Abstract
Apoptosis of renal tubular and glomerular cells during kidney disease involves activation of Fas ligand (FasL)-dependent death pathway. The significance of FasL in neonates with septic acute kidney injury (AKI) is unresolved, but an increase in renal FasL production, and/or infiltration of circulating FasL into the kidneys may occur following initial septic insult. Here, we examined whether soluble Fas ligand (sFasL) levels are altered during early phase of septic AKI in neonates. Six hours of polymicrobial sepsis elicited by cecal ligation and puncture (CLP) elevated serum C-reactive protein (CRP) (a bacteremia and sepsis marker) concentration in anesthetized and mechanically ventilated neonatal pigs. Serum creatinine and urea nitrogen concentrations were increased by ∼39% and 46%, respectively, following 6 h of CLP in the pigs. The urinary level of NGAL, an early marker of AKI was also elevated by ∼71% in the septic pigs. The basal concentration of sFasL in the serum and urine of neonatal pigs was similar. Six hours of CLP significantly increased serum and urine sFasL levels in the pigs by ∼24% and 68%, respectively. However, there was no evidence of caspase activation to suggest an induction of cellular apoptotic process in the kidneys of the septic pigs. These findings suggest that an increase in circulating and urinary sFasL during early septic AKI in neonatal pigs is not associated with renal apoptosis.
Introduction
The immune response to pathogen exposure includes the release of a wide variety of vasoactive and inflammatory mediators that can alter vascular resistance, hemodynamics, and/or provoke cell death.Citation1,Citation2 Pro-inflammatory cytokines, including interferon-gamma (IFN-γ) and tumor necrosis factor (TNF) alpha, whose levels are elevated during sepsis activate cellular signal transduction pathways that promote apoptotic cell death.Citation1,Citation2 Binding of TNF-α to its receptor TNFR1 results in the formation of a signaling complex containing adaptor proteins, including TNFR-associated death domain protein, receptor-interacting protein 1, TNF-receptor-associated factor 2, and Fas-associated death domain protein (FADD).Citation2,Citation3 The recruitment of these proteins and succeeding activation of the initiator and executioner caspases trigger apoptosis via cleavage of cellular proteins.Citation2,Citation3
Fas death receptor (Apo-1/CD95/TNFRSF6) and its cognate ligand FasL are members of the TNF superfamily that regulate the physiological and pathophysiological mechanisms of immune response.Citation4,Citation5 Activation of Fas by FasL is a major trigger of the extrinsic pathway of apoptosis.Citation4,Citation5 Similar to the TNF-α/TNFR1-mediated death-signaling cascades, binding of FasL to Fas induces trimerization of the receptor, and sequential recruitment of FADD and pro-caspase-8 to form an active death-inducing signaling complex (DISC).Citation4,Citation5 DISC formation results in the activation of caspases and apoptotic events.Citation4,Citation5 In the kidneys, the FasL/Fas system has been demonstrated in glomerular mesangial cells, fibroblasts, and tubular epithelial cells.Citation6,Citation7 Renal FasL is upregulated in rats and mice with proliferative glomerulonephritis and lupus nephritis, respectively.Citation7 FasL-positive infiltrating lymphocytes in the interstitium and Fas expression in renal tubular epithelium were found to be significantly higher in renal allograft biopsy specimens from kidney transplant recipients with acute rejection when compared with recipients with no rejection.Citation8 Increased FasL and/or Fas expressions have also been linked to renal tubular apoptosis in animal models of chronic kidney disease, endotoxemia, and renal ischemia/reperfusion injury.Citation9–13
FasL is synthesized as a transmembrane protein that can be cleaved by metalloproteinases to produce a soluble form (sFasL).Citation14,Citation15 Similarly, Fas can also occur as both a membrane-bound Fas and a soluble isoform (sFas).Citation16 However, sFas is derived from alternative splicing of Fas mRNA.Citation16 Fas and FasL expressions are upregulated in peripheral blood mononuclear cells of critically ill adult patients with multiple organ dysfunction syndromes.Citation17 An increase in serum sFas levels in adult trauma patients who developed sepsis has also been reported.Citation18 Plasma sFas concentration was found to be elevated during severe sepsis with multiple organ failures, whereas plasma sFasL was only increased in multiple organ failures associated with liver injury in children.Citation19 Whether sFasL/sFas alterations in critically ill children correlate with renal injury is unclear.
