Abstract
Essential mixed cryoglobulinemia (type II) has turned out to be secondary to hepatitis C virus (HCV) infection in the large majority of patients. Interferon might be anticipated to be effective only in HCV-associated cryoglobulinemias. We found that interferon was highly effective in an HCV-positive patient with true essential type II mixed cryoglobulinemia. The patient presented with symptomatic cryoglobulinemic vasculitis without underlying immunologic, infectious, or neoplastic diseases. Tests for HCV viremia, a reverse-transcriptase–polymerase-chain-reaction (RT-PCR) assay, and anti-HCV antibodies (third-generation assays) were positive before therapy. The patient had severe cryoglobulinemic vasculitis with purpura, peripheral neuropathy, and membranous proliferative glomerulonephritis. The cryocrit before therapy was 6 percent in the patient. Recombinant interferon alfa-2a (Roferon-A, Hoffmann–LaRoche, Basel, Switzerland) was administered at a dose of 3 million units per day for three months and 3 million units every other day for the subsequent nine months, a protocol adopted for HCV-associated cryoglobulinemia. The patient had a complete clinical response, with the disappearance of serum cryoglobulins and all signs of cutaneous vasculitis and with the normalization of kidney-function results and urinary values in the patient with nephropathy. The patient has remained in complete remission for more than one year since the withdrawal of therapy. True essential mixed cryoglobulinemia with HCV infection complicated with glomerulonephritis represents a therapeutic challenge.
INTRODUCTION
Essential mixed cryoglobulinemia (type II) has turned out to be secondary to hepatitis C virus (HCV) infection in the large majority of patients. Interferon is now considered the treatment of choice. It is generally believed that the efficacy of interferon in patients with mixed cryoglobulinemia depends on the suppression of HCV viremia, as viral antigens seem to be a key factor in cryoprecipitates. As a corollary, interferon might be anticipated to be effective only in HCV-associated cryoglobulinemias. The patient had severe cryoglobulinemic vasculitis with purpura, peripheral neuropathy, and membranous proliferative glomerulonephritis. True essential mixed cryoglobulinemia without evidence of HCV infection represents a therapeutic challenge. Herein our findings warrant a trial with interferon in such cases.
CASE PRESENTATION
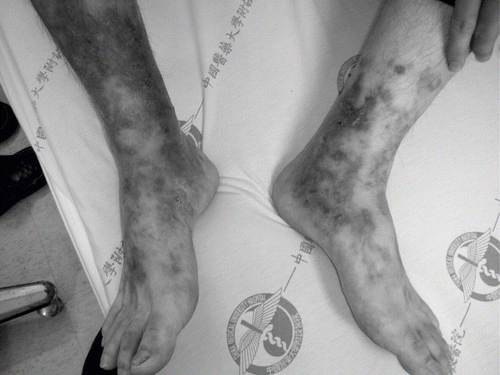
A 65-year-old female patient developed frequent recurrence of ulceration over bilateral lower legs with severe painful sensation noted four years previously. The patient was first visited OPD on April 3rd, 2006, due to purpuric macules over bilateral lower legs off and on for two years. The lesion got worse during cold weather. However, there was no painful sensation, abdominal pain, or arthralgia. In addition, ulceration over lower legs was noted over bilateral lower legs at that time. She visited our OPD on May 28th, 2006. Meanwhile, she was admitted to our ward because the wounds on bilateral lower limbs worsened. Upon admission, her temperature was 36°C, blood pressure 69 mmHg, pulse rate 97 per minute, and respiratory rate 22 per minute. Her general appearance was ill looking. Physical examination revealed showed clear breathing sound in the both lung fields and no pitting edema of lower extremities, and multiple skin lesions were observed over bilateral lower legs. Gangrene change was noted over bilateral lower legs. In addition, multiple irregular or stellate shaped erythematous macules, progressing to pustules and tightly adherent crust on bilateral lower legs, was found, especially on medial ankles (see ). The laboratory data at admission showed white blood cell 11,320 (neu-segm 70.0%, lympho 