Abstract
Midodrine is an alpha-agonist that causes peripheral vasoconstriction, resulting in increased blood pressure. It has been reported to be safe and effective in patients with end stage renal disease (ESRD) and is widely used for hemodialysis-associated hypotension. We report a case report of midodrine-induced ischemia in a patient on hemodialysis and review the literature relating to the safety of midodrine in patients with end stage renal disease.
INTRODUCTION
Hemodialysis-associated hypotension is a common complication in patients with end stage renal disease (ESRD) undergoing chronic hemodialysis (HD). There are two common types of hemodialysis-associated hypotension. One occurs usually during HD treatment (i.e., intradialytic hypotension, IDH) and the other is a more chronic, persistent form of hypotension. As many as 10–30% of treatments are complicated by symptomatic hypotension,Citation[1] and 5–10% of the dialysis population suffers from chronic hypotension.Citation[2] Hemodialysis-associated hypotension contributes significantly to morbidity and mortality in this populationCitation[3],Citation[4] and may be refractory to multiple therapeutic modalities, including reduced ultrafiltration, high dialysate sodium, cool dialysate, and increased dialysate calcium concentration.
The exact mechanism of HD-associated hypotension is not well known; however, it has been ascribed to multiple factors, including excessive ultrafiltration, autonomic dysfunction, and cardiac disease. Among them, autonomic insufficiency is thought to be a primary contributing cause of hemodialysis hypotension. Since the introduction of midodrine for the treatment of symptomatic orthostatic hypotension due to autonomic failure of various etiologies, there has been much interest in using it in ESRD patients with HD-associated hypotension. Its use in HD was first described by Blowey et al.Citation[5] in a case report in 1996. Subsequently, Flynn and coworkersCitation[6] reported a case series of 21 patients in whom the short-term use of midodrine was associated with a decrease in IDH and was without significant adverse events. Since then, several investigators have reported a beneficial effect of midodrine in HD patients with IDHCitation[7–12] or chronic HD hypotension.Citation[2],Citation[13],Citation[14] A systematic review of these studies examining midodrine use for dialysis-associated hypotension had reached the same conclusions.Citation[15]
Midodrine is well tolerated in patients with orthostatic hypotension and normal renal function, with only 7.9% of 3,030 patients who received up to 15 months of treatment reporting adverse events.Citation[16] The most commonly reported adverse events were pilomotor reactions (goose bumps, formication, tingling, and chills; 55%), followed by gastrointestinal complaints (nausea, heartburn, stomatitis; 12.6%), cardiovascular effects (tachycardia, palpitations, supine hypertension, bradycardia; 9.4%) and central nervous system (CNS) effects (headache, dizziness, restlessness, excitability and irritability; 8.4%), sleep disturbances (3.7%), hot flashes (2.1%), allergic skin reaction (1%), and increased desire to urinate (0.5%). Generally, midodrine-induced reactions were mild and transient, responding to a decrease in midodrine dosage. The tolerability profile of midodrine has been confirmed in patients with normal renal functionCitation[17],Citation[18] as well as in HD population.Citation[2],Citation[5–10],Citation[13–15]
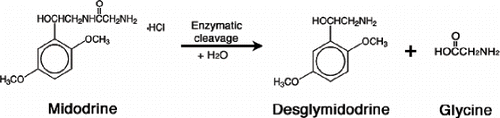
Midodrine hydrochloride is an oral pro-drug that forms an active metabolite, desglymidodrine, which is an alpha1-agonist (see ). This agent increases both arteriolar and venous tone, improving blood return to the heart, which results in a rise in standing, sitting, and supine systolic and diastolic blood pressure in patients with hypotension.Citation[6],Citation[16] Midodrine is rapidly absorbed in the gastrointestinal (GI) tract, converted to the active metabolite in the systemic circulation, and renally cleared. The bioavailability of desglymidodrine is 93% and reaches peak levels in approximately 60–90 minutes, with a half-life of three hours in patients with normal renal function. However, in patients with ESRD, the half-life is increased to an average of 9–10 hours.Citation[5] Both the pro-drug and its active metabolite are effectively removed by HD given their low binding to proteins (24–31%) as well as their molecular weight of 290.75 daltons, shortening desglymidodrine half-life to 1.4 hours. Midodrine is specific for the alpha-1 receptor and is unable to cross the blood-brain barrier, thereby minimizing the cardiac and CNS effects in patients treated with this drug.
Figure 1. Conversion of midodrine (pro-drug) to its active metabolite desglymidodrine via enzymatic hydrolysis.