Acute kidney injury (AKI), a disorder characterized by an abrupt deterioration of renal function is a significant problem in neonatal intensive care unit.Citation20 Sepsis, one of the leading causes of AKI in hospitalized neonates, remains life-threatening disease, with severe sepsis contributing up to 26% of neonatal mortality.Citation21 Although FasL is locally produced in normal kidneys,Citation7 its significance in septic AKI in neonates is poorly understood. However, an increase in renal FasL production and/or circulating FasL infiltration into the kidneys in response to inflammation may occur following initial septic insult. In the present study, we used a neonatal pig model to test the hypothesis that FasL shedding is elevated during the early phase of septic AKI in neonates. We also examined whether changes in sFasL levels are associated with renal apoptosis in septic neonates.
Materials and methods
Animals
The use of animals in this study was in compliance with the NIH Guide for the Care and Use of Laboratory Animals. All experimental procedures were approved by and carried out in accordance with the guidelines of the Institutional Animal Care and Use Committee of the University of Tennessee Health Science Center. Neonatal pigs (male; 3–5 days old; Nichols Hog Farm, Olive Branch, MS) were used in this study. The neonatal pigs were anesthetized and maintained as we have previously described.Citation22 Briefly, the animals were anesthetized with ketamine hydrochloride (20 mg/kg) and xylazine (2.2 mg/kg) intramuscularly and maintained on α-chloralose (50 mg/kg, intravenously) at 37 °C. The pigs were intubated via tracheostomies and mechanically ventilated. The right femoral artery was catheterized for continuous measurement of mean arterial pressure (MAP). A femoral vein was also catheterized for anesthetic and fluid administration and blood sampling. Arterial blood gas and pH were measured periodically and ventilation adjusted to maintain PCO2, PO2, and pH at physiological ∼30 mmHg, >85 mmHg, and 7.4, respectively.
Polymicrobial sepsis induction
To induce sepsis in an anesthetized and mechanically ventilated neonatal pig, ∼4 cm midline laparotomy was performed to expose the cecum and terminal ileum. The cecum was ligated below the ileocecal valve with a non-absorbable silk suture and punctured with a 22-gauge needle in 6–7 places. Fecal matter was then extruded from the punctured cecum into the peritoneum. The cecum was returned to the abdominal cavity, and the incision site was stitched in two layers. Sham-operated neonatal pigs were subjected to the same procedure, but without cecal ligation and puncture (CLP). Following the surgical procedure, the animals were given 1 mL of saline intraperitoneally once and maintained on isotonic fluid intravenously at a rate of 200 μL/min throughout the experiment. Animals were monitored for 6 h. The zero-time point for each experiment represents the time the CLP surgical procedure was completed.
Kidney injury and apoptosis assay
Kidney function in the neonatal pigs was evaluated by measuring serum concentration of creatinine and urea nitrogen. Serum creatinine concentration was determined by mass spectrometry (isotope dilution LC-MS/MS) at the UAB/UCSD O’Brien Core Center for Acute Kidney Injury Research (University of Alabama at Birmingham). Blood urea nitrogen (BUN) level was measured using a colorimetric detection kit (Arbor Assays, Ann Arbor, MI). Neutrophil gelatinase-associated lipocalin (NGAL) was measured in the urine using the pig NGAL ELISA kit (BioPorto Diagnostics, Hellerup Denmark). sFasL concentration was quantified in serum and urine using the porcine sFasL ELISA kit (MyBioSource Inc., San Diego, CA).
Apoptosis was investigated in kidney cryosections (10 μm thickness) using the FLICA 660 caspase-3/7 apoptosis assay kit (ImmunoChemistry Technologies, Bloomington, MN), following the manufacturer's instructions. Images were acquired using a Zeiss LSM 710 confocal microscope. The FLICA 660 caspase-3/7 detection probe was excited at 660 nm and emission collected at 680–690 nm. We also measured the expression of pro-apoptotic proteins caspase-3 and Bax in renal cortical lysates of the pigs using Western immunoblotting.
Western immunoblotting
Slices of neonatal pig kidney cortex were homogenized in ice-cold RIPA buffer. Protein lysates were mixed with sample buffer containing a reducing agent (Life Technologies, Grand Island, NY) and boiled at ∼70 °C for 10 min. SDS-polyacrylamide gel electrophoresis was used to analyze the protein samples. Immunoreactive proteins were visualized and documented using the Kodak Imaging system (Carestream Molecular Imaging, Rochester, NY).
