Abstract
Objective: To investigative clinical and pathological characteristics of IgA nephropathy with chronic renal failure.
Method: Clinical and pathological findings from 65 cases of IgA nephropathy with chronic renal failure were reviewed. Pathological characteristics of all the cases were analyzed according to WHO definition and Oxford Classification. Evaluating the severity of pathological lesions by the Katafuchi R semiquantitative scoring system, and analyzing their relationship with clinical indexes of renal function.
Results: Of all 65 cases the male and female ratio was 1.4, and the mean age was 37 ± 13 years old. Levels of systolic pressure, mean arterial pressure (MAP), blood urea nitrogen (BUN), serum creatinine (Scr), uric acid (UA), album (Alb), serum IgG and 24 h urinary protein were related with eGRF level (p < 0.05, respectively). The most common pathological type was proliferative sclerosis glomerulonephritis (PSGN) and M1S1E0T0 according to WHO definition and Oxford Classification, respectively, and most of the 65 cases had glomerulosclerosis. Simple IgA deposition was the most common immunopathologic type. Of all the cases, 44.6% accompanied with C3 while 4.6% with C1q. Further analysis revealed there were no relationships between severity of pathological lesion and levels of clinical indexes (Scr and eGRF) (p > 0.05).
Conclusion: IgA nephropathy with chronic renal failure usually occurred in young adults, and it had severe clinical condition and pathological changes, while there was no significant relationship between them.
Introduction
IgA nephropathy (IgAN) has become the most common primary glomerulonephritis worldwide. Previously, IgAN was regarded as a naïve disease and studies were rarely performed about clinicopathological analysis of IgAN with chronic renal failure. Therefore, to better understand IgAN with chronic renal failure well, we collected 65 cases and analyzed their general, clinical and pathological characteristics. Furthermore, we studied the relationship between some clinical indicators of renal function and histopathological features separately, providing help for clinical treatment and prognosis evaluation.
Subjects and methods
Entry and exclusion criteria
The 65 cases with IgAN and chronic renal failure were identified in a retrospective review of all renal biopsies received at the Suzhou Municipal Hospital from January 2010 to May 2015 and the following inclusion criteria were included: (1) diagnosis of IgAN, based on predominant mesangial IgA containing immune complexes detected by immunofluorescence and light microscopy; (2) eGRF evaluated according to CKD-EPI formula was less than 60 mL/min. Besides, any patients with systemic diseases (systemic lupus erythematosus, diabetes mellitus, the Henoch–Schönlein purpura and liver cirrhosis, etc.) were excluded by clinical history, physical examination, and laboratory test results. All the cases were divided into two groups by eGRF level (group A, 31 ≤ eGRF ≤60 mL/min group B, eGRF ≤30 mL/min). The scientific and ethics committee of our hospital approved the study protocol and we obtained informed consent from all patients before the study.
Clinical and laboratory data collection
For all 65 patients included in our study, general, clinical, and laboratory characteristics were collected. The clinical parameters included sex, age, course of disease, and blood pressure. Laboratory data included blood biochemical indicators, such as serum albumin (Alb), blood urea nitrogen (BUN), serum creatinine (Scr), uric acid (UA), triglyceride (TG) and total cholesterol (CHOL), serum immunoglobulin and complement (IgA, IgG, IgM, C3, C4), urinary protein, and urinary red blood cell count (URBC).
Renal pathology evaluation
All specimens having more than eight glomeruli were examined for light microscope and immunofluorescence tests. The pathology diagnosis was determined by using the 1995 WHO classificationCitation1 for glomerular disease. We also assessed cases using the Oxford classification scoring system proposed by the working group of the International IgA Nephropathy Network and the Renal Pathology Society and categorized them with independent histologic lesions which were mesangial hypercellularity (M), segmental glomerulosclerosis (S), endocapillary hypercellularity (E), and tubular atrophy/interstitial fibrosis (T).Citation2 Further pathological evaluation was conducted by the Katafuchi R semiquantitative score system,Citation3 including glomerular mesangial proliferation (score 1–4), segmental glomerulosclerosis (score 0–3), tubular atrophy(score 0–3) and interstitial fibrosis (score 0–3).