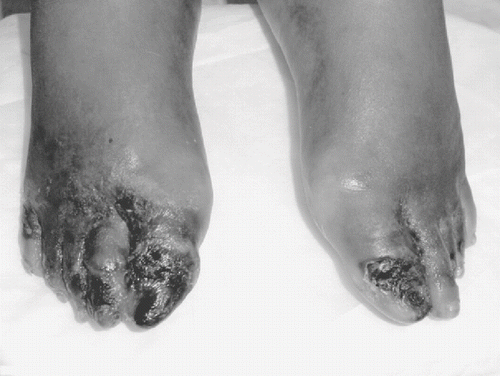
Though midodrine has been reported to be safe, effective, and well tolerated with minimal side effects in ESRD patients, studies in this population have contained small numbers of patients with short-term follow-up. We describe a case of a woman with ESRD on HD who developed ischemia of her lower extremities while taking midodrine. We believe this to be the first report describing midodrine-induced ischemia in a patient with ESRD.
CASE REPORT
The patient is a 48-year-old African-American woman with ESRD secondary to lupus nephritis who has been receiving HD since 1981. Her other medical problems include HD-associated amyloidosis with peripheral neuropathy, secondary hyperparathyroidism necessitating parathyroidectomy two years prior, and renal cell carcinoma in both kidneys requiring bilateral native nephrectomies performed six months prior to presentation. Since the nephrectomies, the patient developed chronic systemic hypotension with pre-hemodialysis systolic blood pressures (SBP) around 80 mmHg. An extensive work-up for secondary causes of hypotension, which included an echocardiogram, thyroid function tests, and ACTH stimulation test, failed to reveal an etiology. After attempting various therapeutic interventions, treatment with midodrine was initiated. Midodrine was titrated to 5 mg daily on non-HD days; on HD days, she took 5 mg 30 minutes prior to HD and then a second dose of 5 mg 6–8 hours after HD. The patient had a subsequent increase in her SBP to 90–110 mmHg and maintenance of blood pressure during HD. The patient did not exhibit any adverse effects to midodrine for the first six months of treatment with this medication. Approximately six months after starting therapy with midodrine, the patient began to report pain in her feet that woke her up from sleep. Upon physical exam, she had indurated and painful toes on both feet with areas of ulceration on the right first toe. She had palpable femoral arterial pulses, and pedal pulses were detectable by Doppler ultrasound bilaterally. Over the next week, the pain and ulcerations progressed to involve more of the right toe, and new ulcerations appeared on the right second toe and left big toe. An angiogram of the lower extremities revealed severe small vessel disease with multiple areas of segmental occlusions in the superficial femoral, popliteal, and tibial vessels. Ankle pressures could not be measured due to vessel calcification.
Midodrine was discontinued approximately seven days after the development of symptoms with subsequent immediate improvement in her pain in both feet. The erythema and ulceration improved on her left foot. The progression of the lesions on her right foot slowed significantly with cessation of the midodrine; however, she developed dry gangrene involving her first through fourth toes (see ) and underwent revascularization of her right leg and subsequently right transmetatarsal amputation. Pathologic examination of the toes revealed gangrenous tissue with no evidence of calcemia uremic arteriolopathy. Eventually, the patient required bilateral below knee amputations due to infection of the necrotic lesions.
DISCUSSION
Midodrine is considered to be well tolerated, safe, and effective for the treatment of HD-associated hypotension that is resistant to most other interventions, and is widely used for this purpose by nephrologists. A recent meta-analysis of 10 studies with five or more hemodialysis patients supports this conclusion.Citation[15] In most of the studies that have evaluated the safety of midodrine, the majority of patients had diabetes as the underlying etiology of ESRD, and some patients had coronary artery disease (CAD) and peripheral vascular disease (PVD). A summary of previous studies of midodrine with data relevant to safety is provided in . Cruz et al.Citation[9] studied 13 patients with IDH for five to eight months. They reported that midodrine 10 mg given 30 minutes before each HD session was well tolerated in all 13 patients, five of whom had cardiomyopathy (CM) with impaired left ventricular (LV) function, and seven of whom had concomitant CAD. None of these patients developed coronary ischemia or exacerbation of heart failure. The same group of investigatorsCitation[10] performed a prospective crossover study of 11 patients comparing 10 mg of midodrine given prior to HD with cool dialysate for nine consecutive HD treatments. They found no adverse events attributable to midodrine. In this study, three patients had CAD, and four had cardiomyopathy. In another study by Cruz et al.,Citation[8] 10 patients were given 5 mg of midodrine 30 minutes prior to HD for 10 consecutive dialysis sessions, with only one patient developing an adverse reaction (scalp paresthesias). Three patients in this study had CAD, and one patient had cardiomyopathy. Montagnac et al.Citation[14] reported a single patient on HD with CAD and PVD who received 10 mg of midodrine twice daily for 4.5 years without significant adverse effects.
Table 1 Safety profile of midodrine in hemodialysis population with HD-associated hypotension
These small studies suggest that midodrine given prior to HD is safe in patients with CAD and PVD. However, the safety of daily midodrine in this patient population has not been well studied. There is a report in the literature suggesting that similar vasoactive agents may induce tissue ischemia. In a letter to the editor, StamatiadisCitation[19] reported a case of a 74-year-old male on continuous ambulatory peritoneal dialysis (CAPD) who developed symptomatic hypotension with negative work-up for secondary causes. Treatment with etilefrine hydrochloride, an alpha/beta-agonist used for the treatment of orthostatic hypotension (not available in the United States), was initiated at the dose of 10 mg three times daily with improvement in patient clinical status and blood pressure. However, shortly after starting treatment, the patient developed extensive bowel ischemia due to thrombosis of the mesenteric artery.
