Abstract
Cytomegalovirus (CMV) is a major pathogen in immunocompromised population and CMV infections in immunocompromised patients cause substantial morbidity and mortality. The common clinical manifestations of CMV infection are pneumonia, hepatitis, colitis and so on, while CMV peritonitis without gut perforation is rare. Reviewing the literature, CMV peritonitis in patients with nephrotic syndrome (NS) had not been reported. Only four cases of CMV peritonitis without gut perforation were reported in adults with other diseases. Two cases were diagnosed by reverse-transcription polymerase chain reaction (RT-PCR) of ascites while the other two cases by histopathological examination of peritoneal tissue. We report four cases of primary nephrotic syndrome complicated with CMV peritonitis. Four cases all diagnosed by RT-PCR of ascites (659–455 000 copies/mL). We mainly discusses the diagnosis and treatment of CMV peritonitis without gut perforation.
Background
Cytomegalovirus (CMV) is a major pathogen in immunocompromised patients, including solid organ transplant recipients, HIV-infected patients, and patients treated with immunomodulating drugs. CMV infection is a substantial cause of morbidity and mortality in immunocompromised hosts [Citation1]. Spontaneous bacterial peritonitis is a serious complication of childhood NS. The most common organisms causing spontaneous bacterial peritonitis are S. pneumoniae and Gram negative organisms, particularly Escherichia coli [Citation2]. To our knowledge, CMV peritonitis without gut perforation had been reported very rarely in immunocompromised patients [Citation3–6] and had not been reported in patients with NS. Here we report four children in the early diagnosis and effective treatment of CMV peritonitis with primary NS.
Case presentation
Case 1
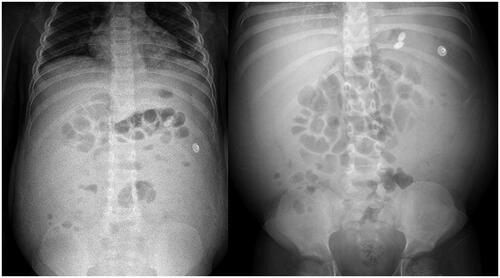
A 7-year-old boy was diagnosed with NS in December 2016, and started oral prednisone therapy, but NS was not modified by prednisone. Cyclophosphamide intravenous pulse therapy (cumulative dose was 188.2 mg/kg) was given from March to August 2017, and prednisone was reduced to 25 mg/day on 21 August 2017. However, urinary protein never turned negative. On 28 September 2017, percutaneous renal biopsy revealed minimal change disease. On 16 October 2017, the boy began to take MMF (mycophenolate mofetil) orally (0.25 g, q12h), and received three times of methylprednisolone pulse therapy (15 mg/kg each time). Urine protein was negative after one week. But MMF was stopped by his parents and proteinuria recurred soon. The boy went to the outpatient clinic because of recurred edema on 17 January 2018. Initial laboratory test of peripheral blood showed albumin (ALB) 10.0 g/L (normal range, 35.0–50.0 g/L), cholesterol (CHOL) 14.0 mmol/L (normal range, 3.1–5.2 mmol/L), urea 11.8 mmol/L (normal range, 2.9–8.6 mmol/L), creatinine (CREA) 43 μmol/L (normal range, 40–60 μmol/L), IgA 0.52 g/L (normal range, 1.45–3.45 g/L), IgM 1.87 g/L (normal range, 0.92–2.04 g/L), IgG 1.36 g/L (normal range, 10.13–15.13 g/L), C3 0.48 g/L (normal range, 0.79–1.17 g/L), C4 0.10 g/L (normal range, 0.17–0.31 g/L). 24-h urine protein was 6.329 g. The boy presented with fever (T 38.5 °C) on the 4th day of admission, accompanied by abdominal pain and diarrhea, but no nausea or vomiting. On physical examination, vitals were within normal range. An abdominal examination showed bulging flanks, mild generalized tenderness, shifting dullness and bowel sounds were normal. Laboratory test of peripheral blood showed the following: white blood cells (WBC) 13.40 × 109/L (normal range, 4.00–10.00× 109/L) (neutrophils (NEUT)% 55.4%; lymphocytes (LY)% 35.6%), C-reactive protein (CRP) 4.73 mg/L (normal range, 0–10.00 mg/L), procalcitonin (PCT) 0.63 ng/mL (normal range, 0–0.05 ng/mL), hemoglobin and platelets were normal. After 3 days of antibiotic therapy, the boy still had fever, abdominal pain. Even worse, the abdominal circumference of the boy gradually increased, the diuretic effect was poor and acute kidney injury occurred. Serum creatinine was 98 μmol/L (creatinine clearance (CCr) was 47.44 mL/min/1.73 m2). Imaging showed that no gas under the diaphragm (). The boy was primally considered as spontaneous peritonitis. Due to the ineffective antibiotic treatment, the increase of ascites and deterioration in renal function, abdominal puncture was performed. The routine biochemical screening tests of ascites are shown in . Cultures of the specimen produced no growth of fungi, mycobacteria, or bacteria and EBV DNA was negative in ascites. Surprisingly, CMV DNA was detected in ascites (854 copies/mL) and urine (1930 copies/mL), but negative in blood. Therefore, the boy received the treatment of intravenous GCV (5 mg/kg q12h for 2 weeks, 5 mg/kg qd for 1 week). After 1-week antiviral treatment, the clinical symptoms were in remission, the renal function returned to normal, the routine biochemical screening tests of ascites were normal and CMV DNA was negative in both ascites and urine. But the boy had persistent proteinuria. He was finally treated with tacrolimus (1 mg every 12 h) and urine protein was negative a month later. CMV DNA was negative in blood and urine during 16 months of follow-up.
