Abstract
Patients on hemodialysis (HD) are prone to atherosclerotic cardiovascular complications. In an attempt to determine the significance of several atherosclerotic and thrombogenic parameters as risk factors for atherothrombotic cardiovascular disease (CVD) in these patients, we compared two groups of non-diabetic HD patients matched for age and sex, selected according to the absence (group 1, n = 30) or presence (group 2, n = 30) of symptomatic atherothrombotic vascular disease affecting the coronary, cerebral, or peripheral arteries. Duration of HD, primary renal disease (PRD), presence of hypertension, EPO treatment, and smoking habits were recorded. Serum total cholesterol (TC), triglycerides (TG), HDL-C, LDL-C, TC/HDL-C ratio, lipoprotein(a) (Lp(a)), fibrinogen (FG), plasminogen (PLG), fibronectin (FN), and hematocrit (HCT) were measured pre-HD in a midweek session. The same blood parameters were also assessed in twenty matched clinically healthy subjects (controls). None of the blood parameters differed between groups 1 and 2, except for serum Lp(a) and FN, which were higher in group 2 (p = 0.005 and p = 0.041, respectively). Both groups were not different regarding PRD, duration of HD, and EPO treatment, but the presence of hypertension and smoking habits were more common in group 2 (p = 0.008 and p = 0.045, respectively). Moreover, multiple stepwise logistic regression analysis with Lp(a), FN, hypertension, and smoking showed that the presence of hypertension (p = 0.016) and the Lp(a) (p = 0.027) and FN (p = 0.024) levels, but not smoking, were independent predictors for the presence of atherothrombotic CVD. Our results suggest that hypertension, abnormal lipid particles, and thrombogenic proteins may contribute to the high prevalence of CVD in HD patients.
INTRODUCTION
Cardiovascular disease (CVD) is the leading cause of morbidity and mortality in chronic uremic patients undergoing hemodialysis (HD).Citation[1] The annual mortality rate due to CV complications is approximately 9%, which in the case of middle-aged patients may exceed the rate noted in general population by more than 50 times. Given that atherosclerotic arterial occlusive disease accidents in the form of myocardial or cerebral infarction represent nearly half of these CV deaths, chronic renal disease has been identified as a “vasculopathic state.”Citation[1–4] The understanding and the management of its determinants have therefore become a major focus of nephrology care. Classic risk factors for CVD disease established by the Framingham study such as age, hypertension, diabetes mellitus, and dyslipidemia are highly prevalent in HD patients.Citation[5],Citation[6] However, there is sufficient data to support the hypotheses that classic CVD risk factors cannot fully explain the excess risk noted in these patients, and a number of non-traditional risk factors have been already proposed in many studies.Citation[7–9] Hyperfibrinogenemia, hyperhomocysteinemia, hyperlipoproteinemia(a), oxidative stress, chronic inflammation, and malnutrition, related to uremic state per se and/or to the dialysis procedure have all been proposed as non-traditional risk factors that play a significant role in the development of atherosclerosis in uremic patients on maintenance HD.Citation[10–15]
Recently, Orem et al.Citation[16] suggested that plasma fibronectin (FN) levels may be a significant predictor of coronary artery disease in the general population. Furthermore, it has been reported that increased FN levels are associated with vascular access occlusion,Citation[17] though to our knowledge, plasma FN levels have not been investigated yet as risk factor for CVD in these patients.
In an attempt to determine the significance of several traditional and non-traditional atherothrombogenic parameters as risk factors for atherothrombotic vascular disease, we compared two age- and sex-matched groups of non-diabetic uremic patients on regular HD according to the absence or the presence of symptomatic vascular disease affecting the coronary, cerebral, and/or peripheral arteries. Diabetic patients are obviously, even in predialytic period, exposed to an increased risk of atherosclerosis, and they also have higher morbidity and mortality than comparable non-diabetic patients.Citation[18] Thus, diabetic patients were not included in our study, as they would have added significant heterogeneity to our study dialysis population, leading to reduced statistical power of our results.
