Abstract
Introduction
Nephrotoxicity is the most important adverse effect of colistin therapy. We investigated the frequency of nephrotoxicity, risk factors related to nephrotoxicity, and its relationship with mortality in patients who received intravenous colistin in intensive care units (ICUs).
Materials and methods
We retrospectively reviewed the data of patients who received intravenous colistin in ICUs between 2011 and 2017. Acute kidney injury (AKI) diagnosis and staging were made based on the Kidney Disease Improving Global Outcome criteria.
Results
There were 149 patients included in the study with 61% being male. The mean age was 58.7 ± 20.3 years. AKI was detected in 96 (64.4%) patients. There were 25 patients with AKI stage 1 (16.8%) and 71 patients with AKI stage 2 or 3 (47.7%). Advanced age (65.0 vs. 47.4 years; p < .001), diabetes mellitus (p < .001), heart failure (p = .01), high APACHE II score (31.7 vs. 28.08, p = .019), and inotrope usage (p = .01) were found as risk factors for AKI. The 14-day mortality rate was higher in the AKI group (p = .027).
Discussion
Higher AKI and mortality rates are observed in patients with diabetes, heart failure, advanced age and the hemodynamically impaired. However, it is a fact that there are no alternative therapies other than colistin in the treatment of multidrug-resistant Gram-negative bacterial infections. Therefore, the development of AKI in this patient group should not be considered a sufficient reason for discontinuing colistin treatment. Understanding the risk factors in this potential nephrotoxic treatment can provide a more careful patient follow-up.
Introduction
In recent years, there is an increase in multidrug-resistant (MDR) Gram-negative bacterial infections, particularly in those admitted to intensive care units (ICUs). The increasing prevalence of infections due to MDR Gram-negative bacteria constitutes a serious threat to public health. Current treatment options are limited and the development of new antimicrobial agents is very slow. Infections due to MDR strains are associated with increased morbidity and mortality and prolonged hospitalization [Citation1]. It has become increasingly challenging to treat infections caused by Gram-negative microorganisms such as Pseudomonas aeruginosa, Acinetobacter baumanii and Klebsiella pneumonia which may gain resistance against cephalosporins, quinolones and carbapenems. Polmyxins which are used in the treatment of MDR Gram-negative bacterial infections are classified into 5 groups (A–E). Polymyxin A, C and D are not used due to severe toxicity issues in clinical practice [Citation2]. Polymyxin B is used in United Kingdom, Brazil, Malaysia, Singapore and India. Colistin, also known as polymyxin E, is preferred worldwide [Citation3] and was introduced into clinical practice in 1959. However, cephalosporins, amikacin and other effective antibiotics replaced colistin over time since nephrotoxic and neurotoxic adverse effects were observed in the 1970s [Citation2,Citation4,Citation5]. The increase in Gram-negative bacterial infections has resulted in de novo colistin use, an antibacterial agent which has been forgotten. Nephrotoxicity is the most important adverse effect related to colistin treatment, which has been reported as 20–60% in different studies [Citation6–9]. Colistin-induced acute kidney injury (AKI) appears to be due to an increase in tubular epithelial cell membrane permeability resulting in cell swelling and cell lysis [Citation10]. Studies on patients with MDR Gram-negative bacterial infections and given intravenous colistin are important. Generally, there is no alternative treatment to colistin. Understanding the risk factors well in this potential nephrotoxic treatment can provide a more careful patient follow-up. In our study, we investigated the frequency of nephrotoxicity, risk factors related to nephrotoxicity and its relationship with mortality in patients received intravenous colistin in intensive care units.
Materials and methods
This retrospective study was performed among patients admitted to tertiary ICUs and received intravenous colistin for MDR Gram-negative infection between April 2011 and December 2017.
The study population included patients older than 18 years who received colistin over 48 h, had a daily 300 mg dose of colistin and had an estimated glomerular filtrate rate (eGFR) value ≥60 mL/min/1.73 m2 at the initiation of colistin therapy. The exclusion criteria were as follows: <18 years of age, colistin therapy shorter than 48 h, the daily colistin dose was not 300 mg, e-GFR value <60 mL/min/1.73 m2 and the presence of acute kidney injury (AKI) at the time of initiation of colistin therapy. The e-GFR value was calculated with the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula. AKI was defined according to Kidney Disease Improving Global Outcome (KDIGO) guidelines () [Citation11]. No urine criterion was used for AKI definition as we failed to obtain data about decreased urine output. The primary endpoint was nephrotoxicity; the secondary endpoint was mortality.
