Abstract
There is increasing evidence indicating that oxidative stress plays an important role in the pathogenesis of rhabdomyolysis-induced myoglobinuric acute renal failure (ARF). During times of war and natural disasters, myoglobinuric ARF can assume epidemic proportions. Thus, early and effective renoprotective treatments are of utmost importance. It has been shown that L-carnitine, used as a safe and effective nutritional supplement for more than three decades, is effective in preventing renal injury in many renal injury models involving oxidative stress. The present study was performed to investigate the effects of L-carnitine in an experimental model of myoglobinuric ARF. Four groups of rats were employed in this study: group 1 served as a control; group 2 was given glycerol (10 mL/kg, i.m.); group 3 was given glycerol plus L-carnitine (100 mg/kg, i.p.), starting at the same time as the glycerol injection; group 4 was given glycerol plus L-carnitine (100 mg/kg, i.p.), starting 48h before the glycerol injection. After glycerol injections, the i.p. injections of L-carnitine were repeated every 24h for four days. Ninety-six hours after glycerol injections, blood samples and kidney tissues were taken from the anesthetized rats. Urea and creatinine levels in plasma, N-acetyl-beta-D-glucosaminidase activity in urine, and malondialdehyde levels and catalase enzyme activity in kidney tissue were determined. Histopathological changes and iron accumulation in the kidney tissue were evaluated. In this study, glycerol administration led to marked renal oxidative stress, as well as severe functional and morphological renal deterioration. L-carnitine, possibly via its antioxidant properties, ameliorates glycerol-induced myoglobinuric kidney injury.
INTRODUCTION
The term rhabdomyolysis refers to the disintegration of striated muscle, which results in the release of potentially toxic intracellular components, including myoglobin, into the systemic circulation.Citation[1] The degree of rhabdomyolysis can manifest from a subclinical rise of creatinine kinase to acute renal failure (ARF).Citation[2] The main pathophysiologic mechanisms of rhabdomyolysis-induced myoglobinuric ARF are renal vasoconstriction, intraluminal cast formation, and direct myoglobin-induced cytotoxicity.Citation[1],Citation[3]
There is also increasing evidence indicating that reactive oxygen species (ROS) plays an important role in the pathogenesis of myoglobinuric ARF.Citation[4–6] During renal vasoconstriction/hypoperfusion, ATP is degraded to hypoxanthine. When xanthine oxidase converts hypoxanthine to xanthine in the presence of molecular oxygen, superoxide radical (O2−) is generated.Citation[7] On the other hand, the porphyrin ring of myoglobin is rapidly catabolized in tubules, thereby releasing its iron content.Citation[1],Citation[8],Citation[9] Any released free iron could participate in the Fenton and Haber-Weiss chemistry, where catalytic amounts of iron are sufficient to yield hydroxyl radicals (OH°) from O2− and hydrogen peroxide (H2O2), collectively known as ROS.Citation[8],Citation[9] Hydroxyl radical is capable of abstracting a hydrogen atom from polyunsaturated fatty acids to initiate lipid peroxidation.Citation[8] The heme group in myoglobin itself, which can redox cycle between different oxidation states, promotes lipid peroxidation reactions.Citation[10] The resulting accumulation of lipid peroxides destroys membrane structure and function.Citation[8]
L-carnitine (β-hydroxy-γ-N-trimethyl ammonium-butyrate) is a vital component in lipid metabolism for the production of ATP through the β-oxidation of long chain fatty acids.Citation[11] In this way, L-carnitine acts as an indirect antioxidant and facilitates the repair of oxidized membrane/lipid bilayers.Citation[12],Citation[13] L-carnitine activity has also been reported to be a direct scavenger of O2− and H2O2.Citation[14] It has been shown that L-carnitine was effective in preventing renal injury in many renal injury models involving oxidative stress.Citation[15–18] In view of the above findings, the present study was designed to investigate the effects of exogenous L-carnitine on glycerol-induced myoglobinuric acute renal failure.
MATERIALS AND METHODS
Animals
Male Spraque Dawley rats, bred in the Experimental Research Center of Trakya University (Edirne, Turkey), were used in this study. The rats were housed in an air-conditioned room with 12 h light-dark cycles, where the temperature (21 ± 2 oC) and relative humidity (60–65%) were kept constant. The study protocol was approved by the Ethics Committee of the Trakya University Faculty of Medicine.