MATERIALS AND METHODS
Sixty (60) non-diabetic uremic patients (30 males, 30 females) on maintenance HD participated in this study, which was approved by the local ethics committee. The entire population of patients was selected from the in-center dialysis units of three hospitals in Athens and was stratified in two groups of patients, matched for age and sex, according to the absence (group 1, n = 30, 15 males, 15 females) or the presence (group 2, n = 30, 16 males, 14 females) of symptomatic atherosclerotic vascular disease affecting the coronary (n = 23), cerebral (n = 2), and peripheral (n = 1) arteries, or in combination the coronary and cerebral (n = 3) or the coronary and peripheral (n = 1) arteries. All subjects were asked to participate in the protocol and gave informed consent. Pertinent information on patient clinical parameters, including primary renal disease, duration of HD, presence of hypertension, erythropoietin (EPO) treatment, and smoking habits were obtained by medical records. No patients suffered from diabetes mellitus, hepatic dysfunction, malnutrition (albumin < 3 g/dL), severe hyperparathyroidism (PTH > 300 ng/L), or infection. No patient used lipid-lowering medication or oral anticoagulants, though the vast majority (80%) of our patients in both groups was on folic acid (5 mg/day) and vitamin B complex (B1, B6, B12) supplementation. Patients were classified as hypertensives if they were on regular antihypertensive treatment, which was also recorded. Only hypertensive patients with adequate blood pressure control (actual BP < 160/95 mmHg in interdialytic period and at the beginning of HD session) were included in the study.
The diagnosis of coronary artery disease was made if the following conditions were met:
medical history was remarkable for prior myocardial infarction, according to electrocardiographic and enzyme criteria by WHO;
corresponding changes in ECG were present, and angina pectoris could be diagnosed on the basis of typical symptoms by the Rose questionnaire; and
coronary angiography showed luminal narrowing >50% in at least one coronary artery.
Cerebral artery disease was accepted if there were a prior non-fatal cerebral infarction (ischemic stroke) with focal neurological deficit, in the absence of arterial embolism, and with evidence of infarction on computerized tomodensitometry or magnetic resonance imaging documented by hospital records. Peripheral vascular disease was diagnosed if symptoms of intermittent claudication defined by the Rose questionnaire were present, or if an absent pedal pulse was observed by clinical examination and Doppler ultrasound. Only the patients who have exhibited symptomatic atherothrombotic vascular disease after initiation of HD therapy were included in group 2. According to the study protocol, patients of both groups were on bicarbonate HD for at least six months before participating in the study, dialyzed with low-flux synthetic membrane (polysulphone (Psu)) or low-flux synthetically modified cellulose membrane (Hemophan), and received low molecular weight heparin as an anticoagulant. The control group consisted of 20 clinically healthy subjects, with no history of hypertension or renal disease and serum creatinine levels <1.2 mg/dL, who were matched by age and sex to maintenance HD patients of both groups.
Blood samples in HD patients were obtained immediately before the midweek HD session and after overnight fasting to determine total cholesterol (TC), triglycerides (TG), HDL-C, LDL-C, TC/HDL-C ratio, lipoprotein(a) (Lp(a)), fibrinogen (FG), plasminogen (PLG), FN, and hematocrit (HCT) values. The same blood biochemical parameters were also measured in the control group. Serum TC, TG, and HDL-C were determined enzymatically with commercial kits, using a biochemical analyzer (Technicon, RA-1000), without freezing the samples, while LDL-C was calculated according to Friedewald formula. TC/HDL-C ratio was also calculated and recorded. Serum Lp(a) was determined by immunochemical method (N Antiserum to Human Coagulation Factors and C1 Inhibitor, Date Behring Inc.). Plasma FN was also determined by immunochemical method (N Antiserum to Human Fibronectin, Date Behring Inc.).
All blood parameters are expressed as mean ± SD, with the exception of the investigated clinical parameters, which were recorded as yes or no. These parameters are expressed as percent (%) and absolute number (n) of patients (in brackets) and were analyzed with the chi-square test. Normally distributed data were analyzed with the Student's t-test, and data not showing Gaussian distribution were analyzed with the Wilcoxon signed rank test. Multiple stepwise logistic regression analysis was used to detect the independence of variables, which predict the presence of atherothrombotic vascular disease. All variables that were found to be statistically different in univariate analysis between the two groups of patients were entered in the multivariate model. Statistical analysis was performed using STATA (version 8) statistical software. p values < 0.05 were considered statistically significant.