Chemicals and antibodies
Doxorubicin was purchased from LC Laboratories (Woburn, MA). Rabbit polyclonal anticaspase-8 and anticaspase-3 antibodies were purchased from Sigma-Aldrich (St. Louis, MO). Mouse monoclonal anti-β-actin, rabbit polyclonal anti-FADD, and rabbit polyclonal anti-Bax antibodies were obtained from Abcam (Cambridge, MA), Bioss Inc. (Woburn, MA), and Abgent Inc. (San Diego, CA), respectively. HRP-conjugated anti-rabbit and anti-mouse secondary antibodies were purchased from Abcam.
Data analysis
All data are expressed as mean ± standard error of mean (SEM). Statistical significance was determined using Student’s t-tests for paired or unpaired data and analysis of variance with Student–Newman–Keuls for multiple comparisons tests. A p values <.05 was considered significant.
Results
Early polymicrobial sepsis induces acute kidney injury in neonatal pigs
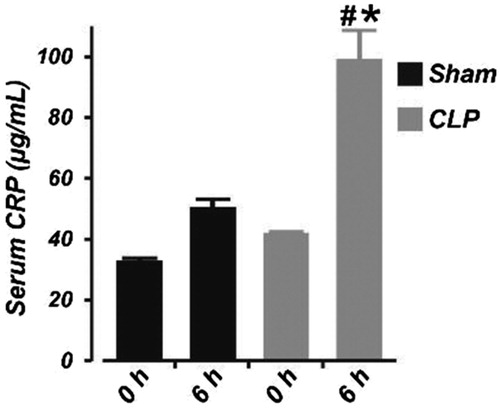
To confirm sepsis, we measured serum level of the bacteremia and sepsis marker CRP using the pig CRP ELISA kit (Immunology Consultants Laboratory, Portland OR). Basal level of CRP in sham-operated and septic neonatal pigs was similar (). There was a slight, but statistically insignificant increase in serum CRP concentration 6 h after sham operation (). By contrast, serum CRP concentration was increased by ∼59% following 6 h of CLP. These data confirmed the presence of sepsis in pigs subjected to CLP. None of the animals died during the study period. Whereas the MAP dropped in control pigs after 6 h of sham operation (possibly due to prolonged anesthesia), hypotension was more significant in the septic pigs (mean ± SEM change in MAP; sham (n = 6) vs. CLP (n = 7): −10.2 ± 3.7 vs. −20.1 ± 3.4; p < .05).
Figure 1. Six hours of cecal ligation and puncture elevates serum concentration of C-reactive protein in neonatal pigs. Mean data obtained from ELISA showing the serum concentration of C-reactive protein at 0 and 6 h in sham-operated (n = 6) and septic (n = 7) neonatal pigs. #p < .05 versus 0 h CLP; *p < .05 versus 0 and 6 h Sham.
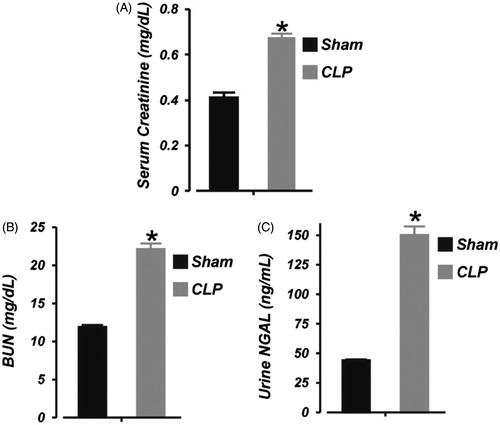
Next, we tested the hypothesis that 6 h of sepsis impairs kidney function in neonatal pigs by measuring serum creatinine and BUN levels in the animals. Serum creatinine and BUN concentrations were increased by ∼39% and 46%, respectively, following 6 h of CLP (). The concentration of urinary NGAL (an early marker of AKICitation23) in neonatal pigs subjected to 6 h of CLP was elevated by ∼71% (). These findings demonstrate renal insufficiency following 6 h of neonatal sepsis.
Figure 2. Early polymicrobial sepsis induces acute kidney injury in neonatal pigs. Mean data illustrating: (A) serum concentration of creatinine (LC/MS-MS assay), (B) serum concentration of urea nitrogen (BUN; colorimetric plate assay), and (C) urine concentration of neutrophil gelatinase-associated lipocalin (NGAL; ELISA) in sham-operated (6 h; n = 6) and septic (6 h; n = 7) neonatal pigs. *p < .05 versus Sham.