Drugs
Glycerol was purchased from Merck KGaA, L-carnitine was purchased from Santa Pharma Chemicals and Pharmaceutics, and all other chemicals and reagents used were of analytical grade.
Study Design
Rats were divided into four groups. The animals were allowed free access to food but deprived of drinking water for 24 h before glycerol injection. Group 1 comprised the control group (n = 8) and received the equivalent volume of saline solution for glycerol. Rhabdomyolysis was induced by injecting glycerol (50% vol/vol in sterile saline), 10 mL/kg body weight; half of the dose was administered in each hind limb muscle in groups 2, 3, and 4. Group 2 animals (ARF group, n = 11) received saline solution i.p. in an equal dose to the L-carnitine in groups 3 and 4, immediately after the glycerol injection. Group 3 animals (ARF-LC group, n = 11) received L-carnitine i.p. (100 mg/kg bw), starting at the same time as the glycerol injection. Group 4 animals (ARF-proLC group, n = 11) received L-carnitine i.p. (100 mg/kg bw), starting 48 h before the glycerol injection. After glycerol injections, the i.p. injections of saline or L-carnitine were repeated every 24 h for four days in the ARF, ARF-proLC, and ARF-LC groups. The animals were placed in individual metabolic cages after the glycerol injection for 24 h urine collection, and allowed free access to food and water (see the flow chart in ). Two rats died during the follow-up period: one rat in the ARF group and one rat in the ARF-LC group. At the end of 96 h, rats were anesthetized with 10 mg/kg xylazine + 50 mg/kg ketamine, a midline abdominal incision was performed, and the animals were neutralized by collecting their blood via intracardiac punction. Kidneys were harvested: the left kidney was deep frozen for further enzymatic analysis, whereas the right kidney was stored in 10% formalin for the histological sectioning. Urine and blood samples were centrifuged immediately (3000 g for 5 min at 4°C), and then samples were stored at –84°C until assay.
Figure 1. Experimental protocol. Abbreviations: ARF = glycerol-administered rats treated with saline solution, ARF-LC = glycerol-administered rats treated with L-carnitine (started at the same time as the glycerol injection, 100 mg/kg, i.p), ARF-proLC = glycerol-administered rats treated with L-carnitine (started 48h before glycerol injection, 100 mg/kg, i.p).
Renal Function
Biochemical analyses of serum and urine were made using the specific Abbott kits in the Abbott C8000 Architect auto analyzer. Creatinine clearance was calculated as μL/min. Urinary N-acetyl-beta-D-glucosaminidase (NAG) activity, a reliable marker of tubular cell damage, was determined using a Bohringer Mannheim BiochemicaR (No: 875406) kit in a Shimadzu UV 160 A spectrophotometer. The urinary NAG activity to urinary creatinine excretion ratio was calculated as mU/mmol.
Malondialdehyde Assays
Kidney tissue samples were homogenized with ice-cold 150 mmol/L KCl to determine malondialdehyde (MDA) levels. Homogenates were centrifuged at 2600 g for 10 min at 4°C. The MDA concentrations of renal tissue, a measure of lipid peroxidation, were assayed in the form of thiobarbituric acid-reactive substances.Citation[19] Supernatant (200 μL) was added to 0.2 mL of 8.1% sodium dodecyl sulphate, 1.5 mL of 20% acetic acid (pH 3.5), 1.5 mL of 0.8% thiobarbituric acid, and 0.6 mL of distilled water. This mixture was heated to 95°C for 60 min. After cooling with tap water, 1.0 mL distilled water, and 5.0 mL n-butanolpyridine mixture (15:1, v/v) were added, and samples were shaken vigorously and centrifuged at 2600 g for 10 min at 25°C. The absorbance of the organic layer was read at 532 nm. MDA was quantified using an extinction coefficient of 1.56 × 105 L/mol per cm, and kidney levels were expressed as nmol MDA/mg tissue.
Catalase Activity Assays
Catalase activity was measured according to Aebi's method.Citation[20] The principle of the catalase activity measurement is based on the determination of the rate constant (k; /s) of the decomposition of hydrogen peroxide at 240 nm. Results are expressed as the rate constant per gram tissue protein.