RESULTS
As shown in , the two groups of non-diabetic HD patients were successfully matched for age and sex. Data describing duration of HD, primary renal disease, smoking habits, presence of hypertension, and EPO treatment in group 1 (absence of CVD) and group 2 (presence of CVD) of patients are also displayed in . Duration of HD and primary renal disease were not significantly different between the two groups. Although chronic pyelonephritis was more frequent in group 1 (20%) vs. group 2 (10%), and hypertensive nephrosclerosis was more frequent in group 2 (23%) vs. group 1 (7%), the two groups were not found to be statistically significant different regarding primary renal disease (see ). The vast majority of patients in both groups were on EPO treatment (80% in group 1 vs. 76.7% in group 2, p = NS) without any significant difference between them (see ). By contrast, there were striking differences in smoking habits and the presence of hypertension between the two groups. Smoking habits were significantly more prevalent in group 2 (presence of atherothrombotic vascular disease, 40%, 12/30, vs. group 1, 16.7%, 5/30; p = 0.0457). Similarly, the presence of hypertension was significantly more common in patients of group 2 (60%, 198/30) compared to patients in group 1 (26.6%, 8/30, p = 0.0086; see ).
Table 1 Comparison of the investigated clinical parameters between the age- and sex-matched groups 1 (absence) and 2 (presence) of CVD of non-diabetic uremic HD patients
Blood biochemistry parameters in the two matched groups of patients are shown in and . Comparison of the studied blood parameters between groups 1 and 2, as well as between HD patient groups and the control group are shown in . Both HD patient groups had significantly higher values of TC, TG, TC/HDL-C ratio, and FG values, as well as lower HDL-C values, than controls. PLG levels differed from controls in neither group, whereas LDL-C, Lp(a) and FN were higher only in group 2 compared to controls. However, when compared with each other, patients in group 2 had significantly higher Lp(a) (approximately twice as much) as well as FN levels than group 1. In particular, the proportion of patients with Lp(a) level >30 mg/dL was 56.7% (17/30) in group 2 compared to 16.7% (5/30) in group 1. There were no other significant differences in the studied blood biochemistry parameters between the two groups of HD patients, as shown in .
Figure 1. Total cholesterol (TC in mg/dL), triglycerides (TG in mg/dL), HDL cholesterol (HDL in mg/dL), LDL cholesterol (LDL in mg/dL), total cholesterol to HDL cholesterol ratio (TC/HDL) and lipoprotein(a) (Lp(a) in mg/dL) concentrations in groups 1 (absence of CVD, n = 30) and 2 (presence of CVD, n = 30). The only statistically significant difference noted was in Lp(a) levels (p = 0.005).
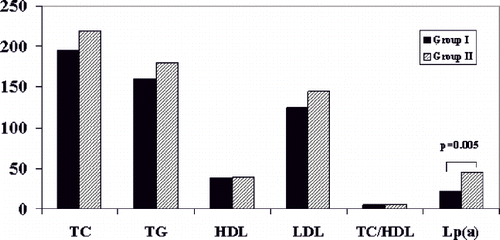
Figure 2. Fibrinogen (FG in mg/dL), plasminogen (PLG in mg/dL), fibronectin (FN in mg/dL) and hematocrit (%) levels in groups 1 (absence of CVD, n = 30) and 2 (presence of CVD, n = 30). The only statistically significant difference noted was in FN levels (p = 0.041).
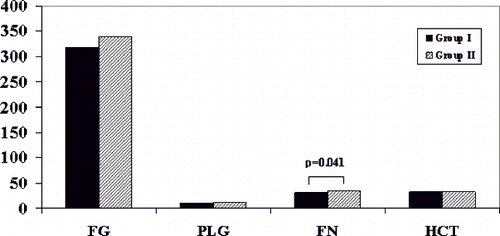
Table 2 Laboratory parameters (mean ± SD) in the age- and sex-matched groups 1 (absence) and 2 (presence) of CVD of HD patients and matched healthy controls
It should be noted that a significant positive correlation was found between FG and FN levels in both patient groups (r = 0.55, p = 0.006 in group 1 and r = 0.60, p = 0.004 in group 2), while in group 2 of patients, an additional positive correlation was detected between FN and Lp(a) levels (r = 0.49, p = 0.025), as well as between age and LDL-C levels (r = 0.51, p = 0.021). No further significant correlations between the studied clinical and laboratory parameters were found in both patient groups.