Early polymicrobial sepsis increases serum and urine sFasL in neonatal pigs
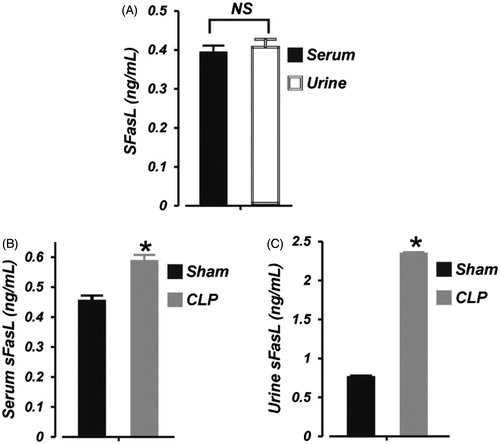
To examine whether sFasL level is altered during early phase of septic AKI in neonates, we measured the basal level of sFasL in neonatal pig serum and urine. As shown in ), the concentration of sFasL in neonatal pig serum and urine was ∼0.39 and 0.41 ng/mL, respectively (p > .05). Six hours of CLP significantly increased serum sFasL level by ∼24% (). Moreover, the concentration of sFasL in the urine was elevated by ∼68% in the septic neonatal pigs (). These data suggest that (1), serum and urine concentration of sFasL in neonatal pigs is similar, (2), serum and urine levels of sFasL is increased during early stages of septic AKI in neonatal pigs, and (3), the kidney is the major source of elevated urine sFasL during septic neonatal AKI.
Figure 3. Early polymicrobial sepsis increases serum and urine sFasL in neonatal pigs. Mean data showing: (A) basal sFasL levels (ELISA) in the serum and urine of neonatal pigs (n = 6), (B) serum sFasL concentration in sham-operated (6 h; n = 6) and septic (6 h; n = 7) neonatal pigs, and (C) urine sFasL concentration in sham-operated (6 h; n = 6) and septic (6 h; n = 7) neonatal pigs. *p < .05 versus Sham. NS: not significant.
Early polymicrobial sepsis does not induce renal apoptosis in neonatal pigs
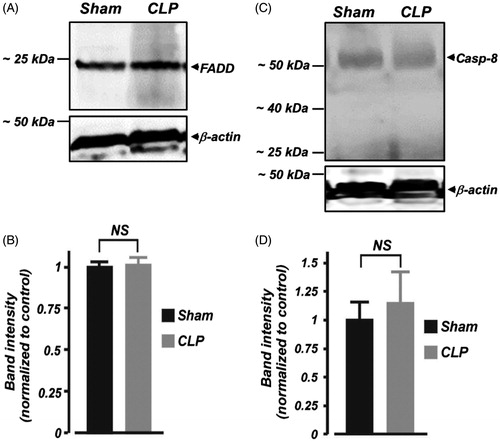
FasL-mediated cell death requires FADD and caspase-8 induction.Citation4,Citation5 Here, we examined whether FADD and caspase-8 protein expressions in the kidney are altered during the early phase of septic AKI in neonates. As shown in and , FADD protein expression was unchanged in kidney cortical samples isolated from septic neonatal pigs. Western blot analysis using a polyclonal anti-caspase-8 antibody detected full length (∼55 kDa) procaspase-8 in the kidney cortical samples (). The expression level of the ∼55 kDa protein was not significantly different in kidney cortical samples from sham-operated neonatal pigs when compared with their septic counterparts (). No further protein fragments representing active caspase-8 were observed in the samples, suggesting a lack of proteolytic processing of inactive caspase-8 in the samples ().
Figure 4. Early polymicrobial sepsis does not alter protein expression of FADD and caspase-8 in neonatal pig kidney cortex. (A and C) Western immunoblot images, (B and D) Mean data demonstrating protein expression levels of FADD and caspase (Casp; ∼55 kDa)-8 in kidney cortex isolated from sham-operated and septic neonatal pigs (6 h; n = 4 each). Smaller protein fragments representing active caspase-8 were not observed in the samples. NS: not significant.
