Abstract
Gadolinium-containing magnetic resonance imaging (MRI) contrast agents have been reported to induce nephrogenic systemic fibrosis in patients with renal dysfunction, and therefore their use has been restricted. However, even without contrast agents, MRI can provide much valuable information on the pathophysiology of renal diseases. This report describes the case of a 90-year-old man with a hydronephrotic and dysfunctioning right kidney induced by testicular cancer evaluated by non-contrast MRI. He was referred to our hospital complaining of lower abdominal distention and appetite loss. Physical examination revealed a painless enlargement of the right scrotum and inguinal lymph node. Laboratory analysis revealed renal dysfunction, after which a non-contrast MRI was performed. T1-, T2-, and diffusion-weighted images (T1WI, T2WI, and DWI, respectively) revealed carcinoma of the right testicle with extensive multiple lymphadenopathy. Moreover, a retroperitoneal metastatic tumor was detected adjacent to the right kidney, which appeared to constrict the right urinary duct. An enlarged right kidney with loss of corticomedullary differentiation was observed on T1WI. The right kidney and enlarged renal pelvis were observed as large signal intensity areas on T2WI. DWI showed an increased signal intensity of the right kidney and a decreased apparent diffusion coefficient. These findings clarified that the retroperitoneal metastasis from the right testicular cancer led to hydronephrosis and dysfunctioning of the right kidney. The present case indicates that non-contrast MRI is useful for the evaluation of renal diseases even in elderly patients with renal dysfunction.
INTRODUCTION
Gadolinium-containing magnetic resonance imaging (MRI) contrast agents have been reported to induce nephrogenic systemic fibrosis (NSF) in patients with renal dysfunction, and thus their use has been restricted.Citation[1–4] However, even without contrast agents, MRI can provide much valuable information on the pathophysiology of renal diseases. In this report, a 90-year-old man with a hydronephrotic and dysfunctioning right kidney induced by testicular cancer was evaluated by non-contrast MRI.
CASE REPORT
A 90-year-old male was referred to our hospital complaining of a gradual onset of appetite loss and abdominal distention. Dysuria was absent. Physical examination revealed a pulse rate of 88 beats per minute in a regular rhythm, blood pressure of 114/68 mmHg, temperature of 36.9°C, a painless enlargement of the scrotum and the inguinal lymph node on the right side, abdominal distention with mild diffuse tenderness, and hypoactive bowel sounds. Flank knocking pain was not noted. Laboratory findings are shown in . Urinalysis revealed proteinuria (>200 mg/dL), microscopic hematuria (5–9 red blood cells/high-power field (HPF)), leukocyturia (1–4 white blood cells/HPF), squamous epithelium (1–2/HPF), hyaline (1–2/HPF), and granular (1–2/HPF) casts. Complete blood count analyses revealed no remarkable changes. Biochemical analysis revealed an elevated serum creatinine (Scr) concentration of 1.4 mg/dL, following which the estimated glomerular filtration ratio (eGFR) was calculated using the following formula:Citation[5]
Table 1 Laboratory findings
which was found to be 47.6 mL/min/1.73 m2. The patient's C-reactive protein concentration (3.81 mg/dL) was found to be elevated. Among tumor markers, only soluble interleukin-2 (sIL-2) receptor concentration was significantly elevated at 8460 U/mL. Although chest roentgenograms revealed bilateral pleural effusion, B-type natriuretic peptide showed an insignificant elevation at 82.3 pg/mL. Moreover, transthoracic echocardiography revealed normal left ventricular motion without severe valvular diseases. We suspected testicular cancer with intra-abdominal and lung metastasis.
Non-contrast pelvic MRI was conducted for the diagnosis of testicular cancer. MRI was performed on a 1.5 Tesla scanner (Avanto, Siemens, Erlangen, Germany) with an eight-element phased-array body coil, which was capable of T1-weighted imaging (T1WI), T2-weighted imaging with fat suppression (T2WI), and diffusion-weighted imaging (DWI) with a b value of 50, 500, and 1000 s/mm2. On T1WI, the right testis was enlarged to 52.5 mm × 43.1 mm with a relatively heterogeneous signal intensity (see ). T2WI revealed a large amount of fluid collected in right testis (see ). On DWI with a b value of 1000 s/mm2, an increased signal intensity area was detected in the right testis (see ). Moreover, abdominal, coronal, and multiplanar reconstruction T1WI showed the diffuse tumorous involvement extended from the right testis to the spermatic cord (see ). Thus, we diagnosed the patient with right testicular cancer extending to the spermatic cord.