Kidney Morphological Studies
Right kidneys were cut longitudinally and fixed in 10% formalin solution. Tissues were embedded in paraffin wax, and 4 μm sections were stained with hematoxylin and eosin. In addition, 4 μm sections of paraffin-embedded tissues were stained with iron stain (procedure number HT 20; Sigma Diagnostics, St Louis, Missouri, USA). The extent of histological renal tubular necrosis, cast formation, and iron accumulation for each animal was evaluated semiquantitatively by a pathologist who analyzed the kidney sections with no knowledge of the treatment groups. Cortical tubular necrosis, cast formation, and iron accumulation were assessed, using a 400-field grid ocular (Nikon, Tokyo, Japan), which has a total screening area of 1 μm2, superimposed on a representative low power figure (magnification 100x) of a 4 μm paraffin section (hematoxylin and eosin or iron staining). Percentages of the areas with tubular necrosis, cast formation, and granular, blue-colored iron accumulation were assigned and recorded for each field of the grid in which tubular structures were seen. The percentage areas of tubular necrosis, cast formation, and iron accumulation were added and divided by the number of fields in which tubular structures were seen. Tubular necrosis, cast formation, and iron accumulation were recorded as a percentage of the areas with a tubular structure.
Statistical Analysis
Data were expressed as mean ± SD. All statistical analyses were conducted with the SPSS/PC 10.0 statistical software package. The Kruskal-Wallis test was used to compare the four groups. In two-group comparisons, the Mann-Whitney U test was used. A Pearson correlation test was performed to evaluate the relationship between the parameters in the glycerol-treated rats. A value of p < 0.05 was accepted as statistically significant.
RESULTS
As shown in , glycerol administration resulted in a significant increase in urea and creatinine levels, as well as in urinary NAG activity; it also significantly decreased creatinine clearance. The urinary NAG/creatinine ratio was significantly decreased in the ARF-LC group when compared to the ARF group. The urinary NAG/creatinine ratio and creatinine clearance were significantly improved in the ARF-proLC group compared with ARF and ARF-LC groups.
Table 1 Laboratory findings of study groups
The levels of kidney tissue MDA significantly increased in rats treated with glycerol as compared to the control rats. The groups treated with L-carnitine showed significantly decreased renal MDA levels as compared to the ARF group. This improvement was found more markedly in the ARF-proLC group than in the ARF-LC group (see ).
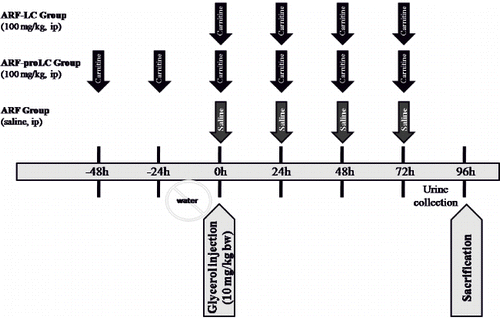
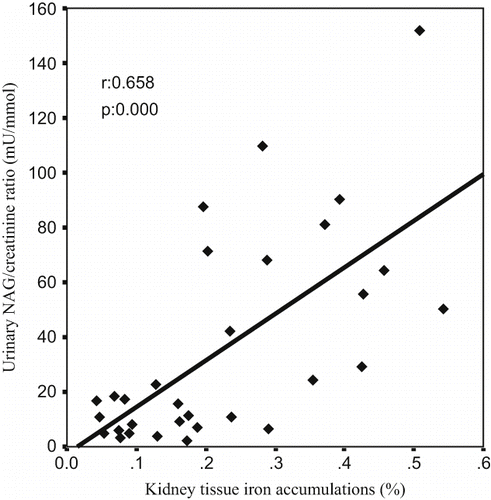
A Pearson correlation test showed some relationships between parameters in glycerol-treated rats (ARF, ARF-LC, ARF-proLC groups). The kidney tissue MDA levels were found to be positively correlated with tubular cell necrosis (r = 0.402, p = 0.025; see ), kidney tissue iron accumulations (r = 0.576, p = 0.001; see ), and urinary NAG/creatinine ratio (r = 0.700, p = 0.000; see ), and negatively correlated with kidney tissue catalase activity (r = –0.392, p = 0.029). Kidney tissue iron accumulations were found to be positively correlated with tubular cell necrosis (r = 0.536, p = 0.002; see ), tubular cast formations (r = 0.451, p = 0.011), and urinary NAG/creatinine ratio (r = 0.658, p = 0.000; see ), and negatively correlated with creatinine clearance (r = –0.509, p = 0.003).
Figure 2. The scattergram shows the relation between kidney tissue MDA levels (in nmol/mg tissue) and tubular cell necrosis (in %) in patient groups (ARF, ARF-LC, and ARF-proLC group).