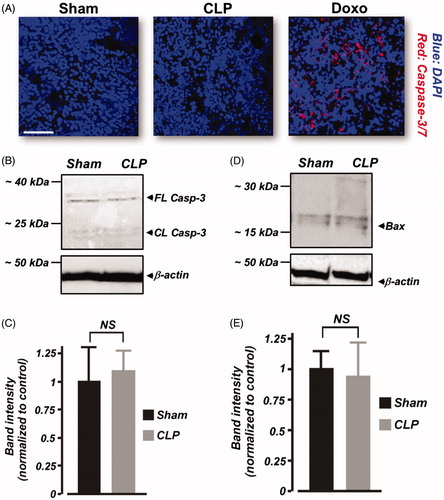
Furthermore, we tested the hypothesis that renal apoptosis is absent following 6 h of CLP in neonatal pigs. The far-red fluorescent dye-labeled FLICA 660 caspase-3/7 probe is nonfluorescent in nonapoptotic cells. However, caspase-3-positive, apoptotic cells produce red fluorescence when stained with the probe. Our data show that kidney slices from sham-operated and septic neonatal pigs were negative for caspase-3 activation (). As a positive technical control to confirm the efficacy of FLICA 660 caspase-3/7 probe, we treated neonatal pig kidney slices with doxorubicin, an inducer of renal apoptosis. As shown in , caspase-3-positive, apoptotic cells were detected in doxorubicin-treated kidney slices. Also, Western immunoblotting showed that full-length (∼35 kDa) caspase-3 protein expression was similar in sham-operated and septic neonatal pig renal cortical lysates (). Cleaved caspase-3 (<20 kDa) immunoreactive bands were essentially absent in samples from both sham-operated and septic neonatal pigs (). Similarly, pro-apoptotic protein Bax immunoreactive bands were weak, and unchanged in the septic kidney samples (). Together, these findings suggest that increased serum and urine sFasL production during early septic AKI in neonatal pigs does not promote renal apoptosis.
Figure 5. Early polymicrobial sepsis does not induce renal apoptosis in neonatal pigs. (A) Three-dimensional reconstruction of z-stack images obtained from kidney sections (n = 4 animals each) of sham-operated and septic neonatal pigs that were probed with the FLICA 660 caspase-3/7 apoptosis assay kit. As a positive control to confirm the efficacy of FLICA 660 caspase-3/7 probe, caspase-3 positive (red fluorescence), apoptotic cells were detected in doxorubicin (Doxo)-treated kidney slices. (B and D) Western immunoblot images. (C and E) Mean data showing protein expression levels of caspase (Casp)-3 and Bax in kidney cortex isolated from sham-operated and septic neonatal pigs (6 h; n = 4 each). Cleaved caspase-3 (<20 kDa) immunoreactive bands were essentially absent in samples from both sham-operated and septic neonatal pigs. Scale bar is 100 μm. NS: not significant.
Discussion
Data here indicate that both circulating and excreted FasL concentrations are significantly increased in neonatal pigs after 6 h of sepsis, and associate with an elevation in the levels of inflammatory and AKI markers. Despite the striking elevation in sFasL production, apoptosis was absent in the kidneys of the septic pigs, suggesting that an early production of sFasL does not result in renal apoptotic cell death during polymicrobial sepsis in neonates.
Although widespread apoptosis of immune cells during sepsis is well established,Citation24–26 whether renal cell death occurs during septic AKI remains controversial. Lipopolysaccharide (LPS) administration triggered renal apoptosis in mice via tumor necrosis factor receptor 1, caspase, and Fas activation.Citation12,Citation27–29 Whereas LPS induced renal tubular apoptosis in infant pigs,Citation30 adult pigs subjected to fecal peritonitis developed AKI without renal tubular necrosis.Citation31 In human, apoptosis was documented in ∼3% of tubular cells in the kidneys of adult patients who died of septic shock compared with ∼0.2% in their nonseptic counterparts.Citation32 Other studies have shown that renal tubular necrosis and apoptosis and glomerular apoptosis are essentially absent in postmortem kidney samples from adult septic patients.Citation26,Citation33 Unlike LPS endotoxemia, CLP model closely mimics the clinical features seen in human sepsis.Citation34–36 Using the CLP model, we show that although renal insufficiency occurred following 6 h of sepsis, there was no evidence of caspase activation to suggest an induction of the cellular apoptotic process. Whether long-term sepsis in neonates induces renal apoptosis requires further investigations.