Finally, multiple stepwise logistic regression analysis with all parameters, which were significantly different between the two patient groups in univariate analysis, indicated that serum Lp(a) (OR = 1.027, p = 0.036) and FN (OR = 1.83, p = 0.045) levels and the presence of hypertension (OR = 4.748, p = 0.016), but not smoking habits (OR = 3.071, p = 0.121) were significant predictors of CVD. All possible interactions between hypertension and Lp(a) or FN levels and smoking were checked, but none reached significance at the level of 5%. In the final multiple logistic regression model, Lp(a) and FN levels, as well as the presence of hypertension, were identified as independent risk factors for atherothrombotic CVD in non-diabetic HD patients, as shown in .
Table 3 Logistic regression analysis with independently significant variables predicting risk for CVD in non-diabetic uremic patients on maintenance HD
DISCUSSION
As mentioned above, despite scientific progress in renal replacement therapy techniques, CV mortality rates in end-stage renal disease (ESRD) patients remain much higher than the general population.Citation[4] The severity and the complexity of CVD in HD patients are constant stimuli for further scientific research in this field. Our study showed that the presence of hypertension and increased FN and Lp(a) levels are independent prognostic factors for symptomatic CVD in non-diabetic HD patients.
Hypertension is very common in HD patients and has been consistently reported as an independent predictor of adverse CV outcomes in these patients,Citation[19] as well as in predialysis ESRD patients.Citation[20] However, a “U” curve association between blood pressure and mortality has been also reported in HD population.Citation[21] The results from the DOPPS study, which included 17,000 HD patients, showed that both low (<120 mmHg) and high (>150 mmHg) systolic blood pressure are associated with high mortality rates.Citation[22] We compared age- and sex-matched HD patients with and without CV disease and found that hypertension increased the risk for symptomatic vascular disease in these patients by a magnitude of four. Our findings are in accordance with several studiesCitation[23–25] that clearly show that hypertension is an independent predictor of left ventricular hypertrophy and ischemic heart disease. Thus, hypertension should be considered a major CVD risk factor and be monitored and treated carefully.
Human FN is a multifunctional glycoprotein and plays a key role in cell adhesion and migration, hemostasis, thrombosis, wound healing, and tissue remodeling.Citation[26] A malfunctioning FN molecule may lead to inadequate wound repair and prolonged wound healing.Citation[27] Although there is considerable range in FN concentrations in adults, there is a tendency for higher concentrations in the presence of inflammation and aging.Citation[28] Experimental studies have provided evidence that alterations in certain domains of the FN molecule induced by inflammation are implicated in atherosclerosis, as molecules including these certain domains have been identified in atherosclerotic lesions.Citation[29] Taking into consideration that atherosclerosis is characterized by chronic inflammation and is a form of prolonged wound healing, which eventually results in luminal narrowing,Citation[30] FN seems to play a significant role in the atherosclerotic process. Indeed, Orem et al.Citation[16] showed that FN concentration is an independent predictor of the presence of coronary artery disease and is positively correlated with CRP, LDL, and total cholesterol concentrations. In accordance to the previous study, Song et al.Citation[31] found that FN levels are higher in patients with ischemic heart disease and hypertension compared to healthy subjects, and that there is a positive correlation between FN levels, total cholesterol, triglycerides, and systolic blood pressure. Our study, in accordance with the previous findings, showed that an increase in FN levels by 1 mg/dL was associated with almost 20% higher risk for symptomatic vascular disease.