23.6%), red blood cell 3.03 × 106/ul, hemoglobulin 9.1 g/dL, hematocrit 27.9%, MCV 92.1 fl., MCH 30.0 pg, MCHC 32.6 g/dL, platelet 547 × 103/ul, glucose 109 mg/dL, aspartate aminotransferase (AST) 34 IU/L, alanine aminotransferase (ALT) 35 IU/L, lactate dehydrogenase (LDH) 188 IU/L, BUN 21 mg/dL, creatinine 1.5 mg/dL, Na133 mmol/L, and K 3.6 mmol/L. Urine routine revealed 3+ for protein and 3+ for occult blood, and the urinary sediment contained 15–20 red cells, 5–10 white cells, and few glandular casts per high power field. The urinary protein excretion level was 3.6 g/day. ANA, anti-dsDNA, anti-Sm, anti-RNP, and rheumatoid factor was negative. We also found C3 of 64 mg/dL (normal, 60–120) and C4 23.5 (normal, 14–24). Cryoglobulin was positive. Tests for the hepatotropic hepatitis A, B, C, and E viruses (RT-PCR or serologic assays) were also negative. Chest x-ray was normal. Cardiac echo was done as well, and only trivial tricuspid regurgitation was found. Abdominal echo was performed, but no abscess or space-occupying lesions were found. Renal echogram showed increased echogenicity of both renal parenchymas. Due to nephrotic range proteinuria, renal biopsy was performed on the seventh day of admission. Two of the 12 glomeruli obtained on PAS staning demonstrated circumferential and cellular crescent formation. Mononuclear cell infiltrate was found over tubules and interstitial area under light microscopy, which is compatible with interstitial nephritis (see ). Immunofluorescence staining demonstrated mesangial deposits of IgA and capillary walls deposits of IgA, IgM, and C3. Under electron microscopy, no electron dense deposit was found. Skin biopsy was performed and leukocytoclastic vasculitis was noted. Immunohistochemical stain of skin biopsy revealed no granular deposition of IgG, IgM, IgA, C3, and C1q. The culture result revealed infection of Aeromonas veronii. Ecthyma gangrenosum was diagnosed. The patient received treatment including vasodilator, intravenous prostaglandin E1, aspirin, plaquenil, prograf, methylprednisolone, leukeran, and antibiotics such as rocephin. After the above treatment, the wounds improved and patient was discharged.
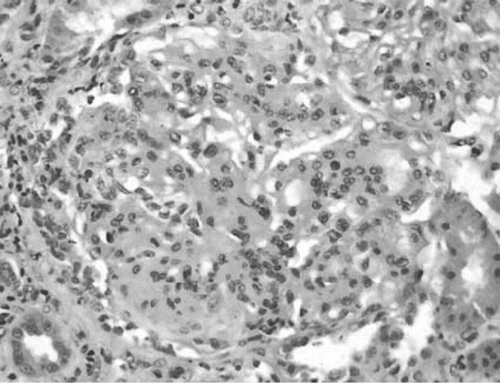
Figure 2.PAS staning demonstrated tuft lobulation and mesangial hypercellularity. This appearance is very characteristic of membranoproliferative GN.
Over December 7th, 2006, to January 3rd, 2007, the patient was admitted again because similar symptoms were flared up. Treatment included antibiotics (ampicillin), trental, persantine, aspirin, plaquenil, methylprednisolone, and prednisolone. Due to progressive distal leg gangrene, low molecular heparin (clexane) was given on December 8th, 2006. Hyperbaric oxygen therapy was performed from December 30th, 2006, to January 7th, 2007; however, her symptoms did not improve. Plasma exchange was performed thrice a week for total six times. Her symptoms improved gradually after plasma exchange. Recombinant interferon alfa-2a (Roferon-A, Hoffmann–LaRoche, Basel, Switzerland) was administered at a dose of 3 million units per day. Her clinical response was improved. Thereafter, she was discharged. Recombinant interferon alfa-2a was administered for three months and 3 million units every other day for the subsequent nine months, a protocol adopted for HCV-associated cryoglobulinemia. In addition, the patient was treated with small doses of glucocorticoids, CellCept, plaquenil, trental (pentoxifylline), persantine (dipyridamole), and aspirin. The patient had a complete clinical response, with the disappearance of serum cryoglobulins and all signs of cutaneous vasculitis and with the normalization of kidney-function results and urinary values. The patient has remained in complete remission for more than one year since the withdrawal of therapy.