Statistical analysis
The SPSS 17.0 software for Windows (SSPS Inc., Chicago, IL) was used for statistical analysis. Continuous variables were expressed as means ± SD and grouped data were expressed as percentages. Comparisons were based on t test or Mann–Witney U test for quantitative data, χ2-test for categorical data. p Values less than 0.05 were considered statistically significant.
Results
General and clinical features
A total of 65 patients were included in this study. There were 38 males and 27 females (male:female ratio was 1.4:1). The mean age was 37 ± 13 years old and the mean disease curse was 19.0 ± 42.1 months.
Blood biochemical results and levels of serum immunoglobulin and complement were listed in . Group A (31 ≤ eGRF ≤60 mL/min) had 42 cases while group B (eGRF ≤30 mL/min) had 23 cases. Compared with group A, group B had higher levels of systolic pressure, mean arterial pressure (MAP), BUN, Scr, UA, and 24 h urinary protein, whereas it had lower levels of serum Alb and IgG (p < 0.05, respectively). Relationship between levels of clinical indicators and eGRF level were analyzed, it revealed that levels of systolic pressure, MAP, BUN, Scr, UA, Alb, serum IgG, and 24 h urinary protein were related with eGRF level (p < 0.05, respectively, ).
Table 1. Clinical characteristics of 65 cases.
Table 2. Relationship between the levels of clinical indicators and eGRF level.
During the first three days after renal biopsy, 3 cases (4.6%) were without urinary test change, 58 cases (89.2%) were presented with microscopic hematuria, and 4 cases (6.2%) were with hematuria and renal hematoma, which were observed on conservative treatment. None of the cases reported the occurrence of serious complications, such as massive hemorrhage, acute renal failure, and so on.
Pathological results
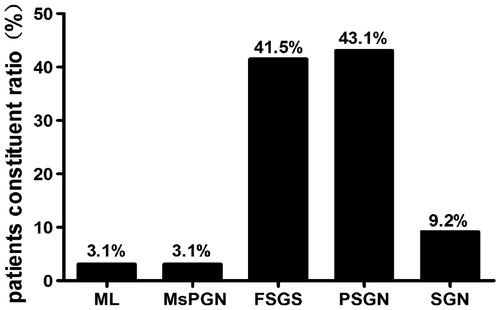
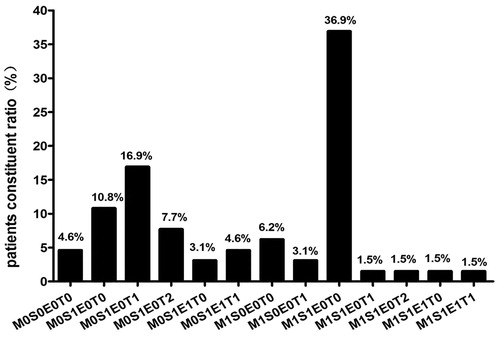
According to WHO definition, the most frequent histological pattern of glomerular injury was proliferative sclerosis glomerulonephritis (PSGN) and focal and/or segmental glomerulosclerosis (FSGS), found in 43.1% and 41.5% of patients, respectively, followed by sclerosis glomerulonephritis (SGN, 9.2%), mesangial proliferative glomerulonephritis (MsPGN, 3.1%) and minimal lesion (ML, 3.1%) (). Based on Oxford Classification, the distribution of pathological diagnoses is shown in . Among 65 cases, the most common pathology diagnosis was M1S1E0T0, accounting for 36.9%, followed by M0S1E0T1 (16.0%).
The pathological lesions of patients were listed in . Sixty-four cases (98.5%) had glomerular sclerosis, of them 62 cases (95.4%) had global sclerosis and 54 cases (83.1%) had segmental sclerosis, revealed that in most of the patients glomerular sclerosis occurred at the time of renal biopsy. All the patients were presented with mesangial cell proliferation, but mild lesion was the most common (72.3%). Most of the cases had interstitial fibrosis/tubular atrophy(72.3%), and part of the cases showed crescent formation (23.1%), capsular adhesion (12.3%), and pericapsular fibrosis (16.9%).