The role of sFasL in Fas-mediated cytotoxicity is contentious, with some uncertainty on the effectiveness on sFasL in triggering apoptosis. Human, but not mouse sFasL promoted apoptosis of Fas-bearing W4 cells.Citation37 sFasL in bronchoalveolar lavage fluid of patients with acute lung injury induced apoptosis of distal lung epithelial cells.Citation38 Similarly, sFasL shed from colon adenocarcinoma cells elicited apoptosis of Fas-expressing Jurkat cells.Citation39 However, other studies have shown that the cytotoxic activity of sFasL may be lacking or less potent.Citation40,Citation41 sFasL may also act as a negative regulator that limits Fas-mediated cell death by competing with mFasL for Fas binding.Citation41–43 FADD, the apoptotic adaptor protein is essential for the induction of Fas-mediated apoptosis by recruiting procaspase-8 to the DISC.Citation4,Citation5 Inflammatory cytokines stimulated apoptosis and increased FADD expression in human proximal tubular cell lines.Citation13 FADD mRNA and protein expressions were also increased in the aorta and lung tissues of mice subjected to CLP.Citation44,Citation45 Furthermore, FADD siRNA administration reduced apoptosis in aortic endothelial cells, spleens, and lungs and improved survival of septic mice.Citation44,Citation45 These reports suggested the involvement of FADD in organ injury during septic shock. In this study, FADD protein expressions were unaltered in renal cortical samples from septic neonatal pigs when compared with the control. Caspase-8, a cytosolic protein is synthesized as an inactive procaspase. When activated, the sequential proteolytic cleavage of procaspase-8 (∼55 kDa) results in intermediate (p43/p41 and p12) and small active fragments (p10 and p18) that trigger the apoptotic cell death pathway.Citation46 Data here suggest that caspase-8 is not activated in the kidneys of neonatal pigs subjected to 6 h of sepsis. Similarly, the executioner caspase-3 and pro-apoptotic protein Bax are not induced in the kidneys of the septic pigs. Taken together, our data indicate that an increase in circulating and urinary sFasL is not associated with renal apoptosis in septic neonatal pigs. Perhaps, an early increase in sFasL production during sepsis is a mechanism to inhibit mFasL/Fas interaction as a defense against renal apoptosis.
The basal concentration of sFasL in the serum and urine of neonatal pigs was similar. However, following 6 h of CLP, sFasL level was ∼44% more in urine compared with serum of the pigs. FasL is locally produced in the kidneys.Citation6,Citation7 FasL expression is also increased in kidney-infiltrating leukocytes during injury.Citation47 Hence, we speculate that FasL that is locally shed in the kidneys contribute significantly to urinary sFasL. Given that newborn kidneys are morphologically and functionally immature, sick neonates are more susceptible to AKI during adverse perinatal conditions.Citation20,Citation48 Hence, identification of circulating and/or excreted immunologic mediators that are altered during early septic AKI may be of importance in timely detection and treatment of AKI in sick neonates. Here, we show that the degree of urinary sFasL elevation in neonatal pigs following 6 h of sepsis is similar to that of NGAL, an early maker of AKI.Citation23 Given that pig and human renal systems are closely related,Citation49 sFasL level may serve as a biomarker for early diagnosis of septic AKI in human neonates. Hence, further studies are needed to explore sFasL importance in septic AKI in human neonates. In conclusion, we present new findings suggesting that circulating and excreted sFasL are increased early in septic AKI in neonates; an effect that is not associated with renal apoptosis.
Acknowledgements
The authors would like to acknowledge the UAB-UCSD O’Brien Core Center for Acute Kidney Injury Research (NIH P30-DK079337) for the assistance with creatinine analysis.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- Aziz M, Jacob A, Yang WL, Matsuda A, Wang P. Current trends in inflammatory and immunomodulatory mediators In sepsis. J Leukoc Biol. 2013;93:329–342.
- Newton K, Dixit VM. Signaling in innate immunity and inflammation. Cold Spring Harb Perspect Biol. 2012;4:1–19.
- Green DR, Llambi F. Cell death signaling. Cold Spring Harb Perspect Biol. 2015;7:1–24.
- Pinkoski MJ, Green DR. Fas ligand, death gene. Cell Death Differ. 1999;6:1174–1181.
- Nagata S. Fas ligand-induced apoptosis. Annu Rev Genet. 1999;33:29–55.
- Ortiz A, Lorz C, Egido J. The Fas ligand/Fas system in renal injury. Nephrol Dial Transplant. 1999;14:1831–1834.