Table 1. Definition and staging of acute kidney injury.
Data related to cause of hospitalization, demographic characteristics, comorbid diseases, other nephrotoxic agents (non-steroid anti-inflammatory drugs, contrast material, vancomycin, piperacillin-tazobactam, aminoglycoside, amphotericine B) if given, colistin dose and duration, APACHE II scores were recorded in all patients. The KDIGO Clinical Practice Guideline was used for AKI staging () [Citation11]. Urine criterion was not used for staging. Only the first colistin episode was included in the analysis of patients who received more than one episode. The creatinine values on days −2, 0 (first day of colistin therapy), 3, 6, 9, 12 and 15 were included in the analysis. Patients with an increase in serum creatinine (≥0.3 mg/dl according to the initial value), but whose serum creatinine value declined to basal values while undergoing treatment, were considered more easily curable causes, such as pre-renal AKI, and not colistin-associated AKI; therefore, AKI was not accepted. AKI was diagnosed based on comparing the values between the last and first days of treatment. All diagnoses of chronic diseases throughout 10 years before index hospitalization were screened on the hospital computing system. Data regarding antibiotics with nephrotoxic potential, contrast material use, and non-steroidal anti-inflammatory drugs (NSAIDs) were obtained from patient files.
Data analysis was done using the SPSS 16.0 for Windows software (SPSS Inc, Chicago, IL, USA). Descriptive statistics were used for demographic data. Frequency and percentages were used for categorical variables. Kolmogorov–Smirnov test was performed to determine the normal distribution of parameters such as age, APACHE II score and basal creatinine. We applied independent samples t-test as parametric tests to those showing normal distribution and Mann–Whitney U test as nonparametric tests to those without normal distribution. Chi-square and Fisher’s exact tests were used to compare the data of no AKI and AKI groups expressed by ratios. A p value <.05 was considered statistically significant.
Results
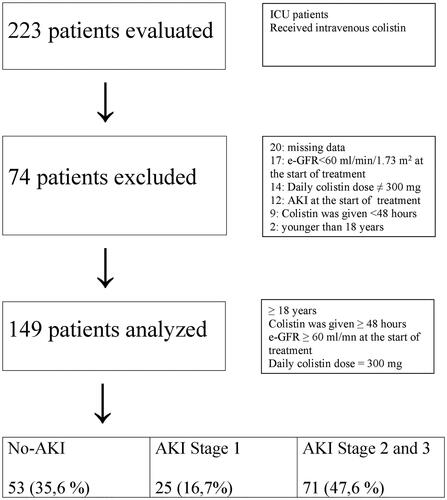
We reviewed 223 patients who received intravenous colistin. There were 20 patients with missing data, 17 patients with eGFR <60 mL/min at the start of treatment, 14 patients not taking 300 mg of colistin daily, 12 patients with AKI at the beginning of the treatment, 9 patients with treatment duration less than 48 h, and 2 patients under 18 years of age; these patients were excluded from the study. The final analysis included 149 patients (). Of these patients, 61% were male and the mean age was 58.7 ± 20.3 years (18–98 years). The most common infections were pneumonia (49.3%) and sepsis (20.3%). A. baumanii, K. pneumonia and P. aeruginosa were the most isolated agents for infection (68.5%, 17.4% and 10.1%, respectively). Colistin treatment was initiated according to culture test results in almost all patients and only one patient received empirical therapy. Colistin was given at a daily dose of 300 mg/day divided into two doses per day. Loading dose was used in 116 patients (77.8%). Loading dose was 300 mg for all patients. The loading dose did not increase AKI risk (p = .116). Mean length of stay was 80.2 ± 60.8 days (13–365 days) and mean duration of colistin use was 12.5 ± 5.4 days (3–36 days).