We report a case in which a patient with ESRD and underlying PVD developed vascular ischemia while on daily midodrine, at a dose that is widely used in patients with chronic hypotension. While this patient had several medical problems that likely contributed to her acute arterial insufficiency, we believe that midodrine played an important role in her presentation. Thus, we believe that nephrologists should exercise caution when prescribing midodrine in patients with underlying PVD, especially in those with diseases that predispose them to the development of vasospasm. Adequately powered, prospective studies with long-term follow-up are needed to determine the safety and efficacy of midodrine in patients with ESRD.
DECLARATION OF INTEREST
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
ACKNOWLEDGMENT
The authors would like to thank Dr. Jaime Uribarri from the Mount Sinai School of Medicine in New York for his invaluable comments and the help in reviewing this manuscript.
REFERENCES
- Perazella MA. Approach to patients with intradialytic hypotension: A focus on therapeutic options. Semin Dial. 1999; 12: 175–181
- Lin YF, Wang JY, Denq JC, Lin SH. Midodrine improves chronic hypotension in hemodialysis patients. Am J Med Sci. 2003; 325(5)256–261
- Daugirdas JT. Preventing and managing hypotension. Semin Dial. 1994; 7: 276–283
- Zager PG, Nikolic J, Brown RH, et al. “U” curve association of blood pressure and mortality in hemodialysis patients. Kidney Int. 1998; 54: 561–569
- Blowey DL, Balfe JW, Gupta I, Gajaria MM, Koren G. Midodrine efficacy and pharmacokinetics in a patient with recurrent intradialytic hypotension. Am J Kidney Dis. 1996; 28(1)132–136
- Flynn JJ, Mitchell MC, Caruso FS, McElligott MA. Midodrine treatment for patients with hemodialysis hypotension. Clin Nephrol. 1996; 45(4)261–267
- Lim PS, Yang CC, Li HP, Lim YT, Yeh CH. Midodrine for the treatment of intradialytic hypotension. Nephron. 1997; 77(3)279–283
- Cruz DN, Mahnensmith RL, Perazella MA. Intradialytic hypotension: Is midodrine beneficial in symptomatic hemodialysis patients?. Am J Kidney Dis. 1997; 30(6)772–779
- Cruz DN, Mahnensmith RL, Brickel HM, Perazella MA. Midodrine is effective and safe therapy for intradialytic hypotension over 8 months of follow-up. Clin Nephrol. 1998; 50(2)101–107
- Cruz DN, Mahnensmith RL, Brickel HM, Perazella MA. Midodrine and cool dialysate are effective therapies for symptomatic intradialytic hypotension. Am J Kidney Dis. 1999; 33(5)920–926
- Alappan R, Cruz D, Abu-Alfa AK, Mahnensmith R, Perazella MA. Treatment of severe intradialytic hypotension with the addition of high dialysate calcium concentration to midodrine and/or cool dialysate. Am J Kidney Dis. 2001; 37(2)294–299
- Hoeben H, Abu-Alfa AK, Mahnensmith R, Perazella MA. Hemodynamics in patients with intradialytic hypotension treated with cool dialysate or midodrine. Am J Kidney Dis. 2002; 39(1)102–107
- Fang JT, Huang CC. Midodrine hydrochloride in patients on hemodialysis with chronic hypotension. Ren Fail. 1996; 18(2)253–260
- Montagnac R, Clavel P, Delhotal-Landes B, Flouvat B, Poulain S, Schllinger F. Use of midodrine (Gutron) to treat permanent hypotension in a chronic hemodialysis patient. Clin Nephrol. 2001; 56(2)162–168
- Prakash S, Garg AX, Heidenheim P, House AA. Midodrine appears to be safe and effective for dialysis-induced hypotension: A systematic review. Nephrol Dial Transplant. 2004; 19: 2553–2558
- McTavish D, Goa KL. Midodrine. A review of its pharmacological properties and therapeutic use in orthostatic hypotension and secondary hypotensive disorders. Drugs. 1989; 38(5)757–777
- Low PA, Gilden JL, Freeman R, Sheng KN, McElligott MA. Efficacy of midodrine vs. placebo in neurogenic orthostatic hypotension. A randomized, double-blind multicenter study. Midodrine Study Group. JAMA. 1997; 277(13)1046–1051
- Jankovic J, Gilden JL, Hiner BC, et al. Neurogenic orthostatic hypotension: A double-blind, placebo-controlled study with midodrine. Am J Med. 1993; 95(1)38–48
- Stamatiadis DN, Gounari PA, Balitsari AV, Bokos JG, Stathakis CP. Hypotension on CAPD: Can use of vasoconstrictive drugs be harmful?. Perit Dial Int. 2002; 22(1)94–95