Abstract
Fibrillary glomerulonephritis (FGN) is a relatively rare cause of renal disease, found in only 0.6–1.5% of native renal biopsies. The pathogenesis of FGN is not well described, and very few associations with disease processes other than hepatitis C virus (HCV) have been made. We describe a case that provides evidence in support of the FGN-HCV association, as well as introduces the association of FGN-HCV and hypocomplementemia. The case is a 53-year-old African-American female demonstrating a classical presentation of FGN complicated by a concomitant HCV infection. Treating an HCV infection with alpha-interferon has been shown to result in subsequent improvement in the nephrotic syndrome and renal function. However, this patient is unique in that she is complicated with hypocomplementemia, creating a complex treatment situation.
Fibrillary glomerulonephritis (FGN) is an uncommon but well-described cause of renal disease due to immunoglobulin-composed fibril deposition in the mesangium and glomerular capillary wall, which is found in approximately 0.6–1.5% of native renal biopsies in the United States.Citation[1–7] The pathogenesis of this disease process in most cases is unknown. The cornerstone for diagnosing FGN is immunofluorescence and electron microscopy (EM), which differentiates FGN from other fibril deposition diseases such as amyloidosis and immunotactoid glomerulopathy. FGN does not show Congo red “apple green” birefringence with polarized light but rather evidence of immunoglobulin deposit. EM demonstrates widespread microfibrils randomly arranged and deposited usually in the mesangium and glomerular basement membrane, which are of 12–30 nm in diameter. This is approximately twice the diameter of amyloid fibrilsCitation[1],Citation[2],Citation[5],Citation[8–11] yet smaller than typical immunotactoid microtubular deposits.Citation[1],Citation[4],Citation[12] Until recently, there have been very few associations of FGN made with any other disease entity.Citation[1],Citation[2],Citation[9],Citation[13],Citation[14] Current evidence suggests an association between FGN and hepatitis C virus (HCV).
We report a case that represents additional evidence in support of the association between FGN and HCV, and adds the association of hypocomplementemia, the first case to report this triad of findings.
CASE REPORT
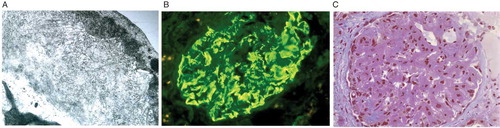
A 53-year-old African-American female with a 33 year history of hypertension (HTN), poorly controlled at all times, and chronic daily NSAID use for 3–4 years presented to the nephrology office as a new consult for proteinuria quantified at 1090 mg/24 hrs. She stated that she has had intermittent hypertensive urgency episodes where she experienced headaches, dizziness, palpitations, and sweats associated with blood pressures of 190s/100s mm/Hg. She had lost 9 kgs in the previous six months and denied a history of hematuria, renal stones, coronary artery disease, stroke, or diabetes. She claimed to smoke one pack of cigarettes per day for 15 years. Her family history was significant for many with HTN, including a son with HTN at age 31. Her medications included verapamil SR (360 mg daily), valsartan/hydrochlorothiazide (160 mg/12.5 mg once daily), albuterol MDI prn, conjugated estrogens/medroxyprogesterone, and vitamins. Physical exam was significant for mild obesity, blood pressure of 148/92 mm/Hg, and alopecia in an otherwise pleasant woman without other abnormal findings. Laboratory results are displayed in . Renal ultrasound showed diffusely echo dense kidneys of normal size, suggesting medical renal disease given her history. A renal biopsy was performed and showed many sclerotic glomeruli. Preserved glomeruli showed mesangial expansion and capillary loop thickening by amorphous material (see ). Congo red stains did not show “apple-green” birefringence with polarized light. Immunofluorescence showed amorphous mesangial and loop deposition of IgG, C3, and C1q, but no IgA or IgM were seen, and there was no light chain restriction when stained with antibodies to kappa and lambda (see ). EM showed mesangial areas expanded by non-branching randomly oriented fibrils of 15–20 nm in thickness also seen in the subendothelial areas of the capillary loops and infiltrating the capillary basement membrane (see ). All of these findings on biopsy with the essential EM are characteristic of FGN, and thus the diagnosis was made.
Table 1 Select laboratory results
Figure 1. A. Histology showing diffuse glomerular infiltration by amorphous material (original 330 ×). B. Immunofluorescence showing amorphous deposits of IgG, C3, and C1q. C. Electron microscopy showing non-branching, randomly oriented fibrils in deposits (original 20,000 ×).
Due to the rapid progression of renal failure with a creatinine clearance of 8.7 mL/min (.15 mL/s) within three months of renal biopsy, the decision was made to start this patient on dialysis. Additional complications due to pseudoaneurysm in the lower pole of the kidney following renal biopsy resulted in recurrent bleeding along with other concurrent medical problems that did not allow for testing various treatment regimens. Unfortunately, within one year of identifying the triad of these conditions, the patient expired.
