Abstract
Background. Previous studies demonstrate that icodextrin is superior to 4.25% dextrose for fluid removal in patients with high and high-average transport membrane. Recent studies reveal that controlling volume status improves malnutrition in peritoneal dialysis (PD) patients. This study hypothesized that icodextrin enhances nutritional and inflammatory status by improving fluid balance. Methods. This retrospective case-control study investigated the effects of icodextrin on patient nutritional profiles over a one-year period. Thirty-two patients who used icodextrin for more than one year were classified as the “icodextrin group.” Ten patients who used glucose-containing dialysate without icodextrin were classified as the control group. Clinical and laboratory parameters were compared between groups. Demographic and laboratory parameters were analyzed at baseline, 3 months, 6 months, and 12 months after starting icodextrin dialysis. Results. Ultrafiltration of icodextrin per exchange in the icodextrin group was 66% higher than that for 4.25% dextrose exchange in the icodextrin group (icodextrin vs. 4.25% dextrose: 492.1 ± 204.5 vs. 296.1 ± 115.3 mL/exchange; p < 0.0001, paired t-test). The increased albumin and normalized protein catabolic rate (nPCR) after icodextrin for one year was unique for the icodextrin group (p < 0.0001 and p < 0.0001, respectively). The inflammatory marker high sensitivity C-reactive protein (hsCRP) decreased significantly only in the icodextrin group (p = 0.0048). Conclusion. Icodextrin dialysate may improve nutritional and inflammatory status in PD patients. However, the long-term clinical effects of icodextrin require further study.
INTRODUCTION
Icodextrin (Extraneal®) is a starch-derived, water-soluble glucose polymer with an average molecular weight of 20,000 Dalton.Citation[1] Iso-osmotic 7.5% icodextrin solution induces transcapillary ultrafiltration (UF) by colloid osmosis in contrast with hyper-osmotic dextrose-based solutions, which induce UF by crystalline osmosis.Citation[1] Minimal absorption of icodextrin produces sustained osmotic pressure facilitating UF during long dwell times (10–14 hours).Citation[1] The availability of an icodextrin-based dialysate is a significant advance in fluid management for patients on peritoneal dialysis (PD).Citation[2] Its use has greatly changed prescription options, results, and technique survival.Citation[3]
Large, randomized, controlled, multi-center clinical trials have confirmed the efficacy and safety of icodextrin in patients on both continuous ambulatory PD (CAPD) and continuous cyclic PD (CCPD).Citation[24],Citation[25] Previous studies suggest that icodextrin is superior to 4.25% dextrose for fluid removal by long-dwell exchange in patients with high and high-average transport membrane.Citation[26–28] Recent studies also indicate the positive effects of icodextrin on peritoneal membrane preservation,Citation[29],Citation[30] left ventricular hypertrophy,Citation[31] and insulin resistance.Citation[4]
Malnutrition can predict mortality in peritoneal dialysis patients.Citation[5],Citation[6] Decreased protein intake, energy deficiency, concurrent chronic illness, catabolic stimulus of the dialysis procedure, loss of nutrients into dialysate, and endocrine disorders are all considered possible causes of malnutrition in peritoneal dialysis patients.Citation[7] Recent studies demonstrate that malnutrition is associated with fluid overload in maintenance dialysis patients.Citation[32],Citation[33] Furthermore, fluid balance is also associated with inflammatory status in peritoneal dialysis patients.Citation[11],Citation[16] Previous studies indicate that controlling volume status improves malnutrition in peritoneal dialysis patients.Citation[11],Citation[34] This study hypothesized that icodextrin would improve nutritional and inflammatory status by optimizing fluid balance. To test this hypothesis, this one-year retrospective case-control study investigated the effects of icodextrin on nutritional profiles of patients on CAPD or CCPD.
