Abstract
Nephrotoxicity is a rare complication caused by anti-tuberculosis therapy-induced oxidative stress. The Cyanobacterium Spirulina fusiformis Voronikhin belonging to Oscillatoriaceae family is used traditionally as a source of antioxidants against oxidative stress. We aimed to investigate the efficacy of S. fusiformis in modifying isoniazid (INH) and rifampicin (RIF)-induced changes in Wistar rat kidneys. Animals were divided into six groups: normal control rats; toxic control (INH & RIF—50 mg/kg b.w./d each; p.o.); INH & RIF + S. fusiformis (400 mg/kg b.w./d); INH & RIF + S. fusiformis (800 mg/kg b.w./d); S. fusiformis (800 mg/kg b.w./d) alone-treated rats; INH & RIF + silymarin (25 mg/kg b.w./d). Study duration was 28 d after which blood and kidneys were analyzed. We also studied the binding and interactions of the transcription factors Liver X Receptor (LXR) and Farnesoid X Receptor (FXR) with INH, RIF, and representative active compounds of S. fusiformis by in silico methods. INH & RIF treatment caused significant (p< 0.05) decrease in antioxidant levels and significant (p< 0.05) increase in the levels of creatinine, urea, and uric acid showing impaired kidney function. Spirulina fusiformis ameliorated these effects in a dose dependent manner. Histological examination of kidneys supported these findings. Results of the in silico analyses showed that selected active components of S. fusiformis interact with LXR and FXR and could be a possible mechanism of action. S. fusiformis rendered protection against anti-tuberculosis drugs-induced oxidative stress in kidney tissues of rats.
Introduction
Tuberculosis is an infection that affects around one-third of the world’s population and leads to millions of deaths around the globe. It also accounts for the highest mortality and morbidity worldwide.Citation1 First-line agents used in tuberculosis treatment are the best defense against its spread but adverse reactions to these drugs prevent their effective usage in certain patients. Though hepatotoxicity due to isoniazid (INH) and rifampicin (RIF) has been reported to be the most important adverse effect, nephrotoxicity has also been reported in a small number of patients. It is also known that RIF is associated with more reported cases of drug-induced kidney injury in anti-tuberculosis treatment (ATT) than the other drugs such as INH and ethambutol.Citation2,Citation3
It was seen that acute kidney injury (AKI) is a transient event during ATT in most patients and does not occur after the drugs have been withdrawn and re-introduced at a later stage. The occurrence of AKI is hypothesized to be due to the formation of immune complexes by anti-rifampicin antibodies that then get deposited in the blood vessels or interstitium causing glomerular endotheliosis and leading to AKI. De Vriese et al.Citation4 have demonstrated that tubular necrosis is caused by the immune complexes. The same has been confirmed in recent case reports from patients undergoing anti-tuberculosis therapy.Citation5 Chan et al.Citation6 have also explained how renal tubular ischemia is caused by endothelial inflammation as a result of immune complex deposition in the blood vessels. This was confirmed in a recent study in Taiwan showed an incidence of 7.1% among patients undergoing anti-tuberculosis therapy. It was also seen that it occurred among the elderly with a median age of 68 and with males more susceptible than females.Citation7 In our current study, we have tried to simulate and evaluate renal injury due to ATT in rats and its use as an animal model of the human condition. We have also tried to study the ameliorative effects of co-administration of Spirulina fusiformis on the renal function and antioxidant status of rats undergoing ATT using INH and RIF as representative drugs. Spirulina species have been known to have anti-tumor, hepatoprotective, metalloprotective, radioprotective, antimicrobial, and anti-inflammatory activities.Citation8 Inflammatory response to immune complexes is thought to play a major role in ATT induced renal damage, along with the generation of reactive oxygen species (ROS) due to induction of cytokines, chemokines, and leucocyte enzymes. The Liver X receptor (LXR) has been shown previously to inhibit inflammatory response and also positively regulate antioxidant enzymes.Citation9 We have tried to look at the interaction between LXR and selected active compounds of S. fusiformis (β-carotene, phycocyanobilin, and vitamin B12) by in silico docking experiments. Farnesoid X receptor (FXR) is another nuclear receptor known to play a role in regulating renal lipid metabolism, fibrosis, inflammation and oxidative stress.Citation10 The selected active compounds of S. fusiformis were also studied for their interaction with FXR by the same in silico methods.
