Abstract
One hundred and four patients receiving hemodialysis and undergoing anaemia treatment with darbepoetin alfa intravenously once weekly were switched to a biweekly dosing schedule and followed for 24 weeks. The darbepoetin alfa dose was adjusted to maintain the target Hb concentration of 11–14 g/dL. A significant decline in the erythropoiesis-stimulating agent resistance index was observed over the 24-week follow-up, beginning with week 16, whereas the mean dose of darbepoetin alfa did not change significantly after switching to the biweekly dosing schedule. Other factors that might affect resistance to erythropoiesis remained unchanged.
INTRODUCTION
Erythropoiesis-stimulating agents (ESAs), administered appropriately, can effectively maintain hemoglobin (Hb) concentrations in most patients who have anaemia associated with chronic kidney disease.Citation[1–3] However, some of these patients do not respond to ESA therapy with the anticipated increase in Hb concentration. If this inadequate response persists, these patients are said to show ESA resistance or ESA hyporesponsiveness. In the European Best Practice Guidelines (EBPG) developed by a committee of the European Dialysis and Transplant Association and the European Renal Association, resistance to ESAs is defined by the continued need for high-dose ESA therapy (>20,000 IU/kg/week for epoetin alfa/beta or >100 mcg/week for darbepoetin alfa).Citation[4]
It is noted in the guidelines that the most common causes of incomplete response to ESAs are iron deficiency, either absolute or functional, and inflammation.Citation[4] Other conditions associated with ESA resistance include chronic blood loss or hemolysis, hemoglobinopathies such as thalassanemia or sickle cell anaemia, hematologic malignancies or premalignant conditions, hyperparathyroidism, vitamin deficiencies and malnutrition, inadequate dialysis, and drug-related adverse effects.Citation[4–7] Resistance to ESA therapy is usually relative rather than absolute, and therefore the patient's ESA dose is generally increased to overcome the ESA resistance and achieve the target Hb concentration. Nevertheless, it is recommended in the EBPG that the specific cause or causes of ESA resistance be evaluated and treated if possible.Citation[4]
The ESA resistance index (REI) was developed to facilitate the study of factors that influence resistance to ESA treatment. The use of this index may not only helps to pinpoint treatable factors and thus lead to improved ESA response, but it also may help investigators to compare the way in which changes in patient care affect ESA response. The REI is defined as the weekly ESA dose in units/kg body weight (or in the case of darbepoetin alfa, in μg/kg body weight) divided by the Hb concentration in g/dL.Citation[8] Previous studies in patients receiving hemodialysis demonstrated that the REI can be affected by the route of ESA administrationCitation[9] and purity of the dialysis fluid used,Citation[10] among other treatment-related factors.Citation[11]
Darbepoetin alfa is an ESA that has the same mechanism of action as endogenous erythropoietin and other ESAs, but differs from recombinant human erythropoietin (epoetin alfa and beta) through its longer serum half-life, lower receptor binding affinity, and greater biological activity.Citation[12] Because of these characteristics, darbepoetin alfa is effective when administered on less frequent (extended) dosing schedules relative to the epoetin.Citation[13],Citation[14] Extended dosing regimens offer increased convenience for patients. In the EU, darbepoetin alfa is approved for once every two weeks (Q2W) administration in dialysis patients.Citation[15] Studies have shown that there is no increase in dose requirements for darbepoetin alfa administered Q2W in patients receiving hemodialysis compared with once weekly (QW) dosing.Citation[14] The current study was designed to determine whether changing the darbepoetin alfa dosing interval from QW to Q2W affected the REI in patients receiving hemodialysis.
SUBJECTS AND METHODS
Study Design
This prospective, open-label, single-center, single-arm study was conducted on patients receiving hemodialysis at Cartagena Hospital, Cartagena, Spain. Patients receiving darbepoetin alfa QW administered by the intravenous (IV) route were switched to a Q2W IV dosing schedule and followed for 24 weeks. The initial Q2W dose of darbepoetin alfa was calculated by doubling the baseline QW dose of darbepoetin alfa. The dose was titrated according our hospital anaemia management protocol to maintain Hb concentrations between 11 and 14 g/dL. Changes in the hemodialysis schedule were not permitted during the study. All patients provided written informed consent.
The primary study endpoint was the change in REI that occurred when patients were switched from darbepoetin alfa QW administration to Q2W dosing. The REI was calculated every four weeks, from 8 weeks before the switch to Q2W administration to 24 weeks after the conversion, using the following equation:
Secondary endpoints included Hb concentrations and darbepoetin alfa doses determined every four weeks over the course of follow-up, as well as the proportions of patients in whom Hb concentrations and darbepoetin alfa doses were maintained, increased, or decreased during the evaluation period. Other parameters recorded at baseline and 24 weeks after dose conversion included laboratory values reflecting iron status (ferritin and transferrin saturation [TSAT]), parathyroid function (parathyroid hormone [PTH]), inflammation (C-reactive protein [CRP]), and dialysis adequacy (Kt/V).