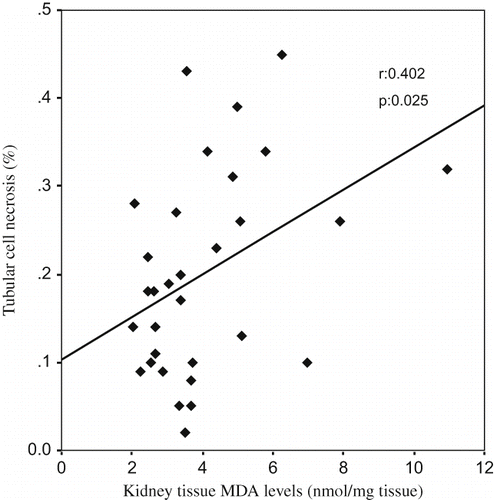
Figure 3. The scattergram shows the relation between kidney tissue MDA levels (in nmol/mg tissue) and kidney tissue iron accumulations (in %) in patient groups (ARF, ARF-LC, and ARF-proLC group).
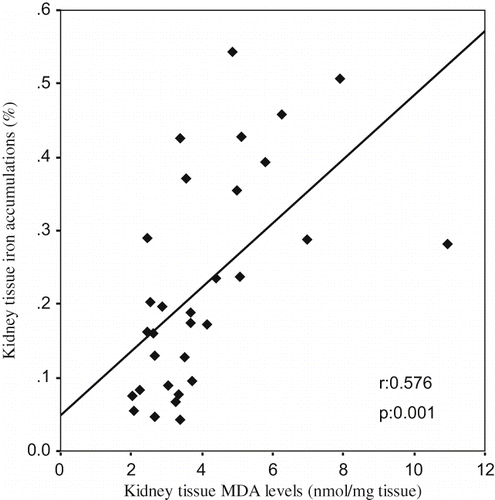
Figure 4. The scattergram shows the relation between kidney tissue MDA levels (in nmol/mg tissue) and urinary NAG/creatinine ratio (in mU/mmol) in patient groups (ARF, ARF-LC, and ARF-proLC group).
Figure 5. The scattergram shows the relation between kidney tissue iron accumulations (in %) and tubular cell necrosis (in %) in patient groups (ARF, ARF-LC, and ARF-proLC group).
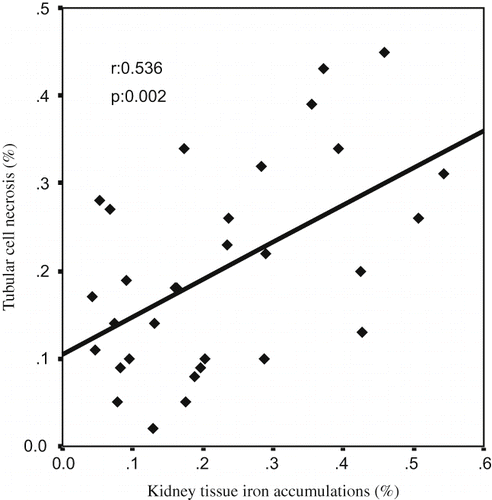
Figure 6. The scattergram shows the relation between kidney tissue iron accumulations (in %) and urinary NAG/creatinine ratio (in mu/mmol) in patient groups (ARF, ARF-LC, and ARF-proLC group).
The histological changes in all groups were examined and the results are given in . The control group did not show any histopathological changes in light microscopic examination (see and ). In contrast, in the ARF group, the basic histological abnormalities were tubular necrosis and cast formation. Necrosis was most severe in the cortical segments of the proximal tubules, and less extensive changes were observed in the medullar segment of the kidney (see ). Blue granular deposits showed iron accumulation in the tubular system (see ). Tubular cell necrosis and iron accumulation decreased significantly in the ARF-LC group when compared to the ARF group (see and ). Tubular cell necrosis and cast formation decreased significantly in the ARF-proLC group than in the ARF group (see ). Moreover, renal iron accumulations decreased significantly in the ARF-proLC group when compared to the ARF and ARF-LC groups (see ).
Figure 7. Hematoxylin and eosin-stained sections of rat kidneys (original magnification × 100). (a) Kidney section of a control rat showing normal architecture. (b) Kidney section of a glycerol + saline-treated rat (ARF group) showing severe tubular necrosis and cast formation. (c) Kidney section of a glycerol + L-carnitine-treated rat, where L-carnitine was started at the same time as the glycerol injection (ARF-LC group). The level of tubular necrosis and cast formation has decreased compared with the ARF group. (d) Kidney section of a glycerol + L-carnitine treated rat, where L-carnitine was started 48h before glycerol injection (ARF-proLC group). The level of tubular necrosis and cast formation has decreased compared with the ARF and ARF-LC groups.