Materials and methods
Animals
Female Wistar albino rats with a mean weight of 138 ± 11 g, from the animal house, VIT University, Vellore, were used for the study. Animals were maintained at a temperature of 27 °C under standard conditions with a 12 h dark-light cycle. The rats were fed with commercially available pellet feed from Hindustan Lever Ltd. (Mumbai, India), and water was made freely available. The experimental procedure was approved by the ethical committee (VIT/IAEC/VIIIth/9) of VIT University, Vellore, India.
Toxin and drug
Commercially available S. fusiformis was obtained from Acumen Pharmaceuticals, Pudhuchery, India, and was administered orally as an aqueous suspension in double-distilled water. INH and RIF were obtained from Novus Life Sciences Pvt. Ltd., Mumbai, India. The standard hepatoprotective drug silymarin was obtained from Microlabs Ltd., Goa, India, and administered orally as an aqueous suspension.
Experimental design
The female Wistar albino rats were divided into six groups of six animals each. The antibiotics and drugs were given orally for a period of 28 d. Group I (normal control) was given saline (0.1 mL) orally. Group II was administered a solution of INH and RIF (50 mg/kg b.w./d) orally. Group III was treated with S. fusiformis (400 mg/kg b.w./d) and Group IV with S. fusiformis (800 mg/kg b.w./d) orally and both the groups were treated with the concomitant administration of INH and RIF (50 mg/kg b.w./d). Group V was treated with S. fusiformis (800 mg/kg b.w./d), while Group VI was treated with INH and RIF along concomitant administration of silymarin orally for 28 d. The dosage of S. fusiformis used in current study was found to be effective in previous studies.Citation11 During the course of the experiment, animals were weighed daily. After 24 h of the last dosage, the rats were decapitated; trunk blood collected, and kidneys procured for biochemical, histopathological, and antioxidant studies.
The trunk blood was collected in clean dry test tubes, allowed to clot and then centrifuged at 3000 rpm for 10 min at 4 °C for serum separation. The serum thus obtained was stored at −70 °C for performing serum protein estimation. Plasma sample was obtained by collecting trunk blood in a tube containing EDTA serving as anticoagulant. Kidney tissues were homogenized in 5% Phosphate buffered saline (PBS) solution. The homogenate was then centrifuged at 3000 rpm for 10 min at 4 °C. The supernatant obtained was used for the estimation of antioxidants. A portion of kidneys was fixed in formalin for histopathological studies.
Biochemical parameters
The levels of total protein, albumin, creatinine, urea, and uric acid were determined in serum using diagnostic kits according to the protocol given by manufacturers. Assays of antioxidant enzymes like catalase (CAT),Citation12 superoxide dismutase (SOD),Citation13 glutathione-S-transferase (G-S-T),Citation14 glutathione peroxidase (GPx)Citation15 and total reduced glutathione (reduced GSH),Citation14 and the lipid peroxidation levelsCitation16 were determined in the kidney tissue homogenate. Total protein was estimated by Lowry’s method using bovine serum albumin as standard.Citation17 Acid phosphatase was estimated in the using by the method of Ref.Citation18
Histopathological analysis
The kidney tissues were excised from all the groups and fixed in 10% neutral formalin solution followed by automated processing and embedding in paraffin wax. A microtome (Leica RM2155) was used to cut the sections to a thickness of 5 μm. This was followed by hematoxylin–eosin staining of the slides and scoring of the renal damage.