Patients
To enroll in the study, patients had to be at least 18 years of age, receiving hemodialysis for at least 6 months, and within a baseline Hb concentration of 11 to 14 g/dL and a serum ferritin concentration of 200 to 500 ng/mL. Patients had to have received QW doses of darbepoetin alfa for at least 12 weeks prior to enrollment, with no more than a 15% change in dose during that period. Patients were required to have had stable Hb concentrations (less than 1.5 g/dL variation in Hb concentration) over the eight weeks prior to enrollment.
Patients were excluded from the study if they had a history of congestive heart failure, major surgery, or red blood cell transfusion within 12 weeks of enrollment; clinical evidence of malignancy; current administration of chemotherapy for cancer or antibiotic therapy for systemic infection; or a positive test for HIV antibody or hepatitis B surface antigen. Pregnant or breastfeeding women were also excluded. Other factors such as clinical evidence of severe hyperparathyroidism, presence of active chronic inflammatory processes, or the expectation of receiving a renal transplant were not reasons for exclusion, but were considered in the analysis of results.
Statistical Analysis
Descriptive data are presented as mean (SD). The Friedman test was used as a nonparametric estimator of mean REI and for evaluation of the clinical significance of this parameter. Student's paired t-test was used for intra-group comparisons of continuous variables, and one-way ANOVA was used for inter-group comparisons. Statistical significance was determined as p < 0.05.
RESULTS
Patient Disposition and Characteristics
A total of 104 patients who met the study eligibility criteria were enrolled. Ninety-one (88%) patients completed the study. The reasons for patient discontinuation from the study were: renal transplantation (n = 4), anaemia due to surgery or hemorrhage (n = 4), protocol violations (n = 3), and death (n = 2; see ). presents the baseline characteristics of the enrolled patients.
Table 1 Baseline characteristics
Clinical Endpoints
Primary and Secondary Outcomes
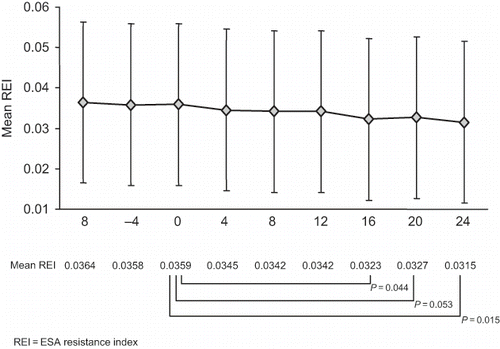
The weekly mean REI is shown in . A clinically significant decline in the REI was observed over the course of follow-up (Friedman's F: p = 0.028). This significant decline in REI (mean [SD]) from baseline was evident by week 16 (0.0359 [0.0241] at baseline vs. 0.0323 [0.0208] at week 16; p = 0.044) and continued until week 24 (0.0315 [0.0208]; p = 0.015).
Figure 2. Change in erythropoiesis-stimulating agent resistance index with darbepoetin alfa over time.
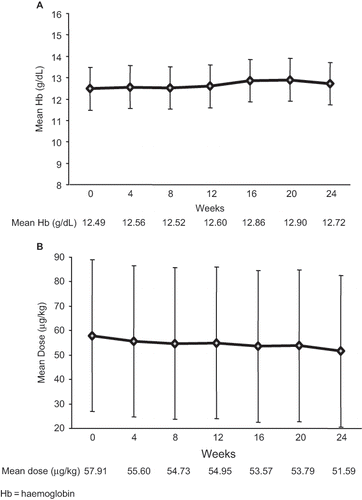
Hb concentrations were maintained over the course of follow-up (see ). At evaluation, the Hb concentrations of 4.4% of the patients were outside the target range of 11 to 14 g/dL. The mean dose of darbepoetin alfa did not change significantly from baseline to 24 weeks after switching from QW to Q2W IV administration (see ).
Figure 3. Change in (a) hemoglobin concentration and (b) dose of darbepoetin alfa after switching from a QW to a Q2W dosing regimen at baseline.
summarizes data obtained on other markers for factors that have been reported to be associated with erythropoietin resistance. There were no significant differences between baseline and week 24 in measures of iron stores (ferritin and TSAT), inflammation (CRP), parathyroid activity (PTH), or dialysis adequacy (Kt/V).