Furthermore, several studies have shown that there is a positive association between Lp(a) and vascular risk. In a meta-analysis of 27 prospective studies, those with Lp(a) in the top tertile had 1.6 higher vascular risk compared to those in the lowest one.Citation[32] Although whether Lp(a) concentration has an additive value over classic CVD risk factors in general population is still controversial,Citation[33] it seems that its evaluation is of greater importance in patients with premature atherosclerosis and renal failure.Citation[34] We showed that an increase in Lp(a) concentration by 1 mg/dL is independently associated with 2.7% higher risk for symptomatic vascular disease. According to our findings, Goldwasser et al.Citation[17] showed that high Lp(a) concentrations (above 57 mg/dL) are independently associated with thrombotic events in HD patients, and that high FN levels could contribute additional risk. From all of the above studies, it seems that there is an interaction between Lp(a) and FN. Experimental studies have shown that Lp(a) can bind to fibrin competing with PLG and subsequently leading to inhibition of endogenous fibrinolytic system.Citation[35] Furthermore, Lp(a) acts on tissue factor and PLG activator within atherosclerotic lesions and induces chemotactic activity within atherosclerotic lesions.Citation[36] Clinical studies have supported these findings, and it seems that Lp(a) plays a proatherogenic/thrombotic role as it attenuates fibrinolysis and promotes coagulation.Citation[37] At the same time, Lp(a) is associated with inflammation, as its concentration in patients with ESRD is influenced by acute phase response.Citation[38] In our previous study, we showed that Lp(a) is almost doubled post-HD with modified cellulose membranes, and that this was related to TNFa release.Citation[39]
Although further studies would be necessary to confirm our findings, it seems that abnormal lipoprotein particles and thrombotic factors in hypertensive uremic patients are implicated in CVD. Thus, high-risk patients should be identified and subjected to sufficient blood pressure control and lipid-lowering therapy.
DECLARATION OF INTEREST
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Locatelli F, et al. Cardiovascular disease in chronic renal failure: The challenge continues. Registro Lombardo Dialisi e Trapianto. Nephrol Dial Transplant. 2000; 15(Suppl)69–80
- Whitaker JN. Effects of pregnancy and delivery on disease activity in multiple sclerosis. N Engl J Med. 1998; 339(5)339–340
- Baigent C, Burbury K, Wheeler D. Premature cardiovascular disease in chronic renal failure. Lancet. 2000; 356(9224)147–152
- U.S. Renal Data System (USRDS). Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, Md 2007
- Bianchi G. Hypertension in chronic renal failure and end-stage renal disease patients treated with hemodialysis or peritoneal dialysis. Nephrol Dial Transplant 2000; 15(Suppl. 5)105–110
- Webb AT, et al. Lipids and lipoprotein(a) as risk factors for vascular disease in patients on renal replacement therapy. Nephrol Dial Transplant. 1995; 10(3)354–357
- Sarnak MJ, et al. Cardiovascular disease risk factors in chronic renal insufficiency. Clin Nephrol. 2002; 57(5)327–335
- Muntner P, et al. The prevalence of nontraditional risk factors for coronary heart disease in patients with chronic kidney disease. Ann Intern Med. 2004; 140(1)9–17
- Longenecker JC, et al. Traditional cardiovascular disease risk factors in dialysis patients compared with the general population: The CHOICE Study. J Am Soc Nephrol. 2002; 13(7)1918–1927
- Bolton CH, et al. Endothelial dysfunction in chronic renal failure: Roles of lipoprotein oxidation and pro-inflammatory cytokines. Nephrol Dial Transplant. 2001; 16(6)1189–1197
- Bostom AG, et al. Hyperhomocysteinemia, hyperfibrinogenemia, and lipoprotein (a) excess in maintenance dialysis patients: A matched case-control study. Atherosclerosis. 1996; 125(1)91–101
- Pawlak K, et al. Oxidative stress—a link between endothelial injury, coagulation activation, and atherosclerosis in hemodialysis patients. Am J Nephrol. 2004; 24(1)154–161