Receptor molecule structures
The 3D structures of the receptor molecules (LXR and FXR) selected for in silico studies were obtained from the protein data bank (http://www.rcsb.org/pdb/home.do). The protein structures were prepared for docking by removing water molecules and adding hydrogen atoms. The active sites were predicted using Q-site finder (http://www.bioinformatics.leeds.ac.uk/qsitefinder/).
Ligand structures
Pubchem (http://www.ncbi.mlm.nih.gov.pccompound) provided the sequences of selected active components of S. fusiformis (phycocyanobilin, β-carotene, and vitamin B12) and these were uploaded in SMILES format on Corina (http://www.molecular-networks.com/node/84) to generate 3D structures of the ligands. The generated 3D structures were used for the in silico docking experiments.
Docking experiment
PatchDock, a server for molecular docking that functions based on shape complementarity principles, was used for the experiment. PatchDock generates scores based on both geometric fit and atomic desolvation energies. The receptor molecule and ligand files were uploaded on PatchDock server in pdb format. A valid e-mail of the user was used for obtaining the completed results.Citation19
Statistical analysis
The data obtained was computed to calculate the mean and standard deviation (SD). One-way analysis (ANOVA) was performed for statistical evaluation of the data.
Results
Serum markers
INH and RIF treated rats showed significantly (p < 0.05) elevated serum creatinine, urea, uric acid, and acid phosphatase levels when compared with the control group. Spirulina treatment significantly (p < 0.05) reduced these levels to near normal indicating the reversal of kidney injury induced by INH and RIF ().
Table 1. Effect of Spirulina fusiformis on kidney functional markers in the serum of INH and RIF intoxicated rats.
Antioxidant levels in kidney homogenate