PATIENTS AND METHODS
Patients Selection
Adult PD patients over the age of eighteen years from the PD unit of Chang Gung Memorial Hospital in Keelung, Taiwan, were enrolled in this study from January 1, 2005, to December 31, 2006. All patients had been on PD for at least one year. Icodextrin had been prescribed for these patients due to insufficient UF, poor blood sugar control, and weight gain due to dialysate glucose exposure. Patients who had taken calcitriol, vitamin C, or iron preparations during the study period were excluded from the study to control for the effects of these supplements. The icodextrin group was comprised of thirty-two patients who had used icodextrin for more than one year. Thirty of these patients were categorized as high or high-average in initial peritoneal equilibration test (PET) studies. Only two of these patients were categorized in low or low average PET categories. Ten patients using glucose-containing dialysate without icodextrin constituted the control group. Only two of these patients were in high or high average PET categories.
Study Design and Parameter Measures
This retrospective case-control study examined clinical and laboratory parameters in both of the above groups. At baseline, all patients underwent a standard PET according to the method described by Twardowski et al.Citation[8] In the icodextrin group, patients received one 7.5% icodextrin exchange (2 L Extraneal®, Baxter Healthcare) with dwelling time of 10 to 14 hours. The exchanges were performed overnight in CAPD patients or during daytime in CCPD patients. The UF volume was compared before (while using 4.25% dextrose solution) and after (while using 7.5% icodextrin dialysate). Demographic and laboratory parameters were collected at baseline and at 3, 6, and 12 months thereafter. Examined data included age, gender, body surface area (BSA), duration on PD, PD modality, underlying disease, serum hemoglobin, hematocrit, blood urea nitrogen (BUN), creatinine, sodium (Na), potassium (K), calcium (Ca), phosphate (P), uric acid, albumin, bicarbonate, aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase, total bilirubin, total cholesterol, triglyceride, fasting glucose, intact parathyroid hormone (iPTH), ferritin, transferrin saturation rate, and high sensitivity C-reactive protein (hsCRP). Serum calcium level was corrected according to albumin level. All patients provided 24-hour urine and dialysate samples at baseline and then after 6 and 12 months to calculate Kt/Vurea. The distribution volume of urea (V) was calculated by Watson equation.Citation[9] Normalized protein catabolic rate (nPCR) was calculated by Randerson equation.Citation[10] Peritoneal equilibration test was performed after starting icodextrin dialysate for one year.
Statistical Analysis
Continuous variables were expressed as mean ± standard deviation. Categorical variables were expressed as frequency counts. Because of the small size of the control group (n = 10), nonparametric analysis with Wann-Whitney test and Fisher's exact test were used to estimate the differences between means in unpaired groups. Repeated measures ANOVA was used to estimate the differences between repeated measures in paired groups. A two-tailed p less than 0.05 was considered statistically significant. All statistical tests were performed using GraphPad Prism, Version 4.0 (GraphPad Software Inc, San Diego, California, USA).
RESULTS
Patient Characteristics
Forty-two peritoneal dialysis patients (thirty-two patients in icodextrin group; ten patients in control group) were enrolled in this study and followed up for one year. All patients completed the study.
lists general and clinical characteristics of patients selected for enrollment in this study at baseline. The two groups did not significantly differ in demographic variables, underlying diseases, or laboratory measures. The dialysis dosages, represented by Kt/Vurea, did not differ between the two groups. Residual renal functions, which were indicated by Kt/Vurea of residual renal function, did not differ between the two groups at the beginning of the study.
Table 1 Patient characteristics of icodextrin and control groups at baseline
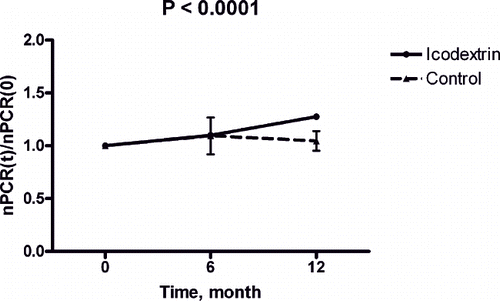
Normalized PCR, an indicator of short-term nutritional status in dialysis patients, did not differ between the icodextrin and control groups before the study.
