Abstract
Introduction
Coexistence of chronic kidney disease (CKD) in the case of acute coronary syndromes (ACS) significantly worsens the outcomes.
Aim
The aim of our study was to assess renal function impact on mortality among patients with ACS.
Materials and methods
The study was based on records of 21,985 patients hospitalized in the Medical University of Bialystok in 2009–2015. Inclusion criteria were ACS. Exclusion criteria were: death within 24 h of admission, eGFR <15 ml/min/1.73 m2, hemodialysis. Mean observation time was 2296 days.
Results
Criteria were met by 2213 patients. CKD occurred in 24.1% (N = 533) and more often affected those with NSTEMI (26.2 (337) vs. 21.2 (196), p = .006). STEMI patients had higher incidence of post-contrast acute kidney injury (PC-AKI) (5 (46) vs. 4.1 (53), p < .001). During the study, 705 people died (31.9%), more often with NSTEMI (33.2% (428) vs. 29.95% (277), p < .001). However, from a group of patients suffering from PC-AKI 57.6% died. The risk of PC-AKI increased with creatinine concentration (RR: 2.990, 95%CI: 1.567–5.721, p < .001), occurrence of diabetes mellitus (RR: 2.143, 95%CI: 1.029–4.463, p = .042), atrial fibrillation (RR: 2.289, 95%CI: 1.056–4.959, p = .036). Risk of death was greater with an increase in postprocedural creatinine concentration (RR: 2.254, 95%CI: 1.481–3.424, p < .001).
Conclusion
PC-AKI is a major complication in patients with ACS, occurs more frequently in STEMI and may be a prognostic marker of long-term mortality in patients undergoing percutaneous coronary intervention (PCI). More attention should be given to the prevention and diagnosis of PC-AKI but necessary PCI should not be withheld in fear of PC-AKI.
1. Background
Cardiovascular diseases are the leading cause of mortality among the Polish population. These are responsible for 46% of deaths – it is almost twice as much as deaths caused by cancer (27%) [Citation1]. Kidney disease affects cardiac function and can change the course and prognosis of patients with acute coronary syndromes (ACS) [Citation2].
A Polish study on the assessment of the incidence of chronic kidney disease (CKD) among the Polish population claims that CKD affects about 5.8%, where in the case of seniors the percentage is 26.9%. Some studies suggest that CKD is the greatest risk factor for cardiovascular diseases occurrence [Citation3–5].
CKD is highly prevalent worldwide and is associated with an increased risk for adverse outcomes in patients hospitalized due to ACS, the co-occurrence of these two diseases increases the risk of death [Citation6].
The gold standard of treatment for patients with ACS is percutaneous coronary intervention (PCI). Many patients after PCI experience a decrease in GFR, which is a certain group manifests itself as post-contrast acute kidney injury (PC-AKI).
Data on the impact of post-procedural eGFR decline on mortality in patients with ACS are still limited – it is known that this condition is associated with more adverse outcomes [Citation7].
Therefore, in our study, we aimed to investigate which factors predispose to contrast-induced acute kidney injury and how it affects mortality among patients with ST-segment elevation myocardial infarction (STEMI) and non-ST-segment elevation myocardial infarction (NSTEMI).
2. Patients and methods
The study was conducted at the Clinical Hospital of Medical University of Bialystok. Based on the medical records of 2258 men and women hospitalized due to ACSs in 2009 − 2015.
The demographic, clinical, and biochemical data of the patients were evaluated. The diagnosis of STEMI and NSTEMI was made by physicians based on the symptoms, level of biochemical markers of myocardial necrosis, and electrocardiographic results.
All patients underwent coronary angiography. During all procedures, an iodine-containing nonionic radiocontrast agent was used. All patients had the same strategy for the prevention of radiocontrast agent – 1000 mL of intravenous hydration with 0.9% NaCl.
CKD-EPI eGFR and creatinine levels were assessed on admission and 48 h after angiography, based on age, gender, race, and creatinine concentration [Citation8]. For the purpose of this study, PC-AKI was defined as an absolute increase of serum creatinine ≥0.5 mg/dL or relative increase ≥25% from the baseline value within the first 48 h of intervention. Currently, newer, more strict criteria are available [Citation9], however, given that the study covers patients hospitalized between 2009 and 2015, thus the end of the study took place before the new guidelines appeared, we decided to use values current for this period.
Around 1.19% of the data were missing during the study period, and these data were excluded from the analysis.
The study protocol was approved by the ethics committees of the Medical University of Bialystok (R-1-002/18/2019).