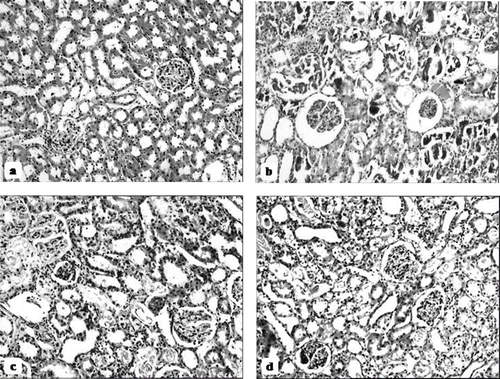
Figure 8. Iron-stained sections of rat kidneys (original magnification × 200). (a) There are no granular deposits in the control rat. (b) Kidney section of glycerol + saline-treated rat (ARF group). There are distinct granular deposits (arrows), showing iron accumulation. (c) Kidney section of glycerol + L-carnitine-treated rat, where L-carnitine was started at the same time as glycerol injection (ARF-LC group). The level of granular deposits (arrow) has decreased compared with the ARF group. (d) Kidney section of glycerol + L-carnitine-treated rats, where L-carnitine was started 48h before glycerol injection (ARF-proLC group). The level of granular deposits (arrow) has decreased compared with the ARF and ARF-LC group.
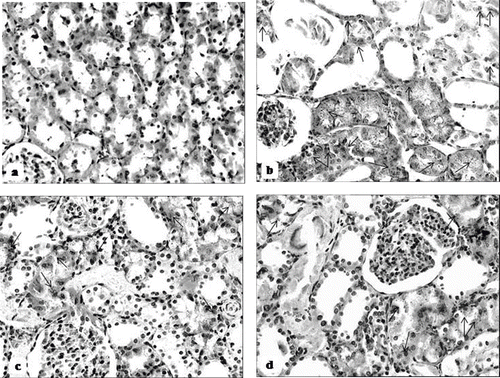
Table 2 Histological findings of study groups
DISCUSSION
In this study, glycerol administration led to a significant increase in plasma urea and creatinine levels and kidney tissue MDA levels, a significant decrease in creatinine clearance and kidney tissue catalase activities, as well as severe renal morphological impairment with an increased renal iron accumulation. Treatment of animals with L-carnitine significantly reduced the renal dysfunction and improved the alterations observed with glycerol injection.
In several studies, it has been demonstrated that ROS play an important role in the pathogenesis of myoglobinuric ARF, based on the findings that in rats with rhabdomyolysis, levels of MDA and F2 isoprostanes in the kidney increase.Citation[1],Citation[3–6],Citation[21] Elevated renal MDA levels, as well as their relations with tubular cell necrosis and the urinary NAG/creatinine ratio in glycerol-administered rats, suggest that lipid peroxidation was an important contributing factor to the development of kidney damage in the present study. Also, we observed that lipid peroxidation, which was connected to functional, biochemical, and morphological renal impairment, was prevented by L-carnitine, implying this molecule's antioxidant effect.
During rhabdomyolysis, reduced oxygen supply to kidney causes the slowing down of intramitochondrial oxidative processes and thus of β-oxidation, leading to the diminish ATP production.Citation[11],Citation[22] L-carnitine plays a major role as a cofactor in the transportation of free fatty acid (FFA) from the cytosol to the mitochondria. FFA degrades to acyl-COA by β-oxidation, and these substances enter the tricarboxylic acid (TCA) cycle. The large amount of oxygen consumed in this reaction and ATP is synthesized in the steps of the electron transport chain and oxidative phosphorylation.Citation[12] Oxygen is reduced to H2O at the end of TCA cycle; thus, ROS formation is reduced. Furthermore, an improvement in renal cell energy metabolism facilitates the repair of oxidized membrane/lipid bilayers.Citation[12],Citation[13] In addition to this indirect antioxidant action, L-carnitine reacts directly with physiologically relevant ROS. More recently, L-carnitine and L-propionylcarnitine (a propionyl ester of L-carnitine) activity as a direct O2− scavenger has been reported.Citation[14],Citation[23] Moreover, it has been also reported that L-carnitine had an effective radical and H2O2 scavenging activity.Citation[14] The superoxide radical and H2O2 may, then, play a role in the generation of additional and more reactive oxidants, including the highly reactive OH°.Citation[8] In this way, the direct antioxidant effects of L-carnitine might contribute to attenuation of oxidative stress in kidney tissue. In the present study, the higher kidney tissue catalase activities, an enzyme responsible for the detoxification of H2O2, found in glycerol-administered rats after treatment with L-carnitine might indicate an improvement in the removal of hydrogen peroxides before they produce new ROS.