Effect of Ultrafiltration and Metabolism
After three months of icodextrin therapy, the mean UF of icodextrin per exchange in the icodextrin group was 66% higher than that in the 4.25% dextrose exchange before starting icodextrin (icodextrin vs. 4.25% dextrose: 492.1 ± 204.5 vs. 296.1 ± 115.3 mL/exchange; p < 0.0001, paired t-test; see ). Laboratory measures at baseline and after 3, 6, and 12 months were analyzed in the control group (n = 10). Creatinine level significantly increased (p = 0.013), and residual renal function (Kt/Vurea) significantly decreased throughout the observation period (p = 0.0098; see ). These laboratory results suggested continuous loss of residual renal function.
Figure 1. Ultrafiltration volume of 4.25% glucose and 7.5% icodextrin solution in icodextrin group patients before and after starting icodextrin dialysis.
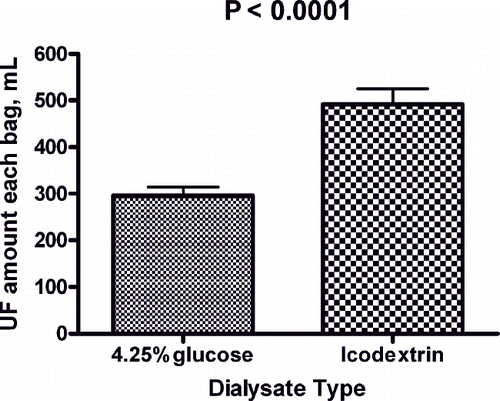
Table 2 Comparisons of laboratory parameters at different timing in control group (n = 10)
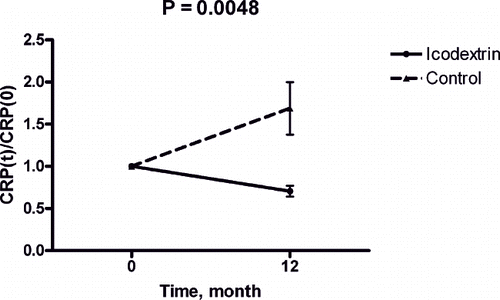
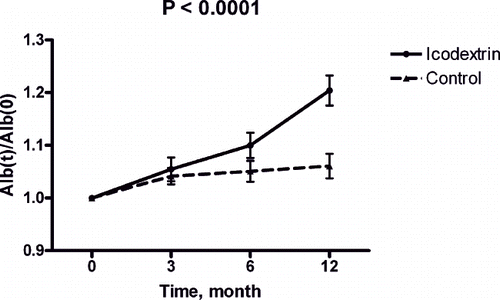
Laboratory measures at baseline and after 3, 6, and 12 months were also studied in the icodextrin group (n = 32). Creatinine levels significantly increased (p < 0.0001), and residual renal function (Kt/Vurea) significantly decreased during the same period (p < 0.0001; see ). Serum bicarbonate, albumin level and normalized protein catabolic rate (nPCR) increased significantly during the one-year observation period (p = 0.0004, p < 0.0001, and p < 0.0001, respectively; see ). High sensitivity CRP and transferrin saturation rate decreased significantly throughout the dialysis period (p = 0.0048 and p = 0.003; see ). This observation suggested that icodextrin might have benefited acid-base balance, nutritional status, inflammatory status, and iron utilization during the one-year observation period.
Table 3 Comparisons of laboratory parameters at different timing in icodextrin group (n = 32)
Hemoglobin and hematocrit levels increased gradually but not significantly (p = 0.06 and p = 0.07; see ). Ferritin levels decreased gradually but not significantly (p = 0.26; see ).
Albumin increased in the icodextrin group but not in the control group (see ). Analysis of nPCR revealed a similar tendency (see ). This observation suggested that nutritional status improved after starting the icodextrin dialysis regimen. Comparison of hsCRP between the two groups suggested that icodextrin evokes less inflammation than dextrose dialysates (see ).
Figure 2. Sequential albumin change during study period in control (broken line) and icodextrin (continuous line) groups.
DISCUSSION
Malnutrition is clearly related to mortality and morbidity in peritoneal dialysis patients. This study revealed improved bicarbonate level, transferrin saturation rate, albumin level, nPCR, and decreased serum hsCRP level after icodextrin. These analytical results suggest that a PD regimen of icodextrin improves nutritional and inflammatory status.