Exclusion criteria
Exclusion criteria were: death within 24 h of admission, eGFR <15 mL/min, and hemodialysis. Total 1.9% (N = 45) of patients were excluded from the study, in majority male (60%, N = 27), 54% (N = 25) hospitalized with STEMI, mean eGFR 31.3 mL/min (SD = 26), median = 21.2 mL/min of which 22.2% (N = 10) were on hemodialysis.
In this group, the main causes of death were cardiovascular diseases 93.3% (N = 42) of which 51.1% of death was reported in 24 h of admission (N = 23).
Long-term observation
The data concerning the cause of deaths that were recorded on April 1, 2019, were obtained from the Statistical Office in Olsztyn, Poland. The records included the causes of deaths that were classified according to codes in the International Classification of Diseases – 10th Revision (ICD-10).
The mean follow-up period from the onset to the death was 1319 (SD = 1070), median – 1230 days. The complete follow-up duration consisted of 2296 days (SD = 1081), median – 2359.
Statistical analysis
The distribution of variables was evaluated using the Kolmogorov–Smirnov test. The two-tailed t-test and Mann–Whitney U test were used for comparative analysis. Spearman’s rank correlation test was applied to evaluate the relationships between the levels of postprocedural decrease of eGFR and biochemical parameters.
The effects of clinical and biochemical factors on death were evaluated by multivariable logistic regression backward stepwise Wald method.
Associations were considered significant at p < .05. All analyses were performed using Statistica 13 software (StatSoft, 2017, Poland).
3. Results
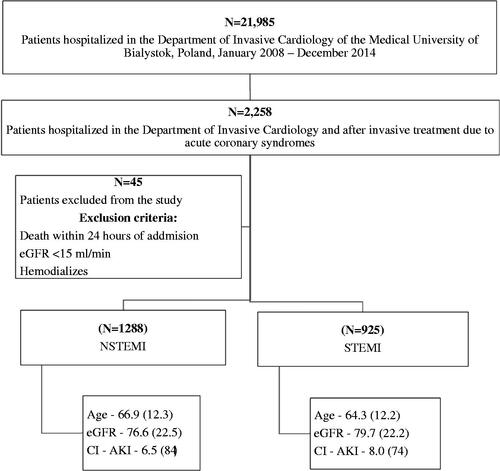
A total of 2213 patients were included into the final analysis – 1288 with NSTEMI and 925 with STEMI. The average age was 65.8 years (SD = 12.2) and men were in the majority (65.2%, N = 1442) (Figure 1).
Comparison of patients with NSTEMI and those with STEMI shows statistically significant differences. The NSTEMI group was older (66.9 (SD = 12.1) vs. 64.3 (SD = 12.2), p < .001), had lower serum LDL (129.7 (SD = 45.1) vs. 140.1 (SD = 44.8), p < .001) and total serum cholesterol concentration (198.4 (SD = 48.9) vs. 207.9 (48.9), p < .001). They were more often burdened with arterial hypertension (77.0% (N = 989) vs. 66.0% (N = 606), p < .001) and diabetes mellitus type 2 (26.4% (N = 340) vs. 18.7% (N = 173), p < .001). The average eGFR was greater in patients with STEMI (79.7 (SD = 20.8) vs. 76.61 (SD = 21.5), p < .001), they also had higher incidence of post-contrast acute kidney injury (5% (N = 46) vs. 4.1% (N = 53), p < .001) ().
Table 1. Characteristics of the studied population with comparison of patients with STEMI and NSTEMI.
The characteristic of the population is presented depending on the presence (N = 533) and absence (N = 1680) of CKD. Patients with CKD more often had comorbidities, such as hypertension (79.7%, N = 425), diabetes mellitus type 2 (40.7%, N = 217), atrial fibrillation (33.6%, N = 179), and they were more likely to die (56.3% (N = 300) vs. 24.1% (N = 405), p < .001). CKD patients have experienced PC-AKI far more often (8.4% (N = 45) vs. 3.2% (N = 54), p < .001) ().
Table 2. Comparison of patients with and without chronic kidney disease.
Table 3. Comparison of patients with and without contrast-induced acute kidney injury.
PC-AKI occurred in 4.5% of patients. This group was statistically older (71.1 (SD = 11.1) vs. 65.6 (SD = 12.2), p < .001), had higher serum creatinine concentration (1.2 (SD = 0.6) vs. 1.0 (SD = 0.3), p < .001), and lower average eGFR (65.6 (SD = 25.7) vs. 78.5 (SD = 20.8), p < .001) (Table 3). They also more often suffered from atrial fibrillation (35.4% (N = 35) vs. 17.2% (N = 363), p < .001), CKD (45.5% (N = 45) vs. 23.1% (N = 488), p < .001), diabetes mellitus (38.4% (N = 38) vs. 22.5% (N = 475), p = .002), and also died more frequently (57.6% (N = 57) vs. 30.7% (N = 648), p < .001).