- Lorz C, Ortiz A, Justo P, et al. Proapoptotic Fas ligand is expressed by normal kidney tubular epithelium and injured glomeruli. J Am Soc Nephrol. 2000;11:1266–1277.
- Akasaka Y, Ishikawa Y, Kato S, et al. Induction of Fas-mediated apoptosis in a human renal epithelial cell line by interferon-gamma: involvement of Fas-mediated apoptosis in acute renal rejection. Mod Pathol. 1998;11:1107–1114.
- Schelling JR, Nkemere N, Kopp JB, Cleveland RP. Fas-dependent fratricidal apoptosis is a mechanism of tubular epithelial cell deletion in chronic renal failure. Lab Invest. 1998;78:813–824.
- Nogae S, Miyazaki M, Kobayashi N, et al. Induction of apoptosis in ischemia-reperfusion model of mouse kidney: Possible involvement of Fas. J Am Soc Nephrol. 1998;9:620–631.
- Furuichi K, Kokubo S, Hara A, et al . Fas ligand has a greater impact than TNF-α on apoptosis and inflammation in ischemic acute kidney injury. Nephron Extra. 2012;2:27–38.
- Ortiz-Arduan A, Danoff TM, Kalluri R, et al. Regulation of Fas and Fas ligand expression in cultured murine renal cells and in the kidney during endotoxemia. Am J Physiol. 1996;271:F1193–F1201.
- Jo SK, Cha DR, Cho WY, et al. Inflammatory cytokines and lipopolysaccharide induce Fas-mediated apoptosis in renal tubular cells. Nephron. 2002;91:406–415.
- Kayagaki N, Kawasaki A, Ebata T, et al. Metalloproteinase-mediated release of human Fas ligand. J Exp Med. 1995;182:1777–1783.
- Tanaka M, Suda T, Haze K, et al. Fas ligand in human serum. Nat Med. 1996;2:317–322.
- Cheng J, Zhou T, Liu C, et al. Protection from Fas-mediated apoptosis by a soluble form of the Fas molecule. Science. 1994;263:1759–1762.
- Papathanassoglou ED, Moynihan JA, McDermott MP, Ackerman MH. Expression of Fas (CD95) and Fas ligand on peripheral blood mononuclear cells in critical illness and association with multiorgan dysfunction severity and survival. Crit Care Med. 2001;29:709–718.
- Paunel-Gorgulu A, Flohe S, Scholz M, Windolf J, Logters T. Increased serum soluble Fas after major trauma is associated with delayed neutrophil apoptosis and development of sepsis. Crit Care. 2011;15:R20.
- Doughty L, Clark RS, Kaplan SS, Sasser H, Carcillo J. sFas and sFas ligand and pediatric sepsis-induced multiple organ failure syndrome. Pediatr Res. 2002;52:922–927.
- Andreoli SP. Acute kidney injury in children. Pediatr Nephrol. 2009;24:253–263.
- Weston EJ, Pondo T, Lewis MM, et al. The burden of invasive early-onset neonatal sepsis in the United States, 2005-2008. Pediatr Infect Dis J. 2011;30:937–941.
- Soni H, Adebiyi A. Pressor and renal regional hemodynamic effects of urotensin II in neonatal pigs. J Endocrinol. 2013;217:317–326.
- Devarajan P. Biomarkers for the early detection of acute kidney injury. Curr Opin Pediatr. 2011;23:194–200.
- Hotchkiss RS, Nicholson DW. Apoptosis and caspases regulate death and inflammation in sepsis. Nat Rev Immunol. 2006;6:813–822.
- Le TY, Pangault C, Gacouin A, et al. Early circulating lymphocyte apoptosis in human septic shock is associated with poor outcome. Shock. 2002;18:487–494.
- Hotchkiss RS, Swanson PE, Freeman BD, et al. Apoptotic cell death in patients with sepsis, shock, and multiple organ dysfunction. Crit Care Med. 1999;27:1230–1251.
- Cunningham PN, Dyanov HM, Park P, Wang J, Newell KA, Quigg RJ. Acute renal failure in endotoxemia is caused by TNF acting directly on TNF receptor-1 in kidney. J Immunol. 2002;168:5817–5823.
- Guo R, Wang Y, Minto AW, Quigg RJ, Cunningham PN. Acute renal failure in endotoxemia is dependent on caspase activation. J Am Soc Nephrol. 2004;15:3093–3102.