Improved fluid status is associated with improved nutritional status, and deteriorating fluid status resulted in the development of malnutrition.Citation[11] In PD patients, volume overload tends to cause low intake of calories and dietary protein. Gastrointestinal edema and poor ingestion may result from fluid overload.Citation[11] Chronic fluid overload status may be associated with higher serum endotoxin and inflammatory cytokines levels in PD and heart failure patients.Citation[12],Citation[13] Elevated levels of circulating tumor necrosis factor (TNF) and other proinflammatory cytokines result from altered gut permeability, bacterial or endotoxin translocation in bowel lumen, and reduced tissue perfusion.Citation[12],Citation[33],Citation[35] Elevated TNF levels suppress appetite and induce anorexia by inhibiting the normal adaptive feeding response to energy deficits.Citation[12],Citation[36],Citation[37] Inflammation also results in loss of muscle mass by activating the ubiquitin-proteasome proteolytic system.Citation[14],Citation[15] Controlling volume status can improve systemic endotoxin levels, inflammation, and malnutrition.Citation[11],Citation[13],Citation[16] Taken together, these factors suggest that icodextrin improves malnutrition and reduces inflammatory status. Serum albumin and nPCR are medium- and short-term nutritional markers in dialysis patients.Citation[5],Citation[6] Icodextrin dialysis significantly increased nPCR and albumin in this study. High sensitivity CRP is a nonspecific marker of inflammatory response.Citation[17] Icodextrin dialysis significantly decreased hsCRP level throughout the study period. These beneficial effects are likely exerted by icodextrin via improved fluid balance.
Icodextrin may also have beneficial effects via mechanisms other than fluid balance. Appetite is regulated by the hypothalamus via several circulating mediators.Citation[18] Leptin, a protein of 167 amino acids, is one of these mediators.Citation[19] Leptin directly affects the hypothalamus by decreasing appetite and increasing metabolism.Citation[18] Recent studies suggest that hypoleptinemia may cause uremic anorexia and malnutrition.Citation[20] Patients under peritoneal dialysis therapy have higher leptin levels than those under hemodialysis therapy.Citation[18] Serum leptin levels progressively increase under PD therapy because of marked increases in body fat mass as a consequence of continuous carbohydrate load.Citation[21] Recent studies indicate that icodextrin administration is followed by a decrease in leptonema.Citation[22] Decreased leptin levels may result from a reduction in body weight due to decreased carbohydrate load, or enhanced elimination of leptin due to ultrafiltration-induced convective transport.Citation[22] Data for serum leptin levels were not available for these patients. Further studies may examine whether leptin levels are decreased in patients using icodextrin dialysates.
This retrospective case-control study did not control for PET categories, as only patients in high or high average PET categories used icodextrin dialysis. However, these patients are associated with poor nutritional status.Citation[23] The specific characteristics of these patients further demonstrate the beneficial effects of icodextrin on the nutritional status of this specific patient group. Prospective randomized studies are needed to clarify the causal relationship between icodextrin dialysis and nutrition-inflammatory status in PD patients with different membrane permeability characteristics. However, because of ethical concerns, performing randomized prospective studies in high permeability PD patients is problematic. Previous well-designed studies have already determined that icodextrin is beneficial for fluid control in high permeability PD patients and is associated with lower mortality and morbidity.Citation[24–28] However, conducting a prospective randomized clinical study to answer this important question would be inappropriate. We believe the current finding of an association between icodextrin dialysis and nutrition-inflammatory status is of clinical interest and important for daily clinical care of PD patients.
In conclusion, icodextrin may improve nutritional and inflammatory parameters in high permeability PD patients. The effect may be beneficial for patients under PD therapy. However, further studies are necessary to evaluate the long-term effects of icodextrin on clinical outcome.
DECLARATION OF INTEREST
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
ACKNOWLEDGMENTS
The authors would like to thank Ted Knoy for his editorial assistance.