Table 3. Different pathological lesions of 65 cases.
Of 65 patients, the distribution of immunoglobulin was A, AM, AG, and AGM in 30 (46.1%), 25 (38.5%), 7 (10.5%) and 1 (4.6%) patients, respectively. The range of IgA fluorescence intensity was from + to ++++ and IgA ++ was the most common, accounting for 55.4%. The distribution of immunofluorescence examinations were listed in . Among the 65 cases, 44.6% were with C3 deposition, 4.6% with C1q deposition, and 1.5% with Fib deposition. Compared with group A, group B had higher rate of C1q deposition (p < 00.05), while there were no difference of IgG, IgM, C3, and Fib deposition rate between two groups.
Table 4. Immunofluorescence tests results of 65 cases.
Relationship between clinical indicator and pathological injury
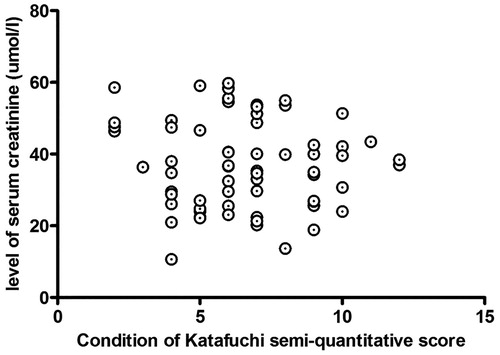
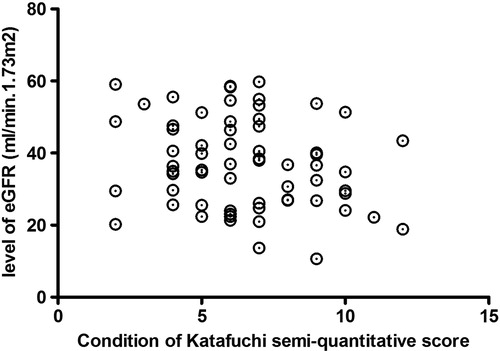
Scr and eGRF are important clinical indicators for renal function evaluation. The Oxford classification was recognized as the most accurate classification to predict the risk of progression of renal disease in IgAN.Citation4 Zeng et al.Citation5 made a multicenter study to verify the Oxford classification of IgAN in Chinese, found that M, S, and T had close relationship with long-term renal outcomes. Therefore, we evaluated mesangial cell proliferation (M), segmental sclerosis (S), and interstitial fibrosis/tubular atrophy (T) by the Katafuchi R semi-quantitative score system, found there were no relationship between levels of Scr and eGRF and degree of pathological injury (p > 0.05, and ).
Discussion
Epidemiology data showed that IgAN usually occurred in young adults and there existed gender difference that males were more common than females.Citation6 In our study, of the 65 IgAN patients with chronic renal failure the mean age is 37 ± 13 years old and the male–female ratio is 1.4, which is consistent with general characteristics of IgAN.
Elegant studies revealed that higher urinary protein, lower serum album, hypertension and presented with renal failure at the time of renal biopsy are indicators related with poor prognosis of IgAN.Citation7,Citation8 In our study, levels of urinary protein and systolic pressure were higher and album level was lower than the normal one, suggested IgAN with chronic renal failure was serious. eGRF is an important indicator reflecting renal function, it is evaluated by several formula based on serum creatinine, such as the Cockcroft–Gault equation, MDRD formula, CKD-EPI formula, and so on, among which CKD-EPI formula was regarded with much more accuracy and predictability.Citation9 Therefore, we divided all the cases into two groups according to eGRF calculated by CKD-EPI formula and made some comparison about the clinical indicators. The results revealed that the lower level of eGRF, levels of systolic pressure, MAP, BUN, Scr, UA and 24 h urinary protein were higher while Alb and serum IgG level was lower, suggested that systolic pressure, urinary protein, BUN, Scr, UA, Alb, and serum IgG could evaluate the state of IgAN with chronic renal failure to some degree.