Administration of INH and RIF caused significant (p < 0.05) increase in the levels of lipid peroxidation and a significant decrease (p < 0.05) in the levels of superoxide dismutase (SOD) and catalase (). In addition, significant (p < 0.05) reductions in GSH-Px, GST, and reduced GSH were observed after the administration of INH and RIF (). Spirulina treatment normalized the lipid peroxidation levels and showed a significant (p < 0.05) reversion in the levels of SOD, catalase, GSH-Px, GST, and reduced GSH to near normal levels ().
Table 2. Effect of Spirulina fusiformis on the activities of anti-oxidant enzymes and lipid peroxidation levels in the kidney tissue homogenates of INH & RIF intoxicated rats.
In silico experiments
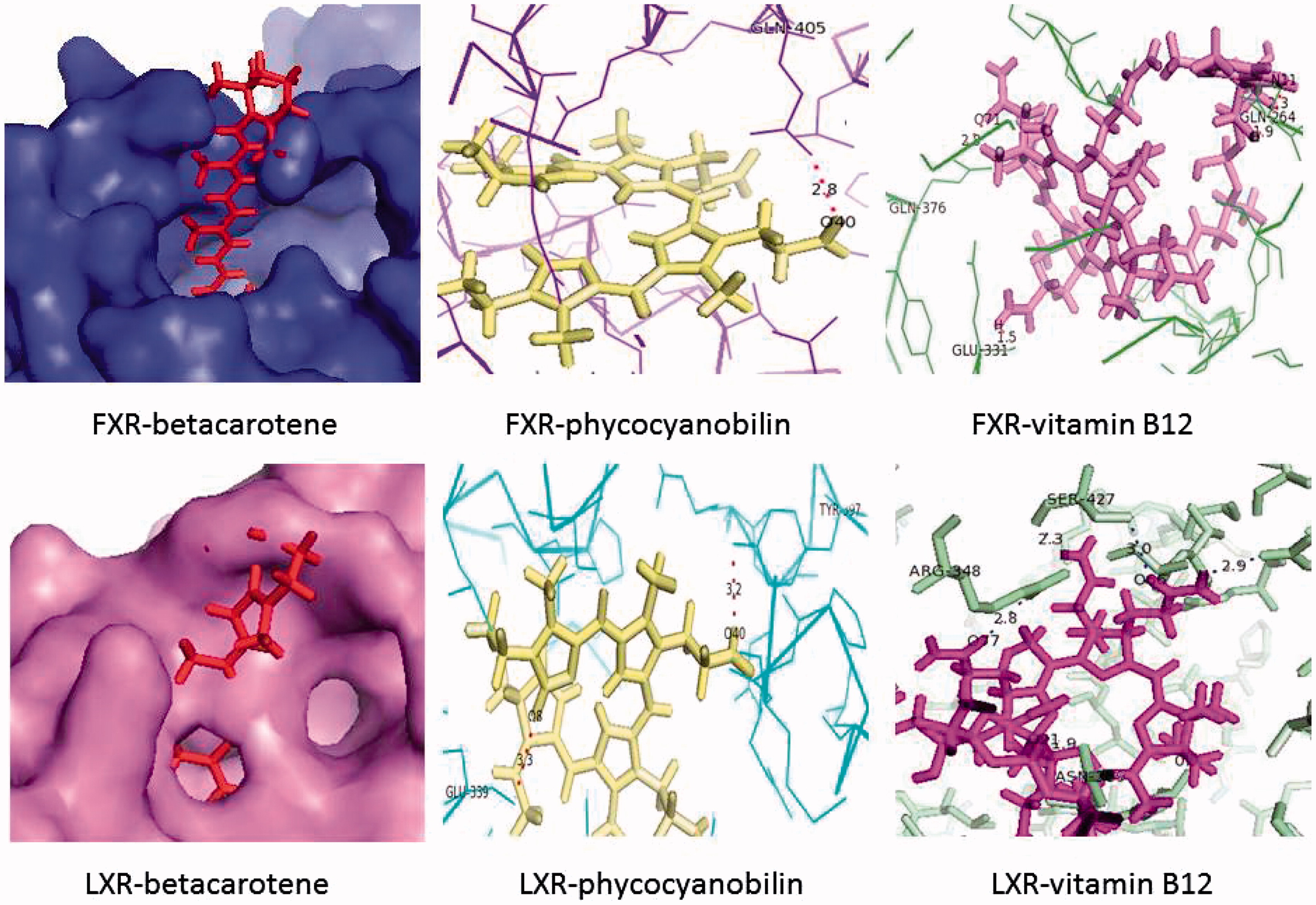
Vitamin B12 and β-carotene interact more with LXR than phycocyanobilin while with FXR, phycocyanobilin and β-carotene interact well with less scores between FXR and vitamin B12. These results are shown in as scores and ACE. The simulated interactions are also seen in .
Table 3. Scores and ACE of in silico docking experiments between the selected active compounds of Spirulina fusiformis (beta carotene, vitamin B12, and phycocyanobilin) and the nuclear receptors LXR and FXR.
Histological assessment of kidney tissue
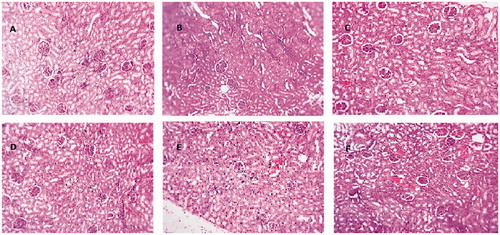
Normal glomerular and tubular histology was seen in the normal control rats (Group I) and there were no observable changes in the kidney tissue morphology. INH and RIF intoxicated rats (Group II) showed glomerular and tubular damage and congestion indicating their nephrotoxic effect. INH + RIF and S. fusiformis (400 mg/kg b.w.) treated rats (Group III) showed normal renal parenchyma with minimal congestion of blood vessels. There was no glomerular or tubular damage in the S. fusiformis treated rats. INH + RIF and S. fusiformis (800 mg/kg b.w.) treated Group IV rats showed congestion of blood vessels. Silymarin treated group showed nearly normal kidney architecture. These results are shown in .