Table 1. Results of peritoneal fluid analysis and culture in 4 cases of NS with CMV peritonitis.
Case 2
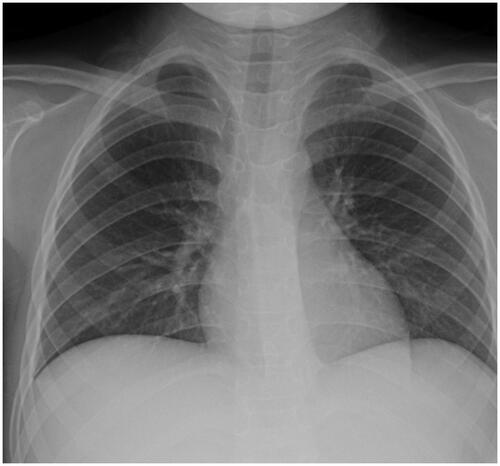
An 8-year-old girl was diagnosed with steroid-sensitive NS in October 2017. Prednisone was reduced to 25 mg/day on 20 January 2018. The girl was seen on 18 February 2018, on account of edema and oliguria. Laboratory data of peripheral blood included ALB 17.4 g/L, CHOL 15.0 mmol/L, CREA 151 μmol/L (CCr was 42.20 mL/min/1.73 m2). 24-h urine protein was 7.972 g. Therefore, intravenous methylprednisolone (40 mg qd) was instead of oral prednisone. On 23 February 2018, the girl presented with pharyngodynia, oral herpes, followed by fever, vomiting, abdominal pain, no diarrhea. Physical examination revealed normal vital signs, severe facial and lower limb edema. Pharyngeal portion had hyperemia and herpes was seen in the retropharyngeal wall. Abdominal examination showed bulging flanks, mild generalized tenderness, shifting dullness and liver was touched 2 cm under the right costal margin. Laboratories of peripheral blood included WBC 11.69 × 109/L (NEUT 79.6%; LY 14.5%), normal hemoglobin and platelets, CRP 18.26 mg/L, PCT 0.67 ng/mL, CREA 250 μmol/L (CCr was 25.5 mL/min/1.73 m2), ALT 102.0 U/L (normal range, <40.0 U/L), IgA 0.95 g/L, IgM 1.20 g/L, IgG 1.69 g/L, C3 0.64 g/L, C4 0.26 g/L. After antibiotic therapy, fever subsided 2 days later, but abdominal pain, abdominal tenderness and ascites were still present. Imaging showed that no gas under the diaphragm (). In order to the reassessment of diagnosis, abdominal puncture was carried out in this patient on 27 March 2018. The routine biochemical screening tests of ascites are shown in . Cultures of the specimen produced no growth of fungi, mycobacteria, or bacteria and EBV DNA was negative in ascites. However, CMV DNA was detected in ascites (2200 copies/mL), blood (1290 copies/mL) and urine (3230 copies/mL). After the treatment of intravenous GCV (the dose was adjusted for renal function: 2.5 mg/kg qd for 1 week; 2.5 mg/kg q12h for 1 week; 5.0 mg/kg qd for 1 week) and protecting liver, edema gradually subsided and abdominal symptoms improved. Also, liver enzymes and renal function returned to normal. The liver could not be touched and CMV DNA in ascites was negative after 2 weeks. The routine biochemical screening tests of ascites were normal, too. After 3 weeks, urine protein turned negative and CMV DNA in blood was negative either. CMV DNA was negative in blood and urine during 8 months of follow-up.