On the other hand, the O2−, given its great affinity for nitric oxide (NO), is able to rapidly remove it by its conversion into toxic peroxynitrite.Citation[24] Reduced bioavailable NO, a potent renal vasodilatator, can contribute to renal vasoconstriction/hypoperfusion and tissue injury in the setting of myoglobinuric ARF.Citation[25] L-carnitine treatment may lead to a reduction of NO inactivation by ROS with enhanced NO bioavailability. Recently, it has been reported that L-carnitine was able to improve the endothelium-dependent relaxation of small mesenteric arteries from SHR by decreasing vascular O2− production, thus increasing NO participation in endothelial function.Citation[26] These findings indicate that vasodilatation and the enhancement of renal perfusion contributed, at least in part, to the renoprotective effect of L-carnitine in the present study.
In our study, tubular iron accumulations were found to be positively correlated with renal MDA levels, the urinary NAG/creatinine ratio, and tubular cell necrosis. Because iron is a transition metal that readily accepts and donates electrons, it greatly facilitates free radical production and may itself become a free radical.Citation[8] Deferoxamine, an iron chelator, has been shown to protect against myoglobinuric ARF in rats.Citation[21],Citation[27] Recently, Gülçin reported that the iron chelating effect of L-carnitine was higher than that of EDTA; the latter is a strong metal chelator.Citation[14] It this way, carnitine may suppress OH° production in the Fenton system by chelating the iron required for the generation of OH°. In our study, we observed that in relation to the ARF group, renal tissue iron accumulations decreased significantly in the L-carnitine treatment groups, especially in the ARF-proLC group. Iron-driven oxidative ROS production plays an important role in myoglobinuric ARF.Citation[21],Citation[27] Therefore, we concluded that the iron chelating activity of L-carnitine might provide additional benefits for myoglobinuric ARF protection.
In addition to the above-mentioned direct and indirect antioxidant effects, it has been shown that carnitine induces haem oxygenase, which is antioxidant and antiapoptotic.Citation[28]
In the present study, the protective effect of L-carnitine against myoglobinuric renal injury was found to be more marked in the ARF-proLC group than in the ARF-LC group. It has been shown that the iron chelating effects of L-carnitine were concentration-dependent.Citation[14] It has also been shown that L-carnitine attenuates oxidative tissue injury on a dose-dependent basis.Citation[16] L-carnitine was initiated two days prior to commencing glycerol administration in the ARF-proLC group. As a matter of course, renal tissue carnitine concentration at glycerol injection time might be higher in the ARF-proLC group. In addition, the total dosage of L-carnitine was higher in the ARF-proLC group (600 mg/kg bw) than in the ARF-LC group (400 mg/kg bw), where L-carnitine treatment started at the same time as glycerol administration.