DISCUSSION
Mixed cryoglobulins (MCs) are proteins that precipitate from cooled serum, and are composed of a polyclonal immunoglobulin G (IgG) bound to another immunoglobulin that acts as an anti-IgG rheumatoid factor (RF). In type II mixed cryoglobulinemia, the antiglobulin component, usually of the IgM class, is monoclonal, whereas it is polyclonal in type III mixed cryoglobulinemia. The majority of MCs are found in patients with connective tissue diseases, infectious or lymphoproliferative disorders, hepatobiliary diseases, or immunologically mediated glomerular diseases (secondary MCs). The etiology is not clear for 30% of all MCs, and this type of cryoglobulinemia is called “essential.” There is a common clinical syndrome in types II and III essential mixed cryoglobulinemia (EMC), characterized by purpura, weakness, and arthralgia. In type II EMC only, in which an IgMk is the monoclonal RF, a membranoproliferative glomerulonephritis (MPGN) occurs with some morphologic and clinical features; this is termed “cryoglobulinemic glomerulonephritis” and is characterized by an acute nephritic syndrome, by the following findings:
the presence of large deposits filling the capillary lumen that sometimes are shown to have a characteristic fibrillar or crystalloid structure by electron microscopy;
the extent of the exudative component consequent to the frequently massive infiltration of monocytes;
a more diffuse and evident thickening of the glomerular basement membrane, which has a double-contoured appearance that is mainly due to the peripheral interposition of monocytes, with less evident mesangial expansion; and
possibly some vasculitis in small and medium-sized renal arteries without concomitant features of segmental necrotizing GN or crescentic GN.Citation[1]
Recently, a number of studiesCitation[2–4] have implicated C virus as a major cause of mixed cryoglobulinemia. Several authors described that up to 95% of “essential” mixed cryoglobulinemia could be attributed to this viral infection. Sixty-two patients have been detected with a C virus infection related to cryoglobulinemia. Nine patients had been histologically examined, with 6 cases showing a membranoproliferative glomerulonephritis pattern, one with associated extraglomerular vasculitis; two with mesangial proliferative pattern and one with membranous glomerulonephritis.
Mixed cryoglobulinemia is a multisystem disorder associated strongly with hepatitis C virus (HCV) infection. The kidney frequently is involved, and glomerulonephritis represents the key factor affecting prognosis. In all, 146 patients with cryoglobulinemic glomerulonephritis underwent biopsies in 25 Italian centers, with 34 cryoglobulinemic controls without renal involvement. Diffuse membranoproliferative glomerulonephritis was the most prevalent histological pattern (83%).Citation[5]
Membranoproliferative glomerulonephritis (MPGN) may be a component of a generalized vasculitis as well as a component of the clinical expression of type-II mixed cryoglobulinemia (MC). Several studies have established a striking association between hepatitis C virus (HCV) infection and MC. The potential role of HCV in the pathogenesis of MPGN, which occurs in almost half of the cases of MC patients, has not been fully investigated, and the demonstration of HCV proteins as the antigenic constituent of the glomerular immune deposits has remained elusive. Kidney biopsy specimens were obtained from 12 HCV RNA, antibody to HCV (anti-HCV)-positive patients with MPGN and type-II MC, and from 8 controls (3 HCV RNA, anti-HCV-negative patients with MPGN and MC and 5 with noncryoglobulinemic “idiopathic” MPGN). Murine monoclonal antibodies developed against c22-3, E2/NS1, c33c, c100-3, and NS5 proteins were used to detect HCV-related antigens by indirect immunohistochemistry. Acid electroelution of tissue sections was performed to enhance the sensitivity of the immunohistochemical method. Specific HCV-related proteins were detected in glomerular and tubulo-interstitial vascular structures in eight (66.7%) HCV-positive MC patients and in none of the HCV RNA, anti-HCV-negative controls. HCV immunoreactive deposits displayed the following two major patterns:
a linear, homogeneous deposition along glomerular capillary walls, including endothelial cells and sub-endothelial spaces; and
a granular bead-like appearance with distinct deposits in mesangial and perimesangial cells.