During the study, 705 people died, more often with NSTEMI (33.2% (428) vs. 29.95% (277), p < .001) of whom 349 (49.5%) died from cardiovascular reasons and 112 from oncological causes (15.9%). In the NSTEMI group, the percentage of cancer deaths was 17.0% (N = 73), cardiovascular deaths were 49.3% (N = 216) vs. 14.01% (N = 39), and 48.1% (N = 133) in the STEMI group. Moreover, 57.6% of patients suffering from PC-AKI died – eight of them died from cancer, 40 due to cardiovascular causes, and two from complications of diabetes. Three deaths, due to acute renal failure, were reported in patients who have not post angiography increase in creatinine concentration. When comparing dead and living patients we can notice that significant differences were found in comorbidities such as diabetes mellitus type 2 (32.2% (N = 227) vs. 19.0% (N = 286), p < .001) and atrial fibrillation (28.9% (N = 204) vs. 12.9% (N = 194), p < .001). The percentage of CKD occurrence was 42.6% (N = 300) among the deceased population and it had a significant impact on mortality (p < .001) ().
Table 4. Comparison of dead and alive patients.
Postprocedural eGFR change rate correlates inversely with platelets count (R = −0.12) in STEMI patients. There is a positive correlation between the postprocedural eGFR change rate in STEMI group and AspAT (R = 0.14) and ALAT (R = 0.08). In the NSTEMI group, a positive correlation was noted between postprocedural eGFR change rate and LDL cholesterol concentration (R = 0.05) ().
Table 5. Spearman’s correlation rank between eGFR change rate and biochemical results.
The risk of CI-AKI increased with an increase in creatinine concentration (RR: 2.99, 95%CI: 1.567–5.721, p < .001) but also with the presence of diabetes mellitus (RR: 2.143, 95%CI: 1.029–4.463, p = .042) and atrial fibrillation (RR: 2.289, 95%CI: 1.056–4.959, p = .036) ().
Table 6. Multivariable logistic regression forward stepwise Wald method – risk ratio for risk of post-contrast acute kidney injury.
Multivariable regression analysis showed that the risk of death in both STEMI and NSTEMI groups was greater with an increase in postprocedural creatinine concentration (for an increase of 1 mg/dl risk ratio was: 2.254 95%CI: 1.481–3.424, p < .001), with age (RR: 1.060, 95%CI: 1.048–1.072, p < .001), the presence of atrial fibrillation (RR: 1.695, 95%CI: 1.251–2.297, p = .034), and increase in fibrinogen concentration (RR: 1.028, 95%CI: 1.018–1.068, p < .001) but also decreased with an increase in hemoglobin concentration (RR: 0.895, 95%CI: 0.826–0.970, p = .007) (–9).
Table 7. Multivariable logistic regression forward stepwise Wald method –risk ratio for risk of death – all population.
Table 8. Multivariable logistic regression forward stepwise Wald method – risk of death in NSTEMI population.
Table 9. Multivariable logistic regression forward stepwise Wald method – risk of death in STEMI population.
4. Discussion
In recent years, the epidemiology of ACSs has changed in Poland and Europe. Non-ST-segment elevation myocardial infarction starts to play a dominant role, which is in line with our study [Citation10,Citation11]. Short-term mortality is higher in the STEMI group, however, in the case of long-term follow-up, the death rate for NSTEMI becomes twice as high in comparison to STEMI [Citation10,Citation12]. These data are reflected in our analysis. Differences in prognosis over time result from different characteristics of patients, people predisposed to NSTEMI are usually older and have more coexisting diseases, especially diabetes and renal failure.
In this investigation, CKD occurred in one out of four people and more often affected those with NSTEMI. Patients with CKD had worse outcomes regardless of the type of ACS. These results can be found in previous studies, CKD of any degree is a potential and independent risk factor for adverse outcomes. [Citation13,Citation14]. Population with CKD had more often comorbidities, such as hypertension, diabetes mellitus, atrial fibrillation, and advanced age which is mostly traditional risk factors for cardiovascular diseases. CKD is also associated with a high burden of cardiovascular diseases, especially coronary artery disease which has an impact on poorer long- and short-term outcomes after ACS [Citation15–17]. These findings are reflected in current guidelines. Individuals with CKD are considered as a group of high risk for cardiovascular diseases and other adverse outcomes which supports calls for more intensive intervention in patients with CKD to prevent them. Therapeutic strategies that have been proved to prevent cardiovascular events in patients with CKD include aggressive blood pressure control, statins, and angiotensin-converting enzyme inhibitors/angiotensin receptor blockers [Citation18].