Figure 2. (A) Control, normal appearing kidney. (B) INH and RIF, Renal parenchyma showing congestion and interstitial damage. (C) INH and RIF + Spirulina 400 mg/kg b.w. Normal appearing renal parenchyma with minimal congestion of interstitial blood vessels. (D) INH and RIF + Spirulina 800 mg/kg b.w. Normal renal parenchyma. (E) Spirulina 800 mg/kg b.w. Normal renal parenchyma. (F) INH + RIF + Silymarin 25 mg/kg b.w. Renal parenchyma showing minimal congestion.
Discussion
INH and RIF are the two major drugs given to treat tuberculosis around the globe. INH is known to be a potent hepatotoxic agent in some patients and RIF is known to increase this effect synergistically. This has been an area of concern and increasing research. Nephrotoxicity due to these compounds on the other hand has lesser incidence and has not been studied much. INH is not known to cause nephrotoxicity but RIF has been proven to cause acute renal failure (ARF) in sporadic cases.Citation4,Citation7 Most acute reactions occur during re-introduction of RIF during therapy though some reports have been during continuous RIF therapy.Citation6,Citation20 RIF-induced ARF is characterized by tubular necrosis and tubulo-interstitial nephritis. It is known that INH and RIF cause liver toxicity and release of toxic metabolites. These can accumulate in the kidney and cause tubular damage.
Our results show that INH and RIF treatment results in increased creatinine, urea and uric acid levels in the rat serum thereby indicating impaired renal function. This is shown to be reversed by treatment with S. fusiformis. It is also seen that the total protein levels and albumin levels have fallen in the rat serum indicating their loss from the blood due to damaged renal function. The INH and RIF treatment is also seen to reduce the antioxidant status of the kidneys thereby reducing their ability to counter the effects of the toxin. As RIF-induced nephrotoxicity usually occurs in humans after re-introduction, it is thought to be due to immune complexes. These immune complexes can lower the antioxidant status of the kidneys by induction of inflammatory mechanisms. Although not established, this could be a possible mechanism in the rat model. It is seen that treatment with S. fusiformis reverses the antioxidant status of the kidneys. Many of the active constituents present in S. fusiformis, such as phycocyanins and phycobilins, have been seen to inhibit lipid peroxidation and scavenge hydroxide and superoxide radicals, thereby increasing the antioxidant status. The protective role of S. fusiformis seen in this study may be due to the antiperoxidative, and antioxidant action of its components. This was comparable with that of the standard drug silymarin. Silymarin is a flavonoid complex that consists of five different compounds (silybin, silydianin, silychristin, taxifolin, and quercetin). Silymarin has been proven to possess significant antioxidant properties and is being used as the standard drug for reference in studies involving animal models.Citation21,Citation22 It was, therefore, chosen as the positive control drug in current study.