REFERENCES
- Wiggins KJ, Rumpsfeld M, Blizzard S, Johnson DW. Predictors of a favourable response to icodextrin in peritoneal dialysis patients with ultrafiltration failure. Nephrology (Carlton). Feb, 2005; 10(1)33–36
- Wolfson M, Ogrinc F, Mujais S. Review of clinical trial experience with icodextrin. Kidney Int Suppl. Oct, 2002, 81: S46–S52
- Amici G, Da Rin G, Agostini A, Velo S, Bocci C. Ten years after MIDAS: Icodextrin lights and shadows. Contrib Nephrol. 2003, 140: 76–81
- Gursu EM, Ozdemir A, Yalinbas B, et al. The effect of icodextrin and glucose-containing solutions on insulin resistance in CAPD patients. Clin Nephrol. Oct, 2006; 66(4)263–268
- Kaysen GA. The microinflammatory state in uremia: causes and potential consequences. J Am Soc Nephrol. Jul, 2001; 12(7)1549–1557
- Wu MS, Lin CL, Chang CT, Wu CH, Huang JY, Yang CW. Improvement in clinical outcome by early nephrology referral in type II diabetics on maintenance peritoneal dialysis. Perit Dial Int. Jan–Feb, 2003; 23(1)39–45
- Wang T, Heimburger O, Bergstrom J, Lindholm B. Nutritional problems in peritoneal dialysis: An overview. Perit Dial Int. 1999; 19(Suppl. 2)S297–S303
- Twardowski ZJ, Nolph KD, Khanna R, et al. Peritoneal equilibration test. Peritoneal Dialysis Bulletin. 1987; 7(3)138–147
- Katzarski K, Charra B, Laurent G, et al. Multifrequency bioimpedance in assessment of dry weight in hemodialysis. Nephrol Dial Transplant. 1996; 11((Suppl.) 2)20–23
- Kopple JD, Jones MR, Keshaviah PR, et al. A proposed glossary for dialysis kinetics. Am J Kidney Dis. 1995; 26(6)963–981
- Cheng LT, Tang W, Wang T. Strong association between volume status and nutritional status in peritoneal dialysis patients. Am J Kidney Dis. 2005; 45(5)891–902
- Aguilera A, Codoceo R, Selgas R, et al. Anorexigen (TNF-alpha, cholecystokinin) and orexigen (neuropeptide Y) plasma levels in peritoneal dialysis (PD) patients: Their relationship with nutritional parameters. Nephrol Dial Transplant. Jun, 1998; 13(6)1476–1483
- Niebauer J, Volk HD, Kemp M, et al. Endotoxin and immune activation in chronic heart failure: A prospective cohort study. Lancet. 1999; 353(9167)1838–1842
- Mitch WE. Malnutrition: A frequent misdiagnosis for hemodialysis patients. J Clin Invest. 2002; 110(4)437–439
- Mitch WE, Goldberg AL. Mechanisms of muscle wasting. The role of the ubiquitin-proteasome pathway. N Engl J Med. 1996; 335(25)1897–1905
- Chung SH, Heimburger O, Stenvinkel P, Wang T, Lindholm B. Influence of peritoneal transport rate, inflammation, and fluid removal on nutritional status and clinical outcome in prevalent peritoneal dialysis patients. Perit Dial Int. 2003; 23(2)174–183
- Selim G, Stojceva-Taneva O, Zafirovska K, et al. Inflammation predicts all-cause and cardiovascular mortality in hemodialysis patients. Prilozi. 2006; 27(1)133–144
- Stenvinkel P. Leptin and its clinical implications in chronic renal failure. Miner Electrolyte Metab. 1999; 25(4–6)298–302
- Rahmouni K, Haynes WG. Leptin and the cardiovascular system. Recent Prog Horm Res. 2004; 59: 225–244
- Johansen KL, Mulligan K, Tai V, Schambelan M. Leptin, body composition, and indices of malnutrition in patients on dialysis. J Am Soc Nephrol. 1998; 9(6)1080–1084
- Vignioble M, Brichard S, Jadoul M, Goffin E. Serum leptin concentration in peritoneal dialysis patients: Determinants, longitudinal evolution and circadian rhythm. Acta Clin Belg. 2001; 56(3)173–179