Case 3
A 3-year-old boy was diagnosed with steroid-resistant NS in November 2017. He was treated with cyclosporine for 3 days and tacrolimus for 2 days, both of which were discontinued due to the side effect of acute kidney injury. On 1 March 2018, a percutaneous renal biopsy revealed minimal change disease (). On 13 March 2018, prednisone was reduced to 10 mg/day. On 4 April 2018, due to a large amount of ascites and shortness of breath, the patient underwent abdominal puncture and drainage. The boy was transferred to our institution on 10 April 2018. Laboratory data of peripheral blood included ALB 14.3 g/L, CHOL 13.0 mmol/L, CREA 29 μmol/L. 24-h urine protein was 5.162 g. The child had fever (T 38.3 °C) and abdominal pain on the 5th day of admission, without nausea, vomiting, diarrhea. Physical examination revealed bulging flanks, tenderness and rebound pain under xiphoid process, shifting dullness. Laboratories of peripheral blood included WBC 12.48 × 109/L (NEUT 79.4%; LY 17.6%), normal hemoglobin and slightly higher platelets, CRP 0.43 mg/L, PCT 0.26 ng/mL, CREA 41 μmol/L, ALT 256.0 U/L. The routine biochemical screening tests of ascites are shown in . Cultures of the specimen produced no growth of fungi, mycobacteria, or bacteria and EBV DNA was negative in ascites. Because of fever and abdominal pain, the boy started experiential antibiotic therapy, which was withdrawn after 48 h as soon as bacterial infection was ruled out and CMV infection was identified. CMV DNA was detected in ascites (659 copies/mL) and urine (2100 copies/mL), but negative in blood. In addition, liver was touched 4 cm under the right costal margin. After the treatment of intravenous GCV (5 mg/kg, q12h for 2 weeks, 5 mg/kg qd for 1 week), clinical symptoms were in remission and CMV DNA was negative in ascites. The routine biochemical screening tests of ascites were normal. The liver couldn’t be touched under the right costal margin and liver enzymes were normal. CMV DNA was negative in blood and urine after 5 months of follow-up.
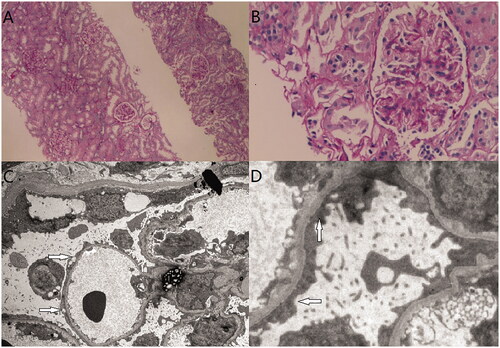
Figure 3. Renal biopsy of case 3. (A) Epithelial cells of renal tubule showed granular and vacuolar degeneration (H&E staining, ×4). (B) Mild proliferation of mesangial cells and stromal cells (H&E staining, ×400). (C & D) electron microscopy image showing minimal change disease, extensive fuzing of podocyte processes of the epithelial cells (arrow).
Case 4
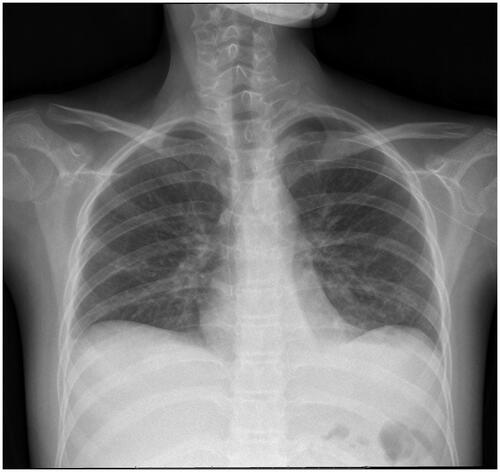
A 13-year-old girl was transferred to our institution on 26 April 2018, with a history of NS lasting for 1-month. She was initially treated with intravenous methylprednisolone 60 mg qd (1.5 mg/kg). Initial laboratories of peripheral blood included ALB 14.0 g/L, CHOL 9.8 mmol/L, CREA 95 μmol/L (CCr was 64.28 mL/min/1.73 m2). 24-h urine protein was 13.470 g. CMV DNA was detected in blood (7550 copies/mL) but negative in urine. Because of a large amount of ascites and poor diuretic effect, abdominal puncture was performed on 28 April 2018. The routine biochemical screening tests of ascites are shown in . Cultures of the specimen produced no growth of fungi, mycobacteria, or bacteria and EBV DNA was negative in ascites. CMV DNA was detected in ascites (769 copies/mL). Therefore, the girl was treated with intravenous GCV (the dose was adjusted for renal function: 2.5 mg/kg, q12h). On 4 May 2018, the child had abdominal pain, cough, and expectoration without fever, vomiting or diarrhea. Physical examination revealed normal vital signs, severe facial and lower limbs edema. There were rales at auscultation of both lungs. Abdominal examination showed bulging flanks, generalized tenderness and rebound tenderness, shifting dullness. Laboratories of peripheral blood included WBC 10.70 × 109/L (NEUT 63.2%; LY 30.4%), normal hemoglobin and platelets, CRP 0.80 mg/L, PCT 0.42 ng/mL, ALT 5.0 U/L, mycoplasma pneumoniae IgM 1:160. The child was treated with oral azithromycin and other intravenous antibiotic treatment. Also, she received intravenous fluids, albumin and diuretics. After 3 days, cough and expectoration mitigated significantly and the rales of lungs disappeared. Urinary output increased gradually. However, there was still abdominal pain and the abdominal circumference increased gradually. Imaging showed that no gas under the diaphragm (). Abdominal paracentesis was performed again on 8 May 2018. The copy number of CMV DNA in ascites increased (455 000 copies/mL) and the renal function improved (CREA 69 μmol/L, CCr 116.78 mL/min/1.73 m2). Considering the improvement of renal function, the dosage of GCV was adjusted (5 mg/kg q12h for 2 weeks; 5 mg/kg qd for 1 week). One week later, the patient’s clinical symptoms were completely relieved, CMV DNA was negative in ascites and the routine biochemical screening tests of ascites were normal. After 3 weeks, she was transferred to the local hospital for further treatment and lost to follow up.