AKI was detected in 96 (64.4%) patients based on the KDIGO criteria. Stage 1 AKI and severe AKI (stages 2–3) were observed in 25 patients (16.8%) and in 71 patients (47.7%), respectively. AKI occurred within the first 3 days in 32.3%, within the first 6 days in 58.3%, and within the first 9 days in 77.1% of patients. It was found that factors such as sex, diagnosis at admission, isolated microorganisms, mechanical ventilation, central catheter, urinary catheter, parenteral/enteral nutrition, trauma history and surgical history had no significant effect on AKI development (). The AKI risk was increased by advanced age, high APACHE II score, inotrope usage, heart failure and diabetes mellitus (DM). AKI was observed in 32 of 35 patients with DM (p < .001). Mean age was 65.0 and 47.4 years in AKI and non-AKI groups, respectively (p < .001). Overall, 101 patients died (67.8%). We considered only deaths that occurred within the first 14 days (59.4%; n = 60) after the start of colistin treatment because deaths occurred over a long period of time (3–168 days); many may not be associated with colistin in such a prolonged period. We found that the 14-day mortality rate was significantly higher in the AKI group compared to the No-AKI group (p = .027). Additionally, mortality was significantly higher in patients who received inotropic agents (p < .001). We compared severe AKI (stages 2–3) with AKI stage 1 and no-AKI groups. The parameters of DM, heart failure, inotropic use, APACHE II score, mean age, and 14-day mortality were not different between the AKI stage 1 and AKI stages 2–3 groups. However, all of these factors were significantly different between the No-AKI vs. AKI stages 2–3 comparison ().
Table 2. Patient demographics and risk factors for acute kidney injury.
Table 3. Comparison of severe AKI with no-AKI and stage 1 AKI.
We did not obtain any information about neurotoxic side effects of colistin from the patient files.
Discussion
Currently, colistin has regained popularity due to an increased rate of MDR Gram-negative microorganism infections. Colistin-related nephrotoxicity rates have been reported as 6–55% in different studies [Citation12]. The majority of these studies were performed using the RIFLE (Risk, Injury, Failure, Lost, End-stage kidney disease) or AKIN (Acute Kidney Injury Network) criteria for staging of AKI [Citation13–15]. AKI was classified by using the KDIGO criteria in current studies [Citation16–18]. We preferred to use KDIGO criteria which is more up to date.
In our study, we evaluated a relatively homogeneous patient group. At the start of treatment, the e-GFR values of all patients were over 60 mL/min/1.73 m2. The daily dose of colistin was 300 mg/day in all patients and 77.8% of the patients were given a loading dose. The loading dose did not increase the risk of AKI (p = .116). We found that AKI developed in 96 of 149 patients who received colistin therapy (64.4%). This was markedly higher than those reported in the literature. In a study by Rashizal et al., colistin-related nephrotoxicity was found to be 23% [Citation15]. In a study on 70 critically ill patients, Dalfino et al. [Citation19] reported AKI incidence as 44%. Miano et al. [Citation16] reported AKI incidence as 51%. There are several other studies that report renal toxicity ranges from 20–60% [Citation5–9,Citation20,Citation21]. AKIN, RIFLE and KDIGO criteria were all used in these studies investigating AKI. The preferred classification criterion partially affects the determined AKI ratio. KDIGO AKI classification is more sensitive in detecting AKI. In the RIFLE classification, patients with a 1.5-fold increase in serum creatinine value compared to the baseline is classified as the at-risk group. Whereas, KDIGO classification, 0.3 mg/dl or 1.5–2-fold increase in basal creatinine is defined as AKI stage 1. Since we utilized the KDIGO classification, our results might reflect a higher AKI rate than literature data. The higher AKI rate could also be related to poorer patient profile; the rate of inotropic agent use was 44.2% (n = 66) and mechanical ventilation rate was 77.1% (n = 115). It should be noted that AKI may also have occurred in patients with severe pneumonia, sepsis, and septic shock due to non-colistin reasons such as pre-renal AKI, acute tubular necrosis secondary to hypotension. In addition, 25 patients (16.7%) had stage 1 AKI. Stage 1 AKI may be associated with colistin, but it can easily occur due to non-colistin reasons in intensive-care conditions.