DISCUSSION
This case demonstrates the classic presentation of a patient with FGN, which includes hypertension, proteinuria, and renal insufficiency. In addition to a rare concomitant HCV infection, our patient was diagnosed with hypocomplementemia. Hypocomplementemia in FGN is rarely reported, as complement levels are typically normal in FGN as compared to other types of glomerulonephritis.Citation[15] However, hypocomplementemia in patients with chronic HCV infection is common.Citation[16],Citation[17]
The literature on the treatment of FGN suggests that optimal treatment regimens typically used for other forms of glomerulonephritis (including the use of corticosteroids and/or cytotoxic agents, such as cyclophosphamide or cyclosporine) are ineffective with FGN, and half of all patients will rapidly progress to end-stage renal disease within 2–4 years.Citation[7],Citation[14] Although there is little information on the combined process of FGN and HCV infection, at least one reported case was treated with alpha-interferon (in response to the active HCV infection) with subsequent improvement in the nephrotic syndrome and renal function. Improvement in FGN following the treatment of the HCV infection supports the hypothesis that in such cases, FGN may not be a primary renal disease but rather a secondary manifestation of an unidentified systemic process.
To our knowledge, this is the first case of FGN, HCV, and hypocomplementemia. Our patient was suffering recurrent retroperitoneal hemorrhages and other concurrent medical problems, and therefore was too unstable to contemplate the use of α-interferon or other therapeutic treatment for the HCV. However, following a thorough review of the literature, the question regarding the best course of treatment for patients with this particular combination of FGN and HCV remains unknown and is further complicated by the addition of hypocomplementemia.
DECLARATION OF INTEREST
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Fogo A, Qureshi N, Horn RG. Morphologic and clinical features of fibrillary glomerulonephritis versus immunotactoid glomerulopathy: Two entities, not one. Am J Kidney Dis. 1993; 22: 448–451
- Iskandar SS, Falk RJ, Jenette JC. Clinical and pathologic features of fibrillary glomerulonephritis. Kidney Int. 1992; 42: 1401–1407
- Rosenstock JL, Markowitz GS, Valeri AM, et al. Fibrillary and immunotactoid glomerulonephritis: Distinct entities with different clinical and pathological features. Kidney Int. 2003; 63: 1450–1461
- Bridoux F, Hugue V, Coldefy O, et al. Fibrillary glomerulonephritis and immunotactoid (microtubular) glomerulopathy are associated with distinct immunologic features. Kidney Int. 2002; 62: 1764–1775
- Duffy JL, Khurana E, Susin M, et al. Fibrillary renal deposits and nephritis. Am J Pathol. 1983; 113: 279–290
- Hvala A, Ferluga D, Vizjak A, Koselj-Kajtna M. Fibrillary noncongophilic renal and extrarenal deposits: a report on 10 cases. Ultrastruct Pathol. 2003; 27: 343–347
- Ivanyi B, Degrell P. Fibrillary glomerulonephritis and immunotactoid glomerulopathy. Nephrol Dial Transplant. 2004; 19(9)2166–2170
- Rosenmann E, Eliakim M. Nephrotic syndrome associated with amyloid-like deposits. Nephron. 1977; 18: 301–308
- Alpers CE, Rennke HG, Hopper J, et al. Fibrillary glomerulonephritis: An entity with unusual immunofluorescence features. Kidney Int. 1987; 31: 781–789
- Mazzucco G, Casanova S, Donini U, et al. Glomerulonephritis with organized deposits: A new clinicopathologic entity? Light, electron microscopic, and immunofluorescence study of 12 cases. Am J Nephrol. 1990; 10: 21–30
- Devaney K, Sabnis SG, Antonovych TT. Nonamyloidotic fibrillary glomerulopathy, immunotactoid glomerulopathy, and the differential diagnosis of filamentous glomerulopathies. Mod Pathol. 1991; 4: 36–45
- Korbet SM, Schwartz MM, Rosenberg BF, et al. Immunotactoid glomerulopathy. Medicine. 1985; 64: 228–243
- D'Agati V, Sacchi G, Truong L, et al. Fibrillary glomerulopathy (FG): Defining the disease spectrum. J Am Soc Nephrol. 1991; 2: 591
- Coroneos E, Truon L, Olivero J. Fibrillary glomerulonephritis associated with hepatitis C viral infection. Am J Kidney Dis. 1997; 29(1)132–135
- Hebert LA, Cosio FG, Neff JC. Diagnostic significance of hypocomplementemia. Kidney Int. 1991; 39: 811–821
- Hepatitis C. virus-associated glomerulonephritis. Adv Intern Med. 1998; 43: 79–97
- Itoh K, Tanaka K, Shiga J, et al. Hypocomplementemia associated with hepatitis C viremia in sera from voluntary blood donors. Am J Gastoenterol. 1994; 89: 2019–2024