Discussion and conclusions
Nephrotic syndrome refers to excessive proteinuria, with associated hypoalbuminemia, edema, and hyperlipidemia. The infectious complications, especially primary peritonitis, are well-known alarming situation in children with NS. Studies have shown incidence of peritonitis in childhood NS ranging from 2.6% to 26.0% [Citation7–10]. Patients with NS have a defective immune response due to increased urinary losses of complement factor B, low IgG levels, inadequate opsonization and impaired T-cell function [Citation11–13]. Ascites formation secondary to hypoalbuminemia also contributes to the development of peritonitis. Furthermore, treatment with immunosuppressive agents further predisposes to an increased risk of infection [Citation2]. The most common pathogens causing spontaneous peritonitis is bacteria and viruses are rarely reported.
Cytomegalovirus is a ubiquitous virus that commonly infects people of all ages throughout the world. The seroprevalence of CMV in the general population ranges from 30% to 97% and increases with age [Citation14]. Infections are usually asymptomatic but can be associated with a wide spectrum of diseases, particularly in immunocompromised persons. In children, the spectrum of disease caused by CMV infection ranges from asymptomatic or mild disease in immunologically normal hosts, to severe and potentially life-threatening disease in immunocompromised children [Citation15]. Children with NS are at high risk of CMV infection due to primary disease itself as well as large-dose and long-term therapy of glucocorticoids or other immunosuppressive agents. The common clinical manifestations of CMV infection are pneumonia, hepatitis, colitis and so on, while CMV peritonitis is rare [Citation3–6]. Literature reports often show secondary infections caused by perforation of digestive tract infection [Citation16–18], and CMV peritonitis without gut perforation is rare owing to the difficulty of diagnosis. This article mainly discusses the diagnosis and treatment of CMV peritonitis without gut perforation.
In our center, although WBC in ascites of four children were less than 250/mm3, they all showed the following traits: (1) local symptoms and signs of peritonitis: fever, abdominal pain, abdominal tenderness or rebound pain, rapid increase of ascites or poor effect of diuretics; (2) CMV DNA was detected in ascites by RT-PCR; (3) The antiviral treatment with ganciclovir was effective while antibiotic didn’t work. After antiviral treatment, CMV DNA in ascites became negative. (4) Excluding other pathogens: cultures of the ascites produced no growth of fungi, mycobacteria, or bacteria. So they were diagnosed with CMV peritonitis. At present, there is no diagnostic standard for viral peritonitis, and there are few reports about viral peritonitis in the literature [Citation3–6,Citation19–30]. Diagnostic methods for CMV infection and CMV-associated disease include isolation of the virus by culture, histology of biopsies, serologic methods, measurement of pp65 antigen in leucocytes, and detection of viral nucleic acids using molecular methods, particularly the polymerase chain reaction [Citation31]. CMV DNA was identified previously in peritoneal fluid of potential kidney transplant recipients treated with peritoneal dialysis [Citation32]. Reviewing the literature, CMV peritonitis in patients with NS had not been reported. Four cases of CMV peritonitis without gut perforation were reported in adults [Citation3–6]. There were two males and two females, the age ranged from 24 to 37 years. There were two cases after kidney transplantation, one case after liver transplantation and one case with acquired immunodeficiency. Three cases received immunosuppression treatment and four cases all had abdominal pain. Two cases were diagnosed by RT-PCR of ascites while the other two cases by histopathological examination of peritoneal tissue. Details are shown in . Therefore, ascites fluid or peritoneal tissue is essential to diagnose CMV peritonitis.