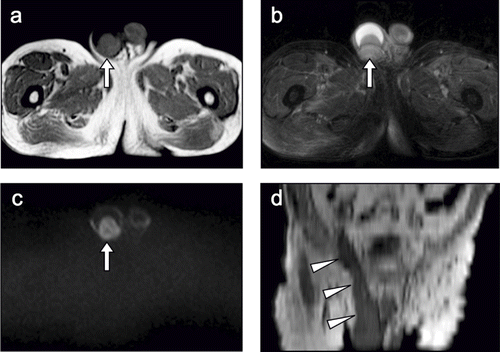
Figure 1. (A) T1-weighted image (T1WI) shows an enlarged right testis (52.5 mm × 43.1 mm) with relatively heterogeneous signal intensity (arrow). (B) T2-weighted image shows a large amount of fluid collected in the right testis (arrow). (C) Diffusion-weighted image shows a high signal intensity area in the right testis (arrow). (D) Abdominal, coronal, and multiplanar reconstruction T1WI shows the diffuse tumorous involvement extending from the right testis to the spermatic cord (arrowheads).
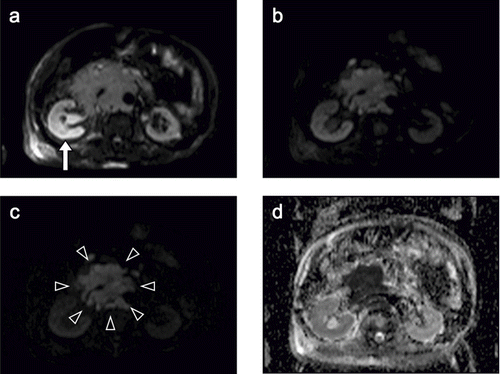
Next, non-contrast abdominal MRI was performed to screen the presence of intra-abdominal metastasis and renal disease. Multiple lymphadenopathy was detected around the right external and common iliac artery and the abdominal aorta. T1WI revealed an enlarged right kidney with loss of corticomedullary differentiation and a normal-sized left kidney with preserved corticomedullary differentiation. Moreover, a retroperitoneal tumor with a low signal intensity was detected adjacent to the right kidney that appeared to constrict the right urinary duct (see ). On T2WI, the right kidney had a significantly higher signal intensity than the left kidney; the right renal pelvis was enlarged and also had a high signal intensity (see ). On DWI with a b value of 50 s/mm2, the right kidney was more clearly depicted as a high signal intensity area than that on T2WI (see ). As the b value increased, the signal intensity of the kidney decreased on both sides, whereas that of the retroperitoneal tumor was relatively well preserved. On DWI with a b value of 1000 s/mm2, the retroperitoneal tumor was clearly highlighted in comparison with the surrounding organs (see and c). The apparent diffusion coefficient (ADC) of the right cortex (1.85) was significantly lower than that of the left cortex (2.49), and that of retroperitoneal tumor was the lowest (0.68; see ). Thus, we concluded that the retroperitoneal metastasis of the testicular cancer led to hydronephrosis dysfunction of the right kidney.
Figure 2. (A) T1-weighted image shows an enlarged right kidney with loss of corticomedullary differentiation (closed-white arrow) and a normal-sized left kidney with preserved corticomedullary differentiation (open-white arrow). A retroperitoneal tumor is depicted adjacent to the right kidney as a low intensity area (arrowheads). (B) T2-weighted image shows that the right kidney (closed-white arrow) has significantly higher signal intensity than the left kidney (open-white arrow), and the right renal pelvis (circle) is enlarged with high signal intensity.
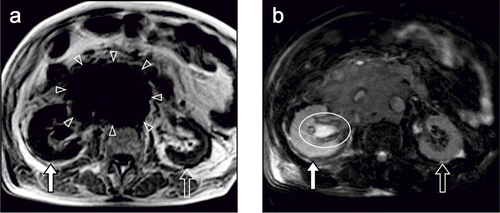
Figure 3. (A) Diffusion-weighted image (DWI) with a b value of 50 s/mm2 showing the right kidney (arrow) as a high signal intensity area more clearly than a T2-weighted image. (B) DWI with a b value of 500 s/mm2. (C) DWI with a b value of 1000 s/mm2 (high b value DWI). As the b value increased, the signal intensity of the kidney decreased bilaterally, whereas that of the retroperitoneal tumor was relatively well preserved. The retroperitoneal tumor in demarcated from the surrounding organs (arrowheads), clearly highlighted by a high b value. (D) An apparent diffusion coefficient (ADC) map shows that the signal intensity is lower in the right kidney than in the left kidney and is very low in the retroperitoneal tumor. The ADC of the right cortex (1.85) was significantly lower than that of left cortex (2.49), and that of the retroperitoneal tumor was the lowest (0.68).