Table 2 Laboratory values for factors associated with erythropoiesis-stimulating agents resistance (n = 91)
Patient Groups
To assess the potential significance of dose changes, patients were grouped according to the change in their biweekly darbepoetin alfa doses at week 24 vs baseline. Among the patients who completed the study (91/104), the darbepoetin alfa dose increased from baseline to week 24 in 24 patients (26%), remained unchanged in 26 patients (29%), and decreased in 41 patients (45%). For the group requiring a dose decrease (see ), the mean darbepoetin alfa Q2W dose was 71.7 μg/kg at baseline vs. 47.2 μg/kg at week 24 (p = 0.001). The darbepoetin alfa Q2W dose was changed more than once during the study period in 60.4% of patients.
Figure 4. Clinical endpoints by change in the status of darbepoetin alfa dose at week 24 vs. baseline.
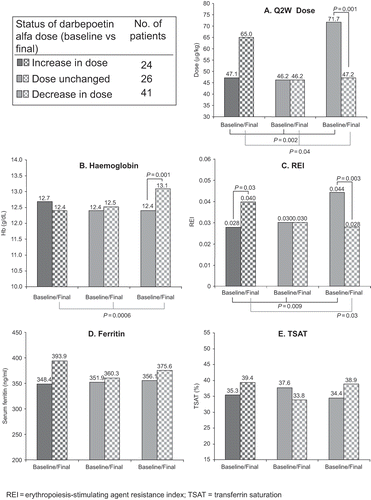
Inter-group comparisons were notable, with significant differences observed between groups for the mean doses of darbepoetin alfa Q2W at baseline and at week 24. The baseline darbepoetin alfa Q2W dose was significantly (p = 0.002) higher in the group requiring a dose decrease than in the other groups. On the other hand, the mean darbepoetin alfa Q2W dose at week 24 was significantly higher in the group requiring a dose increase than in the other groups (p = 0.04).
As shown in and , changes in Hb concentration (p = 0.0006) and REI (p = 0.009) in the three dose response groups demonstrated significant inter- and intra-group differences. The REI was higher at week 24 than at baseline in the group requiring a dose increase (p = 0.03), but there was no significant change in Hb concentration over the study period in this group. The REI declined significantly by the end of the study in the group requiring a dose decrease (p = 0.003), and patients in this group experienced a significant increase in mean Hb concentration from baseline to week 24 (p = 0.001).
There were no clinically relevant differences in measures of iron stores between baseline and week 24 or between dose response groups (see and ).
DISCUSSION
It is well documented that response to ESA therapy in renal anaemia is affected by a number of patient-related factors, such as inflammation and iron status.Citation[4] Recent studies in patients receiving ESAs for renal anaemia have reported an association between ESA resistance and various treatment-related factors. In a prospective, two-arm study, the regimen of epoetin therapy was switched for a group of hemodialysis patients.Citation[9] Fifty-six patients receiving epoetin SC were switched to epoetin IV, and 56 patients receiving epoetin SC or IV were switched to darbepoetin alfa, maintaining the route of administration. Over the course of the study, the REI increased in the group of patients switched to epoetin IV. In contrast, the REI decreased in patients switched to darbepoetin alfa independent of the administration route.
In the present group of hemodialysis patients receiving darbepoetin alfa, data indicate that ESA resistance also may be affected by the dosing schedule. After switching from a QW to a Q2W dosing schedule, Hb concentrations were effectively maintained and darbepoetin alfa dosing requirements decreased or remained the same in the majority of patients. Overall, dose requirements for the Q2W regimen were similar to those for QW treatment, with a slight decrease in the mean dose. The REI appeared to decline after switching to the Q2W extended dosing schedule. Thus, the Q2W dosing schedule for darbepoetin alfa may offer patients the benefit of a reduction in ESA resistance, with the potential for maintaining or even decreasing the darbepoetin alfa dose over time.
The etiology of the observed reduction in ESA resistance during this study in hemodialysis patients is unknown. The use of the REI index, which was developed to assist in the characterization of resistance to ESA treatment, may help in customizing anaemia care for those patients on hemodialysis. Additionally, the REI index might also assist in the comparisons of changes in patient care and their effects on ESA use. Patients who require changes in dose upon conversion from QW to Q2W administration of darbepoetin alfa might have certain clinical characteristics that could help to explain the improved ESA response. Studies have demonstrated an association between epoetin resistance and iron status, hyperparathyroidism, inflammation, and dialysis adequacy.Citation[5–8],Citation[11] However, no change was apparent between baseline and week 24 in this study in markers for iron status, inflammation, hyperparathyroidism, and dialysis adequacy, and changes in darbepoetin alfa dose requirements from baseline to week 24 appeared to be independent of iron status. Therefore, these factors were not likely to have been responsible for the decrease in the REI over the study period, and the reason for the difference remains under investigation.