Table 2. Summary of relevant data of four patients in the literature.
AKI is a common complication in children with NS. It is multifactorial in origin and can be secondary to intravascular volume depletion, acute tubular necrosis, interstitial nephritis, nephrotoxic medications, infections, or renal vein thrombosis [Citation33–34]. In case 4, despite the increase of viral load in ascites, urinary output increased and renal function was improved after the treatment of intravenous fluids, albumin and diuretics. This suggests that the poor renal function of the child may be caused by hypovolemia. In case 2, the child’s renal function was poor when NS recurred, and the renal function deteriorated after CMV infection. However, after antiviral treatment, the child's renal function was improved to normal and NS was relieved. Patel AM [Citation35] pointed out that aggressive treatment of the CMV infection can result in resolution of NS, reversal of foot process effacement, and preservation of kidney allograft function. We have observed that CMV infection can promote and aggravate NS, and its effective treatment is beneficial to the remission of NS. In case 1, acute kidney injury occurred during the recurrence of NS and CMV infection. It was difficult to judge whether it was caused by NS or CMV infection. However, after antiviral treatment, the renal function of the child improved, but there was still serious proteinuria. Farah MA [Citation36] reported that a 72- year- old male transferred to intensive care unit with methicillin-resistant Staphylococcus aureus bacteremia , respiratory failure, and dialysis-dependent acute kidney injury. While he recovered from bacteremia, he remained difficult to wean from respiratory support, had labile blood pressure, and manifested persistent diarrhea. The colon biopsy specimen described tissue-invasive CMV infection. Polymerase chain reaction testing confirmed viremia with 8900 copies/mL viral DNA. After Intravenous GCV initiation, diarrhea and respiratory failure resolved, while renal function recovered to the patient’s baseline. CMV is most likely to invade ductal epithelial cells and replicate in distal tubular epithelial cells, so we deduce that CMV infection may be one of the inducting or aggravating factors of acute kidney injury in children with NS. The relationship between CMV infection and acute kidney injury deserves further study.
The 2013 international consensus guidelines on the management of cytomegalovirus in solid organ transplantation [Citation37] recommend that the initial treatment of CMV disease in children younger than 12 years should be with intravenous GCV at a dose of 5 mg/kg every 12 h with appropriate adjustments for renal function. For children older than 12 years, they can be given oral valganciclovir (VGCV) (900 mg every 12 h) or intravenous GCV (5 mg/kg every 12 h). The recommended length of treatment is determined by the monitoring of weekly CMV viral loads and continuing treatment until one or two consecutive negative samples are obtained with a minimum treatment course of 2 weeks [Citation38–40]. However, in 2018 guidelines [Citation41], significant updates from prior recommendations relate to body surface area-based dosing for younger children and infants and consideration for initial oral VGCV therapy for CMV infection and mild to moderate CMV disease. It is still recommended the initial treatment of severe CMV disease in children with intravenous GCV. In situations where secondary prophylaxis is used, antiviral prophylaxis may be administered either with intravenous GCV or oral VGCV. Intravenous GCV is usually dosed at 5 mg/kg per day [Citation42]. The duration of prophylaxis typically ranges from 2 to 4 weeks. However, the optimum duration of prophylaxis for a sequential approach has not been defined [Citation43–45]. The duration of secondary prophylaxis depends on immunosuppression regimen, age, presence of other opportunistic infections, and other risk factors [Citation41]. In our center, after antibiotic treatment, four children still had abdominal pain and ascites. Symptoms disappeared after intravenous ganciclovir treatment and CMV DNA was negative in ascites of four children. It is worth mentioning that in case 4, the dose of ganciclovir was reduced due to poor renal function. Later, the renal function of the patient recovered and the copy number of CMV DNA in ascites increased, which reminded us that the dose of ganciclovir was insufficient. So we adjusted the dose of ganciclovir and the patient’s condition was improving day by day. In the literature, four patients also received antiviral therapy. Symptoms in three patients disappeared while one patient died due to severe lung infiltration (details are shown in ). All of cases demonstrate that CMV treatment guidelines can be successfully applied to CMV peritonitis and have a good prognosis.