In this study, we have demonstrated the protective effects of L-carnitine by reducing the severity of glycerol-induced myoglobinuric ARF. Rhabdomyolysis can result from both traumatic and nontraumatic causes such as glycerol-induced ARF.Citation[1],Citation[3],Citation[29] A traumatic etiology becomes more prominent after extraordinary events such as massive earthquakes. Unfortunately, many countries are located on or very close to earthquake zones, and new disasters are expected, which might cause thousands of casualties in crowded cities.Citation[29] Thus, early and effective renoprotective treatments are of the utmost importance. The two most widely used experimental models of rhabdomyolysis-induced ARF are either myoglobin infusion into dehydrated/volume depleted animals or intramuscular hypertonic glycerol injection.Citation[1],Citation[3],Citation[30] The latter causes myolysis, hemolysis, acidosis, and intravascular volume depletion similar to trauma-induced rhabdomyolysis.Citation[30] Therefore, we speculate that L-carnitine treatment may be beneficial in traumatic rhabdomyolysis-induced ARF. However, clinical trials need to be conducted to determine the usefulness and the appropriate dose of L-carnitine in traumatic rhabdomyolysis-induced myoglobinuric ARF. We consider that i.v. administration is preferable because after oral doses of 1–6 g, the absolute bioavailability of L-carnitine is only 5–18%, mainly due to its poor absorption.Citation[31] Unabsorbed oral L-carnitine undergoes bacterial degradation in the gastrointestinal tract to form trimethylamine. In healthy subjects, trimethylamine, a volatile amine, is rapidly converted to trimethylamine-N-oxide and is excreted efficiently by the kidneys. Patients with kidney failure are unable to excrete trimethylamine-N-oxide effectively and experience the accumulation of trimethylamine-N-oxide and trimethylamine.Citation[32],Citation[33] It has been shown that both molecules can form N-nitrosodimethylamine, a known carcinogen.Citation[34] Moreover, trimethylamine may contribute to “uremic breath.”Citation[35] On the other hand, the i.v. form of L-carnitine is fully bioavailable and undergoes a negligible metabolism.Citation[33],Citation[36] It has been shown that L-carnitine can safely be used intravenously with no significant side effects, at doses up to 6 g/day, even during long-term (seven months) administration in humans.Citation[37],Citation[38]
In the current study, we have functionally, biochemically, and histopathologically demonstrated the protective effects of L-carnitine by reducing the severity of glycerol-induced myoglobinuric kidney damage. Our data suggest that the putative mechanism of the protective effect of L-carnitine may involve the inhibition of lipid peroxidation by scavenging ROS and/or chelating of iron. Our findings show that L-carnitine, used as a safe and effective nutritional supplement for more than three decades, could be a beneficial agent in the prevention and treatment of glycerol-induced myoglobinuric acute renal failure.
DECLARATION OF INTEREST
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
ACKNOWLEDGMENTS
This work was supported by Trakya University, Scientific Researchs Committee (project number TUBAP-448).
REFERENCES
- Zager RA. Rhabdomyolysis and myohemoglobinuric acute renal failure. Kidney Int. 1996; 49: 314–326
- Vanholder R, Sever MS, Erek E, Lameire N. Rhabdomyolysis. J Am Soc Nephrol. 2000; 11: 1553–1561
- Abassi ZA, Hoffman A, Better OS. Acute renal failure complicating muscle crush injury. Semin Nephrol. 1998; 18: 558–565
- Ustundag S, Yalcin O, Sen S, Cukur Z, Ciftci S, Demirkan B. Ren Fail. 2008; 30: 727–735
- Stefanovic V, Savic V, Vlahovic P, Cvetkovic T, Najman S, Mitic-Zlatkovic M. Reversal of experimental myoglobinuric acute renal failure with bioflavonoids from seeds of grape. Ren Fail. 2000; 22: 255–266
- Chander V, Chopra K. Molsidomine, a nitric oxide donor and L-arginine protects against rhabdomyolysis-induced myoglobinuric acute renal failure. Biochim Biophys Acta. 2005; 1723: 208–214
- Paller MS, Hoidal JR, Ferris TF. Oxygen free radicals in ischemic acute renal failure in the rat. J Clin Invest. 1984; 74: 1156–1164
- Emerit J, Beaumont C, Trivin F. Iron metabolism, free radicals, and oxidative injury. Biomed Pharmacother. 2001; 55: 333–339
- Paller MS, Hedlund BE. Role of iron in postischemic renal injury in the rat. Kidney Int. 1988; 34: 474–480