We had selected three of the most important active components of S. fusiformis for in silico docking experiments against nuclear receptors (LXR and FXR) known to protect against inflammatory responses and oxidative stress. It is known that β-carotene and its products retinoic acid and retinaldehyde activate retinoid X receptor and causes activation of anti-inflammatory genes.Citation23 The receptors chosen for the analysis of LXR and FXR are known to be involved in lipid metabolism. As lipid metabolism is linked to the antioxidant status of the tissue, these receptors can play a major role in ameliorating the inflammatory effects and oxidative stress due to immune complexes of the drugs. It is further known that RXR dimerizes with LXR and FXR and causes the activation of many genes.Citation24 It has also been shown in many previous studies that vitamin B12 plays a major role in the metabolism of lipids through receptors such as cubilin.Citation25,Citation26 The third active compound selected, phycocyanobilin, is known for its antioxidant properties and may play an important role in bringing down the oxidative stress induced by the accumulation of immune complexes and their resulting inflammatory response.Citation27,Citation28 In our docking experiments, we have tried to look at the affinity of these three selected active compounds to LXR and FXR during any direct interaction. We have tried to assess their binding to the nuclear receptors independently. β-Carotene is found to have high affinity to the two receptors, thereby substantiating previous reports of its involvement in lipid metabolism and anti-inflammatory effect.Citation29 Vitamin B12 interacts well with LXR but not as closely with FXR. Phycocyanobilin is found to interact more with FXR than with LXR. The experiments show that these compounds have simulated binding capabilities with the receptors. This raises the possibility that these can activate LXR and FXR thereby modulating lipid metabolism, inflammatory response, and antioxidant status of the kidneys. This gives a possible mechanism by which the inflammation and oxidative stress either due to deposition of immune complexes in the interstitium or toxic effects of drug metabolites are ameliorated by the administration of S. fusiformis. These need to be proven by further studies on whether these interactions are translated to the in vivo environment.
Thus, our study shows that concomitant treatment with S. fusiformis protects the kidneys from the oxidative stress induced by INH & RIF. Since Spirulina can be taken as a food supplement and has very little or no known harmful effects, it can be an ideal drug for co-administration with anti-tuberculous therapy. This is especially significant considering the increasing incidence of renal injury among elderly populations undergoing ATT. Although showing promising results in the rat model further study is necessary to look at its effectiveness and mechanisms before it can be used clinically. Although oxidative stress has been proven to occur in the rat kidneys, nephrotoxicity could not be established conclusively. Other animal models and specific studies may provide a better representation of INH and RIF-induced nephrotoxicity. It is also necessary to study the mechanism of nephrotoxicity due to RIF, since it occurs in select individuals sporadically and especially during re-introduction of the drug which could be due to induction of an immune component. Further studies would make the use of anti-tuberculosis treatment much safer with minimal toxic effects of the ATT drugs.
Funding information
The authors would like to thank Council of Scientific and Industrial Research (CSIR), India, for providing Junior Research Fellowship grant.
Acknowledgements
The authors would like to thank VIT University for providing the necessary facilities for conducting experiments and analyzing results.
Disclosure statement
The authors report that they have no conflicts of interest in this work.
References
- WHO Tuberculosis. WHO. Available at: http://www.who.int/mediacentre/factsheets/fs104/en/. Accessed December 30, 2015.
- Hirsch DJ, Bia FJ, Kashgarian M, Bia MJ. Rapidly progressive glomerulonephritis during antituberculous therapy. Am J Nephrol. 1983;3:7–10.
- Park DH, Lee SA, Jeong HJ, Yoo T-H, Kang S-W, Oh HJ. Rifampicin-induced minimal change disease is improved after cessation of rifampicin without steroid therapy. Yonsei Med J. 2015;56:582–585.
- De Vriese AS, Robbrecht DL, Vanholder RC, Vogelaers DP, Lameire NH. Rifampicin-associated acute renal failure: Pathophysiologic, immunologic, and clinical features. Am J Kidney Dis Off J Natl Kidney Found. 1998;31:108–115.
- Rosati S, Cherubini C, Iacomi F, et al. Acute rifampicin-associated interstitial tubulopathy in a patient with pulmonary tuberculosis: A case report. J Med Case Rep. 2013;7:106.
- Chan WC, O’Mahoney GMS, Yu DYC, Yu RYH. Renal failure during intermittent rifampicin therapy. Tubercle. 1975;56:191–198.
- Chang C-H, Chen Y-F, Wu V-C, et al. Acute kidney injury due to anti-tuberculosis drugs: A five-year experience in an aging population. BMC Infect Dis. 2014;14:23.
- Deng R, Chow T-J. Hypolipidemic, antioxidant, and antiinflammatory activities of microalgae Spirulina. Cardiovasc Ther. 2010;28:e33–e45.