Further examination, surgery, chemotherapy, and radiotherapy were indicated but not performed, according to the wishes of the patient and his family. They were satisfied that the diagnosis was made safely and non-invasively.
DISCUSSION
In an elderly patient with renal dysfunction, we were able to clarify using non-contrast MRI that right testicular cancer with multiple retroperitoneal metastasis induced hydronephrosis and dysfunction of the right kidney.
Gadolinium-contrast MRI has been widely used in patients with renal dysfunction instead of iodine contrast-enhanced angiography or computed tomography (CT) because gadolinium-containing MRI contrast agents have lower renal toxicity than iodine contrast agents.Citation[6],Citation[7] However, gadolinium-containing MRI contrast agents have been reported to induce NSF in patients with renal dysfunction.Citation[1],Citation[2] NSF was primarily reported as scleromyxedema-like cutaneous diseases in renal-dialysis patientsCitation[8] and referred to as nephrogenic fibrosing dermopathy (NFD). More recent autopsy studies of NFD patients have clarified that fibrosis is not limited to the skin and might involve other organs, including muscles, bones, the heart, lungs, the liver, kidneys, and the intestine.Citation[9],Citation[10] Based on this, NFD has been classified as nephrogenic systemic fibrosis (NSF).
Although the use of gadolinium-containing MRI contrast agents have been restricted in renal dysfunction patients, MRI can provide much valuable information on the pathophysiology of renal diseases even in the absence of contrast agents.Citation[11],Citation[12] On T1WI, the cortex can be clearly differentiated from the medulla, referred to as corticomedullary differentiation, in a normally functioning kidney, whereas loss of corticomedullary differentiation can be observed in a dysfunctioning kidney.Citation[13] Moreover, solid renal lesions have lower signal intensities than the renal parenchyma on T1WI, leading to easy detection. On T2WI, the water content in tissues can be evaluated, which helps to characterize cysts and intraparenchymal abscess and differentiate hydronephrosis. DWI can provide information on the Brownian motion of water molecules mostly in the extracellular space, which is useful for tissue characterization. The signal intensity is high if water molecules are restricted in their motion, whereas the signal intensity is low if water molecules can diffuse freely. The b value is an important imaging parameter that affects the contrast of DWI. DWI with a b value of 50 s/mm2 (low b value DWI) has both water diffusion- and T2-contrast, and suppresses background and blood flow signals more effectively. Thus, a low b value DWI sometimes detects abnormalities more easily than T2WI. In contrast, DWI with a b value of 1000 s/mm2 has more pure water diffusion contrast and can evaluate the change in water diffusion more accurately than low b value DWI. An ADC map is reconstructed from diffusion-weighted images obtained using two or more b values, allowing us to quantitatively evaluate water diffusion. The ADC is low if water molecules are restricted in their motion, whereas the ADC is high if water molecules can diffuse freely. The ADC of the kidney is significantly higher than that of the other abdominal organs because water diffusion is less restricted in the kidney—probably due to the high blood supply and water transport function.Citation[14] Moreover, by the measurement of ADC, split renal function can be evaluated quantitatively.Citation[15],Citation[16] In a dysfunctioning kidney, the ADC is significantly lower than that in a normal functioning kidney because water diffusion is more restricted in dysfunctioning kidney—probably due to loss of nephron, fibrosis, and reduced water transport function.
In the present case, after physical and laboratory examination, we initially suspected testicular cancer with intra-abdominal metastasis. However, we did not suspect hydronephrosis because of the lack of significant symptoms, and we could not clarify the etiology of the renal dysfunction. For the evaluation of testicular cancer and renal disease, we performed an imaging study. We did not select iodine contrast-enhanced CT or gadolinium contrast-enhanced MRI, but rather performed a safer non-contrast MRI because of the patient's renal dysfunction. Non-contrast MRI safely and non-invasively clarified that the retroperitoneal metastasis from the right testicular cancer induced the right hydronephrotic and dysfunctioning kidney.