This study is limited by the open-label, single-arm design. Nonetheless, the findings reported here are compelling and may have clinical implications. Darbepoetin alfa is approved in Europe for QW or Q2W administration to patients with chronic kidney failure in the maintenance phase of anaemia management.Citation[15] Studies on extended dosing of darbepoetin alfa have shown that Hb concentrations are successfully maintained and that extended dosing regimens with darbepoetin alfa are generally well tolerated. At least 95% of patients in these studies remained on the extended dosing regimens during the evaluation periods.Citation[13],Citation[14] The Q2W dosing is more convenient than the QW schedule for some patients receiving hemodialysis and for the dialysis centers. Reduced ESA resistance under Q2W dosing also would be an important benefit, but further studies will be needed to confirm these results and to identify potential mechanisms for the association of ESA resistance with dosing schedule.
In conclusion, Hb levels were effectively maintained after switching a group of hemodialysis patients from a darbepoetin alfa QW schedule to a Q2W schedule. Results indicate that the Q2W extended-dosing regimen with darbepoetin alfa may reduce ESA resistance.
DECLARATION OF INTEREST
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
ACKNOWLEDGMENTS
The authors would like to thank Miguel Ángel González for his patient collaboration. The authors would also like to thank Linda Henson for her assistance in drafting the manuscript.
Notes
The authors state that they have had no involvements that might raise the question of bias in the work reported or in the conclusions, implications, or opinions stated. In addition, the authors state that they received no funding support for this work.
REFERENCES
- Eschbach JW, Egrie JC, Downing MR, Browne JK, Adamson JW. Correction of the anemia of end-stage renal disease with recombinant human erythropoietin. Results of a combined phase I and II clinical trial. N Engl J Med. 1987; 316: 73–78
- Bennett WM. A multicenter clinical trial of epoetin beta for anemia of end-stage renal disease. J Am Soc Nephrol. 1991; 1: 990–998
- Nissenson AR, Swan SK, Lindberg JS, et al. Randomized, controlled trial of darbepoetin alfa for the treatment of anemia in hemodialysis patients. Am J Kidney Dis. 2002; 40: 110–118
- Locatelli F, Aljama P, Barany P, et al. Revised European Best Practice Guidelines for the management of anaemia in patients with chronic renal failure. Nephrol Dial Transplant. 2004; 19(Suppl. 2)1–47
- Smrzova J, Balla J, Barany P. Inflammation and resistance to erythropoiesis-stimulating agents—what do we know and what needs to be clarified?. Nephrol Dial Transplant. 2005; 20(Suppl. 8)2–7
- Madore F, Lowrie EG, Brugnara C, et al. Anemia in hemodialysis patients: Variables affecting this outcome predictor. J Am Soc Nephrol. 1997; 8: 1921–1929
- Macdougall IC, Cooper A. The inflammatory response and epoetin sensitivity. Nephrol Dial Transplant. 2002; 17(Suppl. 1)48–52
- Perez-Garcia R, Rodriguez-Benitez P, Villaverde MT, Valderrabano F. [Is the index of response to erythropoietin (IRE) a good marker of adequate dialysis?]. Nefrologia. 2001; 21: 606–607
- Molina M, Garcia Hernandez MA, Navarro MJ, De Gracia MC, Ortuno T. [Change of EPO treatment from subcutaneous epoetin to intravenous epoetin or darbepoetin alpha]. Nefrologia. 2004; 24: 564–571
- Molina M, Navarro MJ, Palacios ME, et al. [Importance of ultrapure dialysis liquid in response to the treatment of renal anaemia with darbepoetin in patients receiving hemodialysis]. Nefrologia. 2007; 27: 196–201
- Rossert J, Gassmann-Mayer C, Frei D, McClellan W. Prevalence and predictors of epoetin hyporesponsiveness in chronic kidney disease patients. Nephrol Dial Transplant. 2007; 22: 794–800
- Egrie JC, Dwyer E, Browne JK, Hitz A, Lykos MA. Darbepoetin alfa has a longer circulating half-life and greater in vivo potency than recombinant human erythropoietin. Exp Hematol. 2003; 31: 290–299
- Carrera F, Disney A, Molina M. Extended dosing intervals with erythropoiesis-stimulating agents in chronic kidney disease: a review of clinical data. Nephrol Dial Transplant. 2007; 22(Suppl. 4)19–30
- Carrera F, Oliveira L, Maia P, Mendes T, Ferreira C. The efficacy of intravenous darbepoetin alfa administered once every two weeks in chronic kidney disease patients on hemodialysis. Nephrol Dial Transplant. 2006; 21: 2846–2850
- Aranesp®. Summary of product characteristics. BredaThe Netherlands 2006, Amgen Europe B.V.,