- Kiss E, Popovic Z, Bedke J, et al. Suppression of chronic damage in renal allografts by Liver X Receptor (LXR) activation relevant contribution of macrophage LXRα. Am J Pathol. 2011;179:92–103.
- Hollman DAA, Milona A, van Erpecum KJ, van Mil SWC. Anti-inflammatory and metabolic actions of FXR: Insights into molecular mechanisms. Biochim Biophys Acta. 2012;1821:1443–1452.
- Rasool M, Sabina EP. Appraisal of immunomodulatory potential of Spirulina fusiformis: An in vivo and in vitro study. J Nat Med. 2009;63:169–175.
- Sinha AK. Colorimetric assay of catalase. Anal Biochem. 1972;47:389–394.
- Marklund S, Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem FEBS. 1974;47:469–474.
- Moron MS, Depierre JW, Mannervik B. Levels of glutathione, glutathione reductase and glutathione S-transferase activities in rat lung and liver. Biochim Biophys Acta BBA – Gen Subj. 1979;582:67–78.
- Rotruck JT, Pope AL, Ganther HE, Swanson AB, Hafeman DG, Hoekstra WG. Selenium: Biochemical role as a component of glutathione peroxidase. Science. 1973;179:588–590.
- Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–358.
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275.
- Kind PRN, King EJ. Estimation of plasma phosphatase by determination of hydrolysed phenol with amino-antipyrine. J Clin Pathol. 1954;7:322–326.
- Schneidman-Duhovny D, Inbar Y, Nussinov R, Wolfson HJ. PatchDock and SymmDock: Servers for rigid and symmetric docking. Nucleic Acids Res. 2005;33:W363–W367. (Web Server issue).
- Min HK, Kim EO, Lee SJ, et al. Rifampin-associated tubulointersititial nephritis and Fanconi syndrome presenting as hypokalemic paralysis. BMC Nephrol. 2013;14:13–18.
- Feyissa T, Asres K, Engidawork E. Renoprotective effects of the crude extract and solvent fractions of the leaves of Euclea divinorum Hierns against gentamicin-induced nephrotoxicity in rats. J Ethnopharmacol. 2013;145:758–766.
- Shahbazi F, Dashti-Khavidaki S, Khalili H, Lessan-Pezeshki M. Potential renoprotective effects of silymarin against nephrotoxic drugs: A review of literature. J Pharm Pharm Sci. 2012;15:112–123.
- Ziouzenkova O, Plutzky J. Retinoid metabolism and nuclear receptor responses: New insights into coordinated regulation of the PPAR-RXR complex. FEBS Lett. 2008;582:32–38.
- Willson TM, Brown PJ, Sternbach DD, Henke BR. The PPARs: From orphan receptors to drug discovery. J Med Chem. 2000;43:527–550.
- Seetharam B. Receptor-mediated endocytosis of cobalamin (vitamin B12). Annu Rev Nutr. 1999;19:173–195.
- Christensen EI, Nielsen R, Birn H. From bowel to kidneys: The role of cubilin in physiology and disease. Nephrol Dial Transplant Off Publ Eur Dial Transpl Assoc – Eur Ren Assoc. 2013;28:274–281.
- TakashiHirata MT. Antioxidant activities of phycocyanobilin prepared from S. platensis. J Appl Phycol J Appl Phycol. 2000;12:435–439.
- Zheng J, Inoguchi T, Sasaki S, et al. Phycocyanin and phycocyanobilin from Spirulina platensis protect against diabetic nephropathy by inhibiting oxidative stress. Am J Physiol Regul Integr Comp Physiol. 2013;304:R110–R120.
- Ciccone MM, Cortese F, Gesualdo M, et al. Dietary intake of carotenoids and their antioxidant and anti-inflammatory effects in cardiovascular care. Mediators Inflamm. 2013; 2013:782137.