Along with the wide spread of renal biopsy and the early diagnosis of IgAN, few studies of IgAN presented with chronic renal failure at renal biopsy were performed. It is concluded that the most common feature of IgAN on light microscopy is mesangial cell proliferation.Citation10 Another study reported that the most frequent diagnosis of IgAN presented with isolated hematuria was FSGS and M1S0E0T0 was the most common according to the Oxford classification.Citation11 Our study showed that the most common pathological type was PSGN and FSGS, and M1S1E0T0 was the most frequent according to the Oxford classification. Further analysis revealed that the vast majority of cases were presented with glomerulosclerosis and the sclerosis rate usually was 50%–75%, all the patients had mesangial cell proliferation but 72% of the cases had mild proliferation, and the great majority of cases with interstitial fibrosis/tubular atrophy but the most common lesion degree was less than 25%. All these suggest that the most important pathological change of IgAN with chronic failure is glomerulosclerosis. Recent studies revealed that there existed cross-talk between mesangial cell, podocyte, and tubular cell and they promote disease progression together.Citation12–14 An elegant study showed that segmental glomerulosclerosis and tubular atrophy and interstitial fibrosis were independent predictive factors of end stage renal disease but not mesangial cell proliferation.Citation15 So, we further speculate that during the progression of IgAN, mesangial cell proliferation is probably the initiating factor, while injury of podocyte and tubular cell are the key elements.
IgAN is characterized by mesangial deposition of IgA immune complexes, with or without IgG and C3.Citation16,Citation17 Our results showed that simple IgA deposition was the most common and IgA fluorescence intensity distribution mainly concentrated in IgA++, which was in accordance with the previous study.Citation18 Not completely in accordance with previous studies, our data showed that most of the cases with IgM and 44.6% of the cases with C3 deposition. Previous research revealed that the presence of C3 activation is associated with more severe renal disease in IgAN patients.Citation19 Subsequent studies also indicated complement activation involved in IgAN, but the pathogenic roles of glomerular deposition of components of the complement cascade are not completely clarified.Citation20,Citation21 The complement system can be activated via three pathways the classical pathway, the alternative pathway, and the lectin pathway. Of three complement activation pathways, the alternative and lectin pathways are involved in IgA nephropathy.Citation22 But, evidence of classical pathway activation, such as C1q deposits, is usually lacking in kidney biopsy specimens of patients with IgAN,Citation23 and the deposition is limited to patients with poor clinical outcomes.Citation24 Being different from the previous reports, our data showed that in 65 cases 3 had glomerular C1q deposition and eGRF level of all three patients less than 30 mL/min. Our result may be related one hand with the activation of the classical pathway may commonly occur in highly damaged kidneys,Citation25 another probably with the collection data is too small, so a further study with large sample data remained initiated.
IgAN with chronic renal failure is a serious disease, so is there any relationship between clinical indicator and pathological changes. Furthermore, we analyzed the relationship between the levels of Scr and eGRF and degree of pathological injury, and found there were no relationships between them. In addition, of all the patients for renal biopsy no one emerged serious complication. Above all, renal biopsy has probably become a necessary and safety examination.
Conclusion
In this study, we showed that IgAN with chronic renal failure had serious clinical characteristic. Pathological analysis revealed that the most significant change was glomerular sclerosis including global and segmental sclerosis, but not mesangial cell proliferation. Therefore, we supposed that mesangial cell probably act as a start in the development of IgAN, while podocyte and tubular cell promote the progression. Correlation analysis indicated clinical characteristic was not related with pathological lesion. So, if necessary, renal biopsy may be optional in consideration of its benefits and risk.
Disclosure statement
The authors have no conflicts of interest to disclose.
References
- Churg J, Bernstein J, Glassock RJ. WHO monograph. Renal Disease: Classification and Atlas of Glomerular Diseases. 2nd ed. Tokyo, Igaku-Shoin; 1995.
- Cattran DC, Coppo R, Cook HT, Feehally J, et al. The Oxford classification of IgA nephropathy: Rationale, clinicopathological correlations, and classification. Kidney Int. 2009;76:534–545.
- Katafuchi R, Kiyoshi Y, Oh Y, et al. Glomerular score as a prognosticator in IgA nephropathy: Its usefulness and limitation. Clin Nephrol. 1988;49:1–8.