An additional burden, especially in the group of CKD patients, was the occurrence of PC-AKI. Contrast-induced acute kidney injury occurred in 99 patients, in every 20th patient with STEMI, however, in the group of patients with CKD history almost 10 percent of the patients were affected. Contrast nephropathy is a common complication, however, the risk of death associated with it is lower than the risk of untreated infarction. PCI is the gold standard procedure [Citation19]. Its effectiveness in reducing mortality is greater than other reperfusion therapy strategies. There are several methods of the prevention of acute renal injury after the administration of contrast, which should be used in high-risk patients (the population with CKD) [Citation20]. However, scientific work so far has focused mainly on the risk of infarction in CKD patients or on the influence of GFR on infarct severity [Citation21,Citation22]. This research allows us to estimate the prognosis in post-infarction patients, after the performed intervention and assess which patients require particular attention. This is a useful marker while considering the whole population with STEMI or NSTEMI infarction.
The studies performed so far report that PC-AKI has a strong impact on an increased risk for adverse clinical outcomes in patients with ACS and increased mortality in post-procedural patients. Our study confirms this theory. During the analysis, it was noted that patients with contrast nephropathy have significantly higher mortality rates [Citation7,Citation23–28].
Patients who are scheduled to have a contrast-enhanced diagnostic or interventional procedure should be evaluated for risk factors of PC-AKI. Previous studies report about various initiators increasing the risk of complications, where the most important is preexisting CKD. Our analysis shows that the risk of PC-AKI is twice as high in patients with diabetes and atrial fibrillation and increases also as the baseline creatinine concentration rises.
One of the most interesting results of our study is a significant role of risk factors, which seems to be underestimated. It is well known that age, diabetes mellitus, prior ACS, time from door to balloon, tachycardia, hypotension, and cardiogenic shock are independent predictors of poor prognosis in patients with ACS. In addition, Shacham et al. have shown that the presence of severe hyperglycemia on admission without previously diagnosed diabetes was an independent risk factor for the development of AKI among STEMI patients undergoing primary PCI [Citation29]. However, in our study, the main prognostic factor was post-procedural creatinine concentration. An increase in post-procedural creatinine concentration of 1 mg/dl in long-term observation resulted in 2.2 times higher death risk ratio. An important impact of renal function was observed in other results. The mortality of patients with contrast-induced acute kidney injury was more than two times higher in the group of CKD in comparison to the rest of the population. In the literature, the impact of CKD on long-term mortality of patients with ACSs was reported with odds ratios between 1.66 and 2.8 [Citation30,Citation31]. In our study, PC-AKI was observed in ninety-nine patients (4.47%). Although the occurrence rate of PC-AKI was higher in the recent studies (11.2 − 16.1%), similarly to our results it was more common in patients diagnosed with STEMI [Citation32–34].
The reason for taking PC-AKI as a strong predictor of death is not only the observation that renal function of half of PC-AKI patients did not return to the baseline concentration but also in almost half of the group it was leading to CKD [Citation35,Citation36]. On the other hand, patients with PC-AKI have more severe coronary artery disease, a higher burden of traditional coronary risk factors, and complications such as diabetes mellitus, hypertension, left ventricular hypertrophy, dyslipidemia, atrial fibrillation, and congestive heart failure, what had an impact on the long-term mortality.
Our results suggest that PC-AKI has a negative impact on outcomes of patients with ACS treated with invasive procedures. More attention should be paid to the prevention and diagnosis of PC-AKI. Nevertheless, necessary PCIs should not be withheld in the fear of PC-AKI.
5. Limitations
Our study had several limitations. Firstly, it was a retrospective, single-center study, but covering a large population. The second limitation is a high percentage of garbage codes in mortality statistics (4–30%). Thirdly, the level of creatinine before ACS and in long-term follow-up is not included in our study. Baseline creatinine values may already reflect impairment from hemodynamic changes in the setting of acute myocardial infarction. Control assessment of creatinine level would let us assess the impact of developing CKD in PC-AKI and non-PC-AKI patients and how the concentration of creatinine returns to baseline levels.