On the other hand, the histological diagnosis of testicular cancer could not be confirmed because an orchiectomy was not performed, according to the wishes of the patient and his family. However, we strongly suspected that the testicular cancer was lymphoma because of the following reasons:
testicular lymphoma is the most common testicular tumor in elderly patientsCitation[17];
the soluble IL-2 receptor was elevatedCitation[18]; and
extensive lymphadenopathy along the spermatic cord is seen more often in testicular lymphoma than in seminoma.Citation[19]
In conclusion, non-contrast MRI can safely and non-invasively provide much valuable information regarding the pathophysiology of renal diseases, even in patients with renal dysfunction. Non-contrast MRI is helpful for the diagnosis, management, and monitoring of the therapeutic response in patients with renal disease and might act as an essential tool for nephrologists in future.
DECLARATION OF INTEREST
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Grobner T. Gadolinium—a specific trigger for the development of nephrogenic fibrosing dermopathy and nephrogenic systemic fibrosis?. Nephrol Dial Transplant. 2006; 21: 1104–1108
- Marckmann P, Skov L, Rossen K, et al. Nephrogenic systemic fibrosis: Suspected causative role of gadodiamide used for contrast-enhanced magnetic resonance imaging. J Am Soc Nephrol. 2006; 17: 2359–2362
- Thomsen HS, European Society of Urogenital Radiology (ESUR). ESUR guideline: Gadolinium-based contrast media and nephrogenic systemic fibrosis. Eur Radiol. 2007; 17: 2692–2696
- http://www.icnfdr.org, Cowper SE. The International Center for Nephrogenic Fibrosing Dermopathy Research (ICNFDR) Web site, 2001–2008
- Levey AS, Coresh J, Greene T, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006; 15(145)247–254
- Runge VM. Safety of approved MR contrast media for intravenous injection. J Magn Reson Imaging. 2000; 12: 205–213
- Townsend RR, Cohen DL, Katholi R, et al. Safety of intravenous gadolinium (Gd-BOPTA) infusion in patients with renal insufficiency. Am J Kidney Dis. 2000; 36: 1207–1212
- Cowper SE, Robin HS, Steinberg SM, Su LD, Gupta S, LeBoit PE. Scleromyxoedema-like cutaneous diseases in renal-dialysis patients. Lancet. 2000; 356: 1000–1001
- Daram SR, Cortese CM, Bastani B. Nephrogenic fibrosing dermopathy/nephrogenic systemic fibrosis: Report of a new case with literature review. Am J Kidney Dis. 2005; 46: 754–759
- Gibson SE, Farver CF, Prayson RA. Multiorgan involvement in nephrogenic fibrosing dermopathy: An autopsy case and review of the literature. Arch Pathol Lab Med. 2006; 130: 209–212
- Michaely HJ, Herrmann KA, Nael K, Oesingmann N, Reiser MF, Schoenberg SO. Functional renal imaging: Nonvascular renal disease. Abdom Imaging. 2007; 32: 1–16
- Nikken JJ, Krestin GP. MRI of the kidney-state of the art. Eur Radiol. 2007; 17: 2780–2793
- Lee VS, Kaur M, Bokacheva L, et al. What causes diminished corticomedullary differentiation in renal insufficiency?. J Magn Reson Imaging. 2007; 25: 790–795
- Namimoto T, Yamashita Y, Mitsuzaki K, Nakayama Y, Tang Y, Takahashi M. Measurement of the apparent diffusion coefficient in diffuse renal disease by diffusion-weighted echo-planar MR imaging. J Magn Reson Imaging. 1999; 9: 832–837
- Toyoshima S, Noguchi K, Seto H, Shimizu M, Watanabe N. Functional evaluation of hydronephrosis by diffusion-weighted MR imaging. Acta Radiologica. 2000; 41: 642–646
- Thoeny HC, De Keyzer F, Oyen RH, Peeters RR. Diffusion-weighted MR imaging of kidneys in healthy volunteers and patients with parenchymal diseases: Initial experience. Radiology. 2005; 235: 911–917
- Tondini C, Ferreri AJ, Siracusano L, et al. Diffuse large-cell lymphoma of the testis. J Clin Oncol. 1999; 17: 2854–2858
- Stasi R, Zinzani L, Galieni P, et al. Detection of soluble interleukin-2 receptor and interleukin-10 in the serum of patients with aggressive non-Hodgkin's lymphoma. Identification of a subset at high risk of treatment failure. Cancer. 1994; 74: 1792–1800
- Saito W, Amanuma M, Tanaka J, Heshiki A. A case of testicular malignant lymphoma with extension to the epididymis and spermatic cord. Magn Reson Med Sci. 2002; 1: 59–63