- Troyanov S, Fervenza FC. Validating the oxford classification of IgA nephropathy. Clin J Am Soc Nephrol. 2011;6:2335–2336.
- Zeng CH, Liu ZH. Validating the oxford classification of IgA nephropathy: chinese results. The Chinese medical association of kidney disease annual meeting in 2011.
- Wang HY. Nephrology [M]. Beijing: People's Medical Publishing House; 2008:994.
- Goto M, Wakai K, Kawamura T, Ando M, Endoh M, Tomino Y. A scoring system to predict renal outcome in IgA nephropathy: A nationwide 10-year prospective cohort study. Nephrol Dial Transplant. 2009;24:3068–3074.
- Xie J, Kiryluk K, Wang W, et al. Predicting progression of IgA nephropathy: New clinical progression risk score. PLoS One. 2012;7:e38904.
- Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612.
- Robert J, Wyatt MD, Bruce A, Julian MD. IgA Nephropathy. N Engl J Med. 2013;368:2402–2414.
- Liu H, Peng Y, Liu H, et al. Renal biopsy findings of patients presenting with isolated hematuria: disease associations. Am J Nephrol. 2012;36:377–385.
- Xiao J, Leung JC, Chan LY, Gou H, Lai KN. Protective effect of peroxisome proliferator-activated receptor-gamma agonists on activated renal proximal tubular epithelial cells in IgA nephropathy. Nephrol Dial Transplant. 2009;24:2067–2077.
- Wang C, Liu X, Ke Z, et al. Mesangial medium from IgA nephropathy patients induces podocyte epithelial-to-mesenchymal transition through activation of the phosphatidyl inositol-3-kinase/Akt signaling pathway. Cell Physiol Biochem. 2012;29:743–752.
- Chan LY, Leung JC, Tsang AW, Tang SC, Lai KN. Activation of tubular epithelial cells by mesangial-derived TNF-alpha: Glomerulotubular communication in IgA nephropathy. Kidney Int. 2005;67:602–612.
- Shi SF, Wang SX, Jiang L, et al. Pathologic predictors of renal outcome and therapeutic efficacy in IgA nephropathy: Validation of the oxford classification. Clin J Am Soc Nephrol. 2011;6:2175–2184.
- Julian BA, Novak J. IgA nephropathy: an update. Curr Opin Nephrol Hypertens. 2004;13:171–179.
- Novak J, Julian BA, Tomana M, Mesteck J. Progress in molecular and genetic studies of IgA nephropathy. J Clin Immunol. 2001;21:310–327.
- Liu H, Peng YM, Liu FY. Renal pathology analysis of 360 hospitalized patients with IgA nephropathy from Hunan province. Chin J Nephrol. 2007;23:542–543.
- Zwirner J, Burg M, Schulze M, et al. Activated complement C3: A potentially novel predictor of progressive IgA nephropathy. Kidney Int. 1997;51:1257–1264.
- Espinosa M, Ortega R, Gomez-Carrasco JM, et al. Mesangial C4d deposition: A new prognostic factor in IgA nephropathy. Nephrol Dial Transplant. 2009;24:886–891.
- Roos A, Rastaldi MP, Calvaresi N, et al. Glomerular activation of the lectin pathway of complement in IgA nephropathy is associated with more severe renal disease. J Am Soc Nephrol. 2006;17:1724–1734.
- Oortwijn BD, Eijgenraam JW, Rastaldi MP, et al. The role of secretory IgA and complement in IgA nephropathy. Semin Nephrol. 2008;28:58–65.
- D’Amico G, Imbasciati E, Di Belgioioso GB, et al. Idiopathic IgA mesangial nephropathy. Clinical and histological study of 374 patients. Medicine (Baltimore). 1985;64:49–60.
- Lee HJ, Choi SY, Jeong KH, et al. Association of C1q deposition with renal outcomes in IgA nephropathy. Clin Nephrol. 2013;80:98–104.
- Maillard N, Wyatt RJ Julian BA, et al. Current understanding of the role of complement in IgA nephropathy. J Am Soc Nephrol. 2015;26:1503–1512.