- Liu Y, et al. Association between cholesterol level and mortality in dialysis patients: Role of inflammation and malnutrition. JAMA 2004; 291(4)451–459
- Boaz M, et al. Comparison of hemostatic factors and serum malondialdehyde as predictive factors for cardiovascular disease in hemodialysis patients. Am J Kidney Dis. 1999; 34(3)438–444
- Shoji T, Nishizawa Y. Chronic kidney disease as a metabolic syndrome with malnutrition—need for strict control of risk factors. Intern Med. 2005; 44(3)179–187
- Orem C, et al. Plasma fibronectin level and its association with coronary artery disease and carotid intima-media thickness. Coron Artery Dis. 2003; 14(3)219–224
- Goldwasser P, et al. Correlates of vascular access occlusion in hemodialysis. Am J Kidney Dis. 1994; 24(5)785–794
- Wirta O, et al. Renal and cardiovascular predictors of nine-year total and sudden cardiac mortality in non-insulin-dependent diabetic subjects. Nephrol Dial Transplant. 1997; 12(12)2612–2617
- Horl MP, Horl WH. Hemodialysis-associated hypertension: Pathophysiology and therapy. Am J Kidney Dis. 2002; 39(2)227–244
- Jungers P, et al. Incidence and risk factors of atherosclerotic cardiovascular accidents in predialysis chronic renal failure patients: A prospective study. Nephrol Dial Transplant. 1997; 12(12)2597–2602
- Zager PG, et al. “U” curve association of blood pressure and mortality in hemodialysis patients. Medical Directors of Dialysis Clinic, Inc. Kidney Int. 1998; 54(2)561–569
- Stidley CA, et al. Changing relationship of blood pressure with mortality over time among hemodialysis patients. J Am Soc Nephrol. 2006; 17(2)513–520
- Mominadam S, et al. Interdialytic blood pressure obtained by ambulatory blood pressure measurement and left ventricular structure in hypertensive hemodialysis patients. Hemodial Int. 2008; 12(3)322–327
- Tomita J, et al. Role of systolic blood pressure in determining prognosis of hemodialyzed patients. Am J Kidney Dis. 1995; 25(3)405–412
- Foley RN, et al. Impact of hypertension on cardiomyopathy, morbidity and mortality in end–stage renal disease. Kidney Int. 1996; 49(5)1379–1385
- Magnusson MK, Mosher DF. Fibronectin: Structure, assembly, and cardiovascular implications. Arterioscler Thromb Vasc Biol. 1998; 18(9)1363–1370
- Yamada KM. Fibronectin peptides in cell migration and wound repair. J Clin Invest. 2000; 105(11)1507–1509
- Mosher DF. Plasma fibronectin concentration: A risk factor for arterial thrombosis?. Arterioscler Thromb Vasc Biol. 2006; 26(6)1193–1195
- Tan MH, et al. Deletion of the alternatively spliced fibronectin EIIIA domain in mice reduces atherosclerosis. Blood. 2004; 104(1)11–18
- Yee KO, Schwartz SM. Why atherosclerotic vessels narrow: The fibrin hypothesis. Thromb Hemost. 1999; 82(2)762–771
- Song KS, et al. Plasma fibronectin levels in ischemic heart disease. Atherosclerosis. 2001; 154(2)449–453
- Danesh J, Collins R, Peto R. Lipoprotein(a) and coronary heart disease. Meta-analysis of prospective studies. Circulation. 2000; 102(10)1082–1085
- Ridker PM, et al. Established and emerging plasma biomarkers in the prediction of first atherothrombotic events. Circulation. 2004; 109(25 Suppl. 1)IV6–IV19
- Ariyo AA, Thach C, Tracy R. Lp(a) lipoprotein, vascular disease, and mortality in the elderly. N Engl J Med. 2003; 349(22)2108–2115
- Hancock MA, et al. Inhibition of plasminogen activation by lipoprotein(a): Critical domains in apolipoprotein(a) and mechanism of inhibition on fibrin and degraded fibrin surfaces. J Biol Chem. 2003; 278(26)23260–23269
- Buechler C, et al. Lipoprotein (a) up-regulates the expression of the plasminogen activator inhibitor 2 in human blood monocytes. Blood. 2001; 97(4)981–986
- Berglund L, Ramakrishnan R. Lipoprotein (a): An elusive cardiovascular risk factor. Arterioscler Thromb Vasc Biol. 2004; 24(12)2219–2226
- Quaschning T, et al. Abnormalities in uremic lipoprotein metabolism and its impact on cardiovascular disease. Am J Kidney Dis. 2001; 38(4 Suppl. 1)S14–S9
- Tzanatos HA, et al. Cytokine release and serum lipoprotein (a) alterations during hemodialysis. Artif Organs. 2000; 24(5)329–333