion as a marker for preeclampsia. Am J Obstet Gynecol. Apr, 2007; 196(4)320, e321–327
- Kincaid-Smith P. The renal lesion of preeclampsia revisited. Am J Kidney Dis. Feb, 1991; 17(2)144–148
- Strevens H, Wide-Swensson D, Grubb A, et al. Serum cystatin C reflects glomerular endotheliosis in normal, hypertensive and pre-eclamptic pregnancies. BJOG. Sep, 2003; 110(9)825–830
- Akbari A, Lepage N, Keely E, et al. Cystatin C and beta trace protein as markers of renal function in pregnancy. BJOG. May, 2005; 112(5)575–578