In a study on 30 patients, nephrotoxicity occurred within the first 5 days in all patients. In the same study, mean age was significantly higher in the AKI group (57.5 vs. 43.3 years; p = .03) [Citation22]. In a multicentre study on 119 patients, Ko et al. [Citation23] reported AKI in 65 patients (54%). AKI was observed in 46 patients within the first 7 days and 19 patients following 7 days. Authors reported higher mortality rates in early AKI groups. In a study by Miano et al. [Citation16], AKI rate within 72 h was 20%. In our study, AKI was detected within 72 h in 32.2% of patients, in 58.3% within 6 days, and 77.1% within 9 days. Our findings suggest that colistin-related AKI is less likely to occur after 9 days.
DM, heart failure, advanced age, high APACHE II score, inotrope usage, higher baseline serum creatinine were significant risk factors for AKI. Based on statistical significance, our results suggest that being at older (≥65 years old) and diabetic are the strongest risk factors for AKI. The nephrotoxic effect of colistin has been a well-known fact for many years. Our data show that the development of AKI is common after initiation of colistin treatment in intensive care patients. However, this is not an effect solely created by colistin alone and in all cases. Conditions such as being in shock, needing inotropic drugs, being ≥65 years of age, diabetic, and diagnosed with heart failure form the basis of colistin nephropathy occurrence. A lower rate of colistin nephrotoxicity may be observed in intensive care patients if they had better haemodynamics, no need for inotropic agents, ≤65 years of age, and without comorbid diseases.
In a multicentre study on 166 patients, Durante-Mangoni et al. [Citation24] classified AKI according to the AKIN criteria. Authors defined severe AKI as AKIN stage 2 and 3. No significant differences were seen in mortality between AKI at any stage or severe AKI and the non-AKI group (p = .32 and p = .54, respectively). The authors concluded that there was no need to discontinue colistin therapy as they observed no significant effect of AKI at any stage on mortality. In contrast to the Durante-Mangoni study, our study was a retrospective cohort design and included only ICU patients. We detected a significant increase in mortality between No-AKI and AKI groups. When we compared advanced stage AKI (stages 2 and 3) with the stage 1 AKI group, we found that the risk factors for AKI development did not differ between the two groups (). When the risk factors were similar, advanced-stage AKI (stages 2–3) patients and early-stage AKI (stage 1) patients had similar mortality. This suggested that the patients died not due to kidney failure, but because of clinical antithesis such as septic shock, which developed because of comorbid conditions. It can be claimed that colistin nephrotoxicity may worsen the clinical course of the patient, but it is difficult to argue that the progression of renal failure is an independent consequence of colistin nephrotoxicity on mortality, regardless of the underlying disease.
Our study represents a large cohort of patients treated with intravenous same dose colistin in ICU patients. Another important aspect of our study is that it included patients with a baseline e-GFR value ≥60 mL/min/1.73 m2. Single-centred, retrospective design and the absence of a control group were some limitations of this study. New studies without these limitations may be useful.
In conclusion, our data demonstrate that AKI is frequently observed in ICU patients treated with colistin. Higher AKI and mortality rates were observed in patients with diabetes, heart failure, advanced age and hemodynamically unstable patients. Unfortunately, there is no alternative therapy to colistin in the treatment of MDR Gram-negative bacterial infections. Therefore, the development of AKI in this patient group should not be considered a sufficient reason for discontinuing colistin treatment. Understanding the risk factors in this potential nephrotoxic treatment can provide a more careful patient follow-up.
Author contributions
All authors have made significant contributions. Gunay: Research idea, data collecting, statistics, data interpretation, data collecting, statistics, interpretation of research. Kaya: Research idea, data collecting, statistics, data interpretation, interpretation of research. Baysal: Statistics, data interpretation, interpretation of research. Yuksel: Data collecting, data interpretation. Arac: Data collecting, data interpretation.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Cerceo E, Deitelzweig SB, Sherman BM, et al. Multidrug-resistant Gram-negative bacterial infections in the hospital setting: overview, implications for clinical practice, and emerging treatment options. Microb Drug Resist. 2016;22(5):412–431.
- Loho T, Dharmayanti A. Colistin: an antibiotic and its role in multiresistant Gram-negative infections. Acta Med Indones. 2015;47(2):157–168.
- Nation RL, Velkov T, Li J. Colistin and polymyxin B: peas in a pod, or chalk and cheese? Clin Infect Dis. 2014;59(1):88–94.