Although currently available antivirals are effective for CMV treatment, safer alternatives are needed. Letermovir is a CMV terminase inhibitor. It was approved for oral or intravenous use by the Food and Drug Administration for CMV prophylaxis in allogeneic stem cell transplant recipients in 2017 [Citation46]. However, the clinical experience in letermovir for treatment is limited [Citation47]. Maribavir and brincidofovir are currently in clinical trials for CMV treatment. Maribavir, a CMV selective inhibitor of UL97 threonine kinase, interferes with viral synthesis, packaging, and egress of virions from the nucleus [Citation48]. Two recently completed studies of maribavir (dosing from 400 to 1200 mg BID) had shown promising results for treatment of CMV in HCT and SOT [Citation49]. Brincidofovir is an orally bioavailable lipid conjugate of cidofovir that has not been associated with kidney or bone marrow toxicity [Citation50]. In trials evaluating hematopoietic stem cell recipients, brincidofovir use has not been shown to reduce clinically significant disease but has been associated with increased adverse events [Citation51]. Other investigational anti-CMV drugs in early stages of development include cyclopropavir and several monoclonal antibodies targeting various CMV proteins [Citation52–53]. However, these new antiviral drugs are currently only studied in adults, and research in children still needs a long way to go.
At present, there were no literature of the relevant studies on CMV prophylaxis in children with NS. However, we can learn from the experience of CMV disease prevention in transplant recipients. CMV antiviral therapy can be initiated when there is evidence of CMV replication in NS children who receive long-term corticosteroids and multiple immunosuppression treatment, but prior to the development of CMV disease. This needs further clinical observation and analysis.
The diagnosis of CMV peritonitis is challenging, because of its rarity and lack of specific clinical signs. However, when NS patients have abdominal pain, refractory ascites and are considered as spontaneous peritonitis, in addition to considering bacterial peritonitis, the possibility of CMV peritonitis should be considered when cultures remain negative and there is no response to antibiotic therapy. CMV DNA detection of ascites or peritoneal histopathology is helpful to diagnose CMV peritonitis. Once CMV peritonitis is diagnosed, antiviral therapy with ganciclovir should be started as early as possible which can reduce the abuse of antibiotics.
Ethics approval
This study was approved by the ethics committee of the First Affiliated Hospital, Sun Yat-sen University. Parents provided written informed consent.
Informed consent
The parent of the children presented in our case report have given written consent to publish our work.
Authors contributions
HL and LC interpreted the results for the case report, drafted, wrote and revised the report, and provided important intellectual review. SW collected the raw data from our hospital work system. ZY, YM and XJ supervised the therapy and critically reviewed the manuscript. LH conceived the idea of the study and did the final proofreading of the manuscript. All authors read and approved the final manuscript.
| Abbreviations | ||
| CMV | = | cytomegalovirus |
| NS | = | nephrotic syndrome |
| RT-PCR | = | reverse-transcription polymerase chain reaction |
| MMF | = | mycophenolate mofetil |
| GCV | = | ganciclovir |
| ALB | = | albumin |
| CHOL | = | cholesterol |
| CREA | = | creatinine |
| WBC | = | white blood cells |
| NEUT | = | neutrophils |
| LY | = | lymphocytes |
| CRP | = | C-reactive protein |
| PCT | = | procalcitonin |
| CCr | = | creatinine clearance |
| VGCV | = | valganciclovir |
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
All meaningful data generated or analyzed in this study are included in the manuscript.
References
- Rose J, Emery VC, Kumar D, et al. Novel decay dynamics revealed for virus-mediated drug activation in cytomegalovirus infection. PLoS Pathog. 2017;13(4):e1006299.
- Teo S, Walker A, Steer A. Spontaneous bacterial peritonitis as a presenting feature of nephrotic syndrome. J Paediatr Child Health. 2013;49(12):1069–1071.
- Hotta K, Fukasawa Y, Wada Y, et al. Cytomegalovirus peritonitis after kidney transplantation diagnosed through histopathological examination. Transpl Infect Dis. 2017;19(4):e12708.
- Lankarani KB, Taghavi SA, Pahlavan SM, et al. Cytomegalovirus peritonitis without gut perforation but with concomitant colitis after a liver allograft transplant. Exp Clin Transplant. 2017;15(1):106–109.
- Wilcox CM, Forsmark CE, Darragh TM, et al. Cytomegalovirus peritonitis in a patient with the acquired immunodeficiency syndrome. Dig Dis Sci. 1992;37(8):1288–1291.
- Meier M, Hiss M, Hafer C, et al. Cytomegalovirus peritonitis after renal transplantation under induction therapy with alemtuzumab in a young woman previously treated with peritoneal dialysis. Nephrol Dial Transplant. 2005;20(8):1771.
- Senguttuvan P, Ravanan K, Prabhu N, et al. Infections encountered in childhood nephrotics in a pediatric renal unit. Indian J Nephrol. 2004;14:85–88.