Immunoglobulin G (IgG) and M (IgM) and C3 fraction deposition in adjacent kidney sections displayed features comparable with those found for HCV deposits. Patients with granular deposits showed more pronounced renal impairment and severe proteinuria. These findings indicate that in MC patients with HCV-associated MPGN, kidney deposits consist of HCV-containing immune complexes that are likely to play a direct pathogenetic role in the renal damage.Citation[6]
Among the several types of chronic glomerulonephritis (GN) described in association with hepatitis C virus (HCV) infection, cryoglobulinemic glomerulonephritis is by far the most frequent. It is usually associated with type II cryoglobulinemia with IgM k rheumatoid factor. It is a membranoproliferative GN, which shows some distinctive histologic features (e.g., intraglomerular monocyte infiltration, intraluminal thrombi due to massive precipitation of cryoglobulins, renal vasculitis) and has a chronic course with acute recurrent episodes that can be controlled by corticosteroids and antiviral therapy (interferon alpha).Citation[7] Membranoproliferative glomerulonephritis in patients with cryoglobulinemia complicating hepatitis C virus have been reported. Antiviral treatment seems to be able to improve the outcome of cryoglobulinemic glomerulonephritis. In the literature, treatment is dominated by antiviral therapy composed first by interferon alpha alone. Combination therapy associating interferon and ribavirin was recently used in renal involvement; it is clearly more effective than interferon alpha alone.Citation[8] Our data support the previous suggestion that the immunomodulatory properties of interferon rather than its antiviral properties may significantly account for its efficacy in cryoglobulinemic vasculitis.
Hepatitis C virus has long been implicated in the pathogenesis of several glomerulopathies, including membranoproliferative glomerulonephritis, mixed cryoglobulinemia, and membranous glomerulonephritis.Citation[9],Citation[10] It is believed that persistent infection with the hepatitis C virus is responsible for an immune complex-mediated glomerulonephritis in this patient. Because hepatitis C has now been pathogenetically linked to several glomerulopathies, testing for this virus should be considered in the serologic work-up of the patient with glomerulonephritis. True essential mixed cryoglobulinemia with evidence of HCV infection warrant a trial with interferon in such cases.
DECLARATION OF INTEREST
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Almirall J, Amengual MJ, López T, Andreu X, Oristrell J, Sala M, Luelmo J, Dalmau B. Type II essential mixed cryoglobulinemia and renal disease. Hepatitis C virus association. Nefrologia. 2002; 22(6)531–539
- Ben Fatma L, Ben Hamida F, Aoudia R, Goucha R, Kaaroud H, Béji S, Barbouch S, Hedri H, Abderrahim E, Elyounsi F, Ben Abdallah T, Ben Moussa F, Kheder A. Membranoproliferative glomerulonephritis in patients with cryoglobulinemia complicating hepatitis C virus: Report of 11 cases. Tunis Med. Mar, 2007; 85(3)220–224
- Roccatello D, Fornasieri A, Giachino O, Rossi D, Beltrame A, Banfi G, Confalonieri R, Tarantino A, Pasquali S, Amoroso A, Savoldi S, Colombo V, Manno C, Ponzetto A, Moriconi L, Pani A, Rustichelli R, Di Belgiojoso GB, Comotti C, Quarenghi MI. Multicenter study on hepatitis C virus-related cryoglobulinemic glomerulonephritis. Am J Kidney Dis. Jan, 2007; 49(1)69–82
- Mazzaro C, Pozzato G, Zorat F, Panarello G, Silvestri F, Barillari G, Mazzoran L, Baracetti S, Crovatto M, Santini GF, Donadon V, Faccini L. Campanacci L Cryoglobulinaemic membranoproliferative glomerulonephritis and hepatitis C virus infection. Ital J Gastroenterol Hepatol. Jan–Feb, 1999; 31(1)45–53
- D'Amico G, Fornasieri A. Cryoglobulinemic glomerulonephritis: A membranoproliferative glomerulonephritis induced by hepatitis C virus. Am J Kidney Dis. Mar, 1995; 25(3)361–369
- Sinico RA, Fornasieri A, D'Amico G. Renal manifestations associated with hepatitis C virus. Ann Med Interne (Paris). Feb, 2000; 151(1)41–45
- Sinico RA, Fornasieri A, D'Amico G. Hepatitis C virus-related proteins in kidney tissue from hepatitis C virus-infected patients with cryoglobulinemic membranoproliferative glomerulonephritis. Hepatology. May, 1997; 25(5)1237–1244
- Sansonno D, Gesualdo L, Manno C, Schena FP, Dammacco F. Membranoproliferative glomerulonephritis associated with hepatitis C virus infection. J Am Soc Nephrol. Dec, 1993; 4(6)1288–1293
- Kaupke J, Vaziri ND. Renal complication of hepatitis C infection. West J Med. May, 1996; 164(5)442–443
- Groves C, Devereux C, McMillan C. A case of cutaneous vasculitis with underlying hepatitis C and cryoglobulinemia. Ulster Med J. January, 2008; 77(1)51–53