- Li J, Nation RL, Milne RW, et al. Evaluation of colistin as an agent against multi-resistant Gram-negative bacteria. Int J Antimicrob Agents. 2005;25(1):11–25.
- Falagas ME, Kasiakou SK, Saravolatz LD. Colistin: the revival of polymyxins for the management of multidrug-resistant Gram-negative bacterial infections. Clin Infect Dis. 2005;40(9):1333–1341.
- Pogue JM, Lee J, Marchaim D, Yee V, et al. Incidence of and risk factors for colistin-associated nephrotoxicity in a large academic health system. Clin Infect Dis. 2011;53(9):879–884.
- Linden PK, Paterson DL. Parenteral and inhaled colistin for treatment of ventilator-associated pneumonia. Clin Infect Dis. 2006;43 (Suppl 2):89–94.
- Hartzell JD, Neff R, Ake J, et al. Nephrotoxicity associated with intravenous colistin (colistimethate sodium) treatment at a tertiary care medical center. Clin Infect Dis. 2009;48(12):1724–1728.
- Shields RK, Anand R, Clarke LG, et al. Defining the incidence and risk factors of colistin-induced acute kidney injury by KDIGO criteria. PLoS One. 2017;12(3):e0173286.
- Ordooei Javan A, Shokouhi S, Sahraei Z. A review on colistin nephrotoxicity. Eur J Clin Pharmacol. 2015;71(7):801–810.
- Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl. 2012;2:1–138.
- Falagas ME, Kasiakou SK. Toxicity of polymyxins: a systematic review of the evidence from old and recent studies. Crit Care. 2006;10(1):R27.
- Ordooei Javan A, Salamzadeh J, Shokouhi S, et al. Evaluation of renal toxicity of colistin therapy with neutrophil gelatinase-associated lipocalin: a biomarker of renal tubular damage. Iran J Kidney Dis. 2017; 11(6):447–455.
- Katip W, Uitrakul S, Oberdorfer P. Clinical outcomes and nephrotoxicity of colistin loading dose for treatment of extensively drug-resistant Acinetobacter baumannii in cancer patients. Infect Drug Resist. 2017;10:293–298.
- Rashizal Sazli MR, Syed Mohamed AF, Wan Mazuan WM, et al. Amin Nordin S. Colistin-associated nephrotoxicity among patients in intensive care units (ICU) of hospitals in Selangor. Med J Malaysia. 2017;72(2):100–105.
- Miano TA, Lautenbach E, Wilson FP, et al. Attributable risk and time course of colistin-associated acute kidney injury. Clin J Am Soc Nephrol. 2018;13(4):542–550.
- Kanic V, Kompara G, Suran D, et al. Impact of KDIGO-defined acute kidney injury on mortality after percutaneous coronary intervention for acute myocardial infarction. Cardiorenal Med. 2018;8(4):332–339.
- Lee TH, Lee CC, Ng CY, et al. The influence of acute kidney injury on the outcome of Stevens-Johnson syndrome and toxic epidermal necrolysis: the prognostic value of KDIGO staging. PLoS One. 2018; 13(9):e0203642.
- Dalfino L, Puntillo F, Ondok MJ, et al. Colistin-associated acute kidney injury in severely ill patients: a step toward a better renal care? a prospective cohort study. Clin Infect Dis. 2015;61(12):1771–1777.
- Kubin CJ, Ellman TM, Phadke V, et al. Incidence and predictors of acute kidney injury associated with intravenous polymyxin B therapy. J Infect. 2012;65(1):80–87.
- Justo JA, Bosso JA. Adverse reactions associated with systemic polymyxin therapy. Pharmacotherapy. 2015;35(1):28–33.
- Deryke CA, Crawford AJ, Uddin N, et al. Colistin dosing and nephrotoxicity in a large community teaching hospital. Antimicrob Agents Chemother. 2010;54(10):4503–4505.
- Ko H, Jeon M, Choo E, et al. Early acute kidney injury is a risk factor that predicts mortality in patients treated with colistin. Nephron Clin Pract. 2011;117(3):c284–288.
- Durante-Mangoni E, Andini R, Signoriello S, et al. Acute kidney injury during colistin therapy: a prospective study in patients with extensively-drug resistant Acinetobacter baumannii infections. Clin Microbiol Infect. 2016;22(12):984–989.