- Ajayan P, Krishnamurthy S, Biswal N, et al. Clinical spectrum and predictive risk factors of major infections in hospitalized children with nephrotic syndrome. Indian Pediatr. 2013;50(8):779–781.
- Alwadhi RK, Mathew JL, Rath B. Clinical profile of children with nephrotic syndrome not on glucorticoid therapy, but presenting with infection. J Paediatr Child Health. 2004;40(1–2):28–32.
- Kumar M, Ghunawat J, Saikia D, et al. Incidence and risk factors for major infections in hospitalized children with nephrotic syndrome. Braz J Nephrol. 2019;41(4):526–533.
- Matsell DG, Wyatt RJ. The role of I and B in peritonitis associated with the nephrotic syndrome of childhood. Pediatr Res. 1993;34(1):84–88.
- Fodor P, Saitua MT, Rodriguez E, et al. T-cell dysfunction in minimal-change nephrotic syndrome of childhood. Am J Dis Child. 1982;136(8):713–717.
- Heslan JM, Lautie JP, Intrator L, et al. Impaired IgG synthesis in patients with the nephrotic syndrome. Clin Nephrol. 1982;18(3):144–147.
- Razonable RR, Humar A. Cytomegalovirus in solid organ transplant recipients-guidelines of the American Society of Transplantation Infectious Diseases Community of Practice. Clin Transplant. 2019;33(9):e13512.
- Cherry JD. Cytomegalovirus. In: Cherry JD, Harrison GJ, Kaplan SL, editors. Feigin and Cherry’s textbook of pediatric infectious diseases. 7th ed. Philadelphia (PA): Elsevier Saunders; 2014. p. 1969.
- Albu E, Mukherjee A, Rao D, et al. Emergency surgery for generalized peritonitis caused by cytomegalovirus colitis in a patient with AIDS. Am Surg. 1999;65(5):397–398.
- Tsai HC, Lee SS, Wann SR, et al. Colon perforation with peritonitis in an acquired immunodeficiency syndrome patient due to cytomegalovirus and amoebic colitis. J Formos Med Assoc. 2005;104(11):839–842.
- Nishimura T, Nakao A, Okamoto A, et al. Ileum perforation due to cytomegalovirus infection in a patient with adult T-cell leukemia. Acute Med Surg. 2016;3(2):178–181.
- Struijk DG, van Ketel RJ, Krediet RT, et al. Viral peritonitis in a continuous ambulatory peritoneal dialysis patient. Nephron. 1986;44(4):384.
- Lal SM, Fowler D, Losasso CJ, et al. Coxsackie virus-induced acute pancreatitis in a long-term dialysis patient. Am J Kidney Dis. 1988;11(5):434–436.
- Abraham G, Vas M, Vas S, et al. Cloudy effluent with atypical lymphocytes in a CAPD patient: case report. Perit Dial Int. 1989;9(4):349–350.
- Lewis SL. Recurrent peritonitis: evidence for possible viral etiology. Am J Kidney Dis. 1991;17(3):343–345.
- Braun A, Querfeld V, Mehls O. “Sterile peritonitis” caused by varicella in a child on maintenance peritoneal dialysis treatment. Pediatr Nephrol. 1991;5(1):95–96.
- Yakulis R, Babinchak TJ. Herpes simplex peritonitis: case report. Clin Infect Dis. 1999;28(6):1212–1215.
- Liesker J, van Elsacker-Niele AM, Blanken R, et al. A cloudy bag and genital ulcers. Clin Nephrol. 2006;65(5):378–379.
- Pauwels S, De Moor B, Stas K, et al. Coxsackievirus B1 peritonitis in a patient treated with continuous ambulatory peritoneal dialysis: a case report and brief review of the literature. Clin Microbiol Infect. 2012;18(10):E431–E434.
- Yoshida K, Miyahira Y, Ishida M, et al. Ascitic fluid due to type II herpes simplex virus infection: report of a case with immunocytochemical confirmation. Diagn Cytopathol. 2013;41(4):354–359.
- Handsfield HH, Ashley RL. Herpes simplex virus peritonitis. Clin Infect Dis. 2000;30(5):839–840.
- Huang N, Liu N, Lu J. Peritonitis secondary to hemorrhagic fever with renal syndrome: a case report in GuangZhou China. BMC Infect Dis. 2020;20(1):36
- Mclean DM, Larke RP, Mcnaughton GA, et al. Enteroviral syndromes in Toronto, 1964. Can Med Assoc J. 1965;92(13):658–661.
- de Jong MD, Galasso GJ, Gazzard B, et al. Summary of the II International Symposium on Cytomegalovirus. Antiviral Res. 1998;39(3):141–162.