- Opatrna S, Racek J, Stehlik P, et al. [Effect of a dialysis solution with icodextrin on ultrafiltration and selected metabolic parameters in patients treated with peritoneal dialysis.]. Cas Lek Cesk. 2002; 141(9)281–285
- Wu CH, Huang CC, Huang JY, Wu MS, Leu ML. High flux peritoneal membrane is a risk factor in survival of CAPD treatment. Adv Perit Dial. 1996; 12: 105–109
- Posthuma N, ter Wee PM, Donker AJ, Oe PL, Peers EM, Verbrugh HA. Assessment of the effectiveness, safety, and biocompatibility of icodextrin in automated peritoneal dialysis. The Dextrin in APD in Amsterdam (DIANA) Group. Perit Dial Int. 2000; 20(Suppl. 2)S106–S113
- Wolfson M, Piraino B, Hamburger RJ, Morton AR. A randomized controlled trial to evaluate the efficacy and safety of icodextrin in peritoneal dialysis. Am J Kidney Dis. Nov, 2002; 40(5)1055–1065
- Finkelstein F, Healy H, Abu-Alfa A, Ahmad S, Brown F, Gehr T, et al. Superiority of icodextrin compared with 4.25% dextrose for peritoneal ultrafiltration. J Am Soc Nephrol. Feb, 2005; 16(2)546–554
- Davies SJ, Woodrow G, Donovan K, Plum J, Williams P, Johansson AC, et al. Icodextrin improves the fluid status of peritoneal dialysis patients: Results of a double-blind randomized controlled trial. J Am Soc Nephrol. Sep, 2003; 14(9)2338–2344
- Woodrow G, Oldroyd B, Stables G, Gibson J, Turney JH, Brownjohn AM. Effects of icodextrin in automated peritoneal dialysis on blood pressure and bioelectrical impedance analysis. Nephrol Dial Transplant. Jun, 2000; 15(6)862–866
- Davies SJ. Exploring new evidence of the clinical benefits of icodextrin solutions. Nephrol Dial Transplant. Jul, 2006; 21(Suppl. 2)ii47–ii50
- le Poole CY, Welten AG, Weijmer MC, Valentijn RM, van Ittersum FJ, ter Wee PM. Initiating CAPD with a regimen low in glucose and glucose degradation products, with icodextrin and amino acids (NEPP) is safe and efficacious. Perit Dial Int. Feb, 2005; 25(Suppl. 3)S64–S68
- Konings CJ, Kooman JP, Schonck M, Gladziwa U, Wirtz J, van den Wall Bake AW, et al. Effect of icodextrin on volume status, blood pressure and echocardiographic parameters: A randomized study. Kidney International. Apr, 2003; 63(4)1556–1563
- Dumler F. Hypoalbuminemia is a marker of overhydration in chronic maintenance patients on dialysis. Asaio J. May–Jun, 2003; 49(3)282–286
- Wang AY, Sanderson J, Sea MM, Wang M, Lam CW, Li PK, et al. Important factors other than dialysis adequacy associated with inadequate dietary protein and energy intakes in patients receiving maintenance peritoneal dialysis. The American Journal of Clinical Nutrition. Apr, 2003; 77(4)834–841
- Jones CH, Wells L, Stoves J, Farquhar F, Woodrow G. Can a reduction in extracellular fluid volume result in increased serum albumin in peritoneal dialysis patients?. Am J Kidney Dis. Apr, 2002; 39(4)872–875
- Freeman LM, Roubenoff R. The nutrition implications of cardiac cachexia. Nutrition Reviews. Oct, 1994; 52(10)340–347
- Fantino M, Wieteska L. Evidence for a direct central anorectic effect of tumor-necrosis-factor-alpha in the rat. Physiology & Behavior. Mar, 1993; 53(3)477–483
- Kirchgessner TG, Uysal KT, Wiesbrock SM, Marino MW, Hotamisligil GS. Tumor necrosis factor-alpha contributes to obesity-related hyperleptinemia by regulating leptin release from adipocytes. The Journal of Clinical Investigation. Dec 1, 1997; 100(11)2777–2782