- Galaris D, Korantzopoulos P. On the molecular mechanism of metmyoglobin-catalyzed reduction of hydrogen peroxide by ascorbate. Free Radic Biol Med. 1997; 22: 657–667
- Di Lisa F, Barbato R, Manebo R, Siliprandi N. Carnitine and carnitine esters in mitochondrial metabolism and function. The carnitine system. A new therapeutical approach to cardiovascular diseases, JW De Jong, R Ferrari. Kluwer Academic Publishers, Dordrecth 1995; 21–38
- Mayes PA. Lipids of physiologic significance. Harper's Biochemistry, RK Murray, DK Granner, PA Mayes, VW Rodwell. Stamford, Appleton and Lange 2000; 160–171
- Arduini A. Carnitine and its acyl esters as secondary antioxidants?. Am Heart J. 1992; 123: 1726–1727
- Gülç I. Antioxidant and antiradical activities of L-carnitine. Life Sci. 2006; 78: 803–811
- Sener G, Ekşioğlu-Demiralp E, Cetiner M, et al. L-Carnitine ameliorates methotrexate-induced oxidative organ injury and inhibits leukocyte death. Cell Biol Toxicol. 2006; 22: 47–60
- Boonsanit D, Kanchanapangka S, Buranakarl C. L-carnitine ameliorates doxorubicin-induced nephrotic syndrome in rats. Nephrology. 2006; 11: 313–320
- Origlia N, Migliori M, Panichi V, et al. Protective effect of L-propionylcarnitine in chronic cyclosporine-a induced nephrotoxicity. Biomed Pharmacother. 2006; 60: 77–81
- Görür S, Bağdatoğlu OT, Polat G. Protective effect of L-carnitine on renal ischaemia-reperfusion injury in the rat. Cell Biochem Funct. 2005; 23: 151–155
- Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979; 95: 351–358
- Aebi H. Catalase in vitro. Methods Enzymol. 1984; 105: 121–126
- Shah SV, Walker PD. Evidence suggesting a role for hydroxyl radical in glycerol-induced acute renal failure. Am J Physiol. 1988; 255: F438–F443
- McHowat J, Yamada KA, Saffitz JE, Corr PB. Subcellular distribution of endogenous long chain acylcarnitines during hypoxia in adult canine myocytes. Cardiovasc Res. 1993; 27: 1237–1243
- Vanella A, Russo A, Acquaviva R, et al. L -propionyl-carnitine as superoxide scavenger, antioxidant, and DNA cleavage protector. Cell Biol Toxicol. 2000; 16: 99–104
- Rubanyi GM, Vanhoutte PM. Superoxide anions and hyperoxia inactivate endothelium-derived relaxing factor. Am J Physiol. 1986; 250: H822–H827
- Valdivielso JM, López-Novoa JM, Eleno N, Pérez Barriocanal F. Role of glomerular nitric oxide in glycerol-induced acute renal failure. Can J Physiol Pharmacol. 2000; 78: 476–482
- Alvarez de Sotomayor M, Bueno R, Pérez-Guerrero C, Herrera MD. Effect of L-carnitine and propionyl-L-carnitine on endothelial function of small mesenteric arteries from SHR. J Vasc Res. 2007; 44: 354–364
- Paller MS. Hemoglobin- and myoglobin-induced acute renal failure in rats: Role of iron in nephrotoxicity. Am J Physiol. 1988; 255: F539–F544
- Calò LA, Davis PA, Pagnin E, et al. Carnitine-mediated improved response to erythropoietin involves induction of haem oxygenase-1: Studies in humans and in an animal model. Nephrol Dial Transplant. 2008; 23: 890–895
- Sever MS. The basic concepts. The crush syndrome, MS Sever. Karger, Basel 2005; 15–35
- Rosenberger C, Goldfarb M, Shina A, et al. Evidence for sustained renal hypoxia and transient hypoxia adaptation in experimental rhabdomyolysis-induced acute kidney injury. Nephrol Dial Transplant. 2008; 23: 1135–1143
- Evans AM, Fornasini G. Pharmacokinetics of L-carnitine. Clin Pharmacokinet. 2003; 42: 941–967
- Bain MA, Faull R, Milne RW, Evans AM. Oral L-carnitine: metabolite formation and hemodialysis. Curr Drug Metab. 2006; 7: 811–816
- Reuter SE, Faull RJ, Evans AM. L-carnitine supplementation in the dialysis population: Are Australian patients missing out?. Nephrology (Carlton). 2008; 13: 3–16
- Ohshima H, Kawabata T. Mechanism of N-nitrosodimethylamine formation from trimethylamine and trimethylaminoxide. IARC Sci Publ. 1978; 19: 143–153
- Simenhoff ML, Burke JF, Saukkonen JJ, Ordinario AT, Doty R. Biochemical profile or uremic breath. N Engl J Med. 1977; 297: 132–135
- Rebouche CJ. Kinetics, pharmacokinetics, and regulation of L-carnitine and acetyl-L-carnitine metabolism. Ann N Y Acad Sci. 2004; 1033: 30–41
- Moretti S, Alesse E, Di Marzio L, et al. Effect of L-carnitine on human immunodeficiency virus-1 infection-associated apoptosis: A pilot study. Blood. 1998; 91: 3817–3824
- Moretti S, Famularo G, Marcellini S, et al. L-carnitine reduces lymphocyte apoptosis and oxidant stress in HIV-1-infected subjects treated with zidovudine and didanosine. Antioxid Redox Signal. 2002; 4: 391–403