- Shulman LM, Rudich C, Sayar Y, et al. Detection of CMV-DNA in cells from peritoneal fluid of IPD/CAPD patients by polymerase chain reaction. Adv Perit Dial. 1992;8:258–264.
- Rheault MN, Zhang L, Selewski DT, et al. AKI in children hospitalized with nephrotic syndrome. CJASN. 2015;10(12):2110–2118.
- Meyrier A, Niaudet P. Acute kidney injury complicating nephrotic syndrome of minimal change disease. Kidney Int. 2018;94(5):861–869.
- Patel AM, Zenenberg RD, Goldberg RJ. De novo CMV-associated collapsing focal segmental glomerulosclerosis in a kidney transplant recipient. Transpl Infect Dis. 2018;20(3):e12884.
- Farah MA, Fulop T, Kokko K, et al. Cytomegalovirus colitis in a critically ill, dialysis-dependent, acute kidney injury patient without immunosuppressive therapy. CN. 2015;84(07):44–49.
- Kotton CN, Kumar D, Caliendo AM, et al. Updated international consensus guidelines on the management of cytomegalovirus in solid-organ transplantation. Transplantation. 2013;96(4):333–360.
- Asberg A, Humar A, Jardine AG, et al. Long-term outcomes of CMV disease treatment with valganciclovir versus IV ganciclovir in solid organ transplant recipients. Am J Transplant. 2009;9(5):1205–1213.
- Chou SW. Cytomegalovirus drug resistance and clinical implications. Transpl Infect Dis. 2001;3(s2):20–24.
- Sia IG, Wilson JA, Groettum CM, et al. Cytomegalovirus (CMV) DNA load predicts relapsing CMV infection after solid organ transplantation. J Infect Dis. 2000;181(2):717–720.
- Kotton CN, Kumar D, Caliendo AM, et al. The Third International consensus guidelines on the management of cytomegalovirus in solid-organ transplantation. Transplantation. 2018;102(6):900–931.
- Kotton CN, Kumar D, Caliendo AM, et al. International consensus guidelines on the management of cytomegalovirus in solid organ transplantation. Transplantation. 2010;89(7):779–795.
- Gerna G, Lilleri D, Callegaro A, et al. Prophylaxis followed by preemptive therapy versus preemptive therapy for prevention of human cytomegalovirus disease in pediatric patients undergoing liver transplantation. Transplantation. 2008;86(1):163–166.
- Lin A, Worley S, Brubaker J, et al. Assessment of cytomegalovirus hybrid preventative strategy in pediatric heart transplant patients. J Pediatric Infect Dis Soc. 2012;1(4):278–283.
- Green M, Kaufmann M, Wilson J, et al. Comparison of intravenous ganciclovir followed by oral acyclovir with intravenous ganciclovir alone for prevention of cytomegalovirus and Epstein-Barr virus disease after liver transplantation in children. Clin Infect Dis. 1997;25(6):1344–1349.
- Prevymis [package insert]. Whitehouse Station (NJ): Merck, Inc; 2017.
- Stern A, Papanicolaou GA. CMV prevention and treatment in transplantation: what’s new in 2019. Curr Infect Dis Rep. 2019;21(11):45.
- Biron KK, Harvey RJ, Chamberlain SC, et al. Potent and selective inhibition of human cytomegalovirus replication by 1263W94, a benzimidazole L-riboside with a unique mode of action. Antimicrob Agents Chemother. 2002;46(8):2365–2372.
- Maertens J, Cordonnier C, Jaksch P, et al. Maribavir versus valganciclovir for preemptive treatment of cytomegalovirus viremia: a randomized, dose-ranging, phase 2 study among hematopoietic stem cell transplant and solid organ transplant recipients. Biol Blood Marrow Transplant. 2017;23(3):s191–s192.
- Marty FM, Winston DJ, Rowley SD, et al. CMX001 to prevent cytomegalovirus disease in hematopoietic-cell transplantation. N Engl J Med. 2013;369(13):1227–1236.
- Marty FM, Winston DJ, Chemaly RF, Mullane KM, et al. A randomized, double-blind, placebo-controlled phase 3 trial of oral brincidofovir for cytomegalovirus prophylaxis in allogeneic hematopoietic-cell transplantation. Biol Blood Marrow Transplant. 2019;25(2):369–381.
- Gentry BG, Kamil JP, Coen DM, et al. Stereoselective phosphorylation of cyclopropavir by pUL97 and competitive inhibition by maribavir. Antimicrob Agents Chemother. 2010;54(8):3093–3098.
- Salazar G, Zhang N, Fu TM, et al. Antibody therapies for the prevention and treatment of viral infections. NPJ Vaccines. 2017;2(1):19.