- Lee SY, Lee YS, Choi HM, et al. Distinct pathophysiologic mechanisms of septic acute kidney injury: role of immune suppression and renal tubular cell apoptosis in murine model of septic acute kidney injury. Crit Care Med. 2012;40:2997–3006.
- Nakajima Y, Mikami O, Yoshioka M, et al. Involvement of apoptosis in the endotoxemic lesions of the liver and kidneys of piglets. J Vet Med Sci. 2000;62:621–626.
- Chvojka J, Sykora R, Krouzecky A, et al. Renal haemodynamic, microcirculatory, metabolic and histopathological responses to peritonitis-induced septic shock in pigs. Crit Care. 2008;12:R164.
- Lerolle N, Nochy D, Guerot E, et al. Histopathology of septic shock induced acute kidney injury: Apoptosis and leukocytic infiltration. Intensive Care Med. 2010;36:471–478.
- Takasu O, Gaut JP, Watanabe E, et al. Mechanisms of cardiac and renal dysfunction in patients dying of sepsis. Am J Respir Crit Care Med. 2013;187:509–517.
- Remick DG, Ward PA. Evaluation of endotoxin models for the study of sepsis. Shock. 2005;24 Suppl 1:7–11.
- Buras JA, Holzmann B, Sitkovsky M. Animal models of sepsis: setting the stage. Nat Rev Drug Discov. 2005;4:854–865.
- Doi K, Leelahavanichkul A, Yuen PS, Star RA. Animal models of sepsis and sepsis-induced kidney injury. J Clin Invest. 2009;119:2868–2878.
- Tanaka M, Suda T, Takahashi T, Nagata S. Expression of the functional soluble form of human fas ligand in activated lymphocytes. EMBO J. 1995;14:1129–1135.
- Matute-Bello G, Liles WC, Steinberg KP, et al. Soluble Fas ligand induces epithelial cell apoptosis in humans with acute lung injury (ARDS). J Immunol. 1999;163:2217–2225.
- Song E, Chen J, Ouyang N, Su F, Wang M, Heemann U. Soluble Fas ligand released by colon adenocarcinoma cells induces host lymphocyte apoptosis: An active mode of immune evasion in colon cancer. Br J Cancer. 2001;85:1047–1054.
- O' Reilly LA, Tai L, Lee L, et al. Membrane-bound Fas ligand only is essential for Fas-induced apoptosis. Nature. 2009;461:659–663.
- Schneider P, Holler N, Bodmer JL, et al. Conversion of membrane-bound Fas(CD95) ligand to its soluble form is associated with downregulation of its proapoptotic activity and loss of liver toxicity. J Exp Med. 1998;187:1205–1213.
- Tanaka M, Itai T, Adachi M, Nagata S. Downregulation of Fas ligand by shedding. Nat Med. 1998;4:31–36.
- Suda T, Hashimoto H, Tanaka M, Ochi T, Nagata S. Membrane Fas ligand kills human peripheral blood T lymphocytes, and soluble Fas ligand blocks the killing. J Exp Med. 1997;186:2045–2050.
- Matsuda N, Yamamoto S, Takano K, et al. Silencing of fas-associated death domain protects mice from septic lung inflammation and apoptosis. Am J Respir Crit Care Med. 2009;179:806–815.
- Matsuda N, Teramae H, Yamamoto S, Takano K, Takano Y, Hattori Y. Increased death receptor pathway of apoptotic signaling in septic mouse aorta: effect of systemic delivery of FADD siRNA. Am J Physiol Heart Circ Physiol. 2010;298:H92–H101.
- Lavrik I, Krueger A, Schmitz I, et al. The active caspase-8 heterotetramer is formed at the CD95 DISC. Cell Death Differ. 2003;10:144–145.
- Miyazawa S, Watanabe H, Miyaji C, Hotta O, Abo T. Leukocyte accumulation and changes in extra-renal organs during renal ischemia reperfusion in mice. J Lab Clin Med. 2002;139:269–278.
- Andreoli SP. Acute renal failure in the newborn. Semin Perinatol. 2004;28:112–123.
- Giraud S, Favreau F, Chatauret N, Thuillier R, Maiga S, Hauet T. Contribution of large pig for renal ischemia-reperfusion and transplantation studies: the preclinical model. J Biomed Biotechnol. 2011;2011:532127.