Finally, we might have potentially underestimated the real incidence of PC-AKI, as our protocol only recommended measurement of creatinine level for 2 days after admission. In addition, a potential underestimation of the phenomenon of PC-AKI may be due to the use of historical definition and creatinine level control at 48 h instead of the commonly accepted 72-h delay. Thus, we might have missed later rises in serum creatinine.
6. Conclusions
Chronic kidney disease affects mostly the population with NSTEMI infarction. Contrast-induced acute kidney disease is a major complication in patients with ACS and occurs more frequently in the STEMI population. The risk of CI-AKI is increased in patients with preprocedural renal insufficiency. CI-AKI may be a prognostic marker of long-term mortality in ACS patients undergoing PCI.
More attention should be paid to the prevention and diagnosis of CI-AKI but necessary PCIs should not be withheld in fear of CI-AKI.
Author contributions
L.K J. M. and H. B.G. designed the study conception and design. M. N, A. K. collected the data. L.K., H.B.G., M.Z.A. performed analysis and interpretation of the data. L.K., J.M. wrote the text in consultation with H.B.G. and S.D. All authors provided critical feedback and helped shape the research, analysis and manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Causes of mortality in Poland, World Health Organization. [cited 2020 Jan 1]. Available from: https://www.who.int/nmh/countries/pol_en.pdf?ua=1.
- Rangaswami J, Bhalla V, Blair John EA, American Heart Association Council on the Kidney in Cardiovascular Disease and Council on Clinical Cardiology, et al. Cardiorenal syndrome: classification, pathophysiology, diagnosis, and treatment strategies: a scientific statement from the American Heart Association. Circulation. 2019;139(16):e840–e878.
- Zdrojewski Ł, Zdrojewski T, Rutkowski M, et al. Prevalence of chronic kidney disease in a representative sample of the Polish population: results of the NATPOL 2011 survey. Nephrol Dial Transplant. 2016;31(3):433–439.
- Zdrojewski Ł, Król E, Rutkowski B, et al. Chronic kidney disease in Polish elderly population aged 75+: results of the WOBASZ Senior Survey. Int Urol Nephrol. 2017;49(4):669–676.
- Ismail MD, Jalalonmuhali M, Azhari J, On behalf of NCVD-PCI investigators, et al. Outcomes of STEMI patients with chronic kidney disease treated with percutaneous coronary intervention: the Malaysian National Cardiovascular Disease Database – Percutaneous Coronary Intervention (NCVD-PCI) registry data from 2007 to 2014. BMC Cardiovasc Disord. 2018;18(1):184.
- Rodrigues FB, Bruetto RG, Torres US, et al. Effect of kidney disease on acute coronary syndrome. Clin J Am Soc Nephrol. 2010;5(8):1530–1536.
- Yang Y, George KC, Luo R, et al. Contrast-induced acute kidney injury and adverse clinical outcomes risk in acute coronary syndrome patients undergoing percutaneous coronary intervention: a meta-analysis. BMC Nephrol. 2018;19(1):374.
- Levey AS, Stevens LA, Schmid CH, for the CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration), et al. A new equation to estimate glomerular filtration rate [published correction appears. Ann Intern Med. 2009;150(9):604–612.
- Margolis G, Gal-Oz A, Letourneau-Shesaf S, et al. Acute kidney injury based on the KDIGO criteria among ST elevation myocardial infarction patients treated by primary percutaneous intervention. J Nephrol. 2018;31(3):423–428.
- Yeh RW, Sidney S, Chandra M, et al. Population trends in the incidence and outcomes of acute myocardial infarction. N Engl J Med. 2010;362(23):2155–2165.
- Fox KA, Eagle KA, Gore JM, GRACE and GRACE2 Investigators, et al. The Global Registry of Acute Coronary Events, 1999 to 2009-GRACE. Heart. 2010;96(14):1095–1101.
- Terkelsen CJ, Lassen JF, Norgaard BL, et al. Mortality rates in patients with ST-elevation vs. non-ST-elevation acute myocardial infarction: observations from an unselected cohort. Eur. Heart J. 2005;26(1):18–26.
- Marenzi G, Cabiati A, Assanelli E. Chronic kidney disease in acute coronary syndromes. World J Nephrol. 2012;1(5):134–145.
- Wright J, Hutchison A. Cardiovascular disease in patients with chronic kidney disease. Vasc Health Risk Manag. 2009;5:713–722.
- Hira RS. Care of patients with chronic kidney disease presenting with acute coronary syndrome: improved, but not good enough. J Am Heart Assoc. 2018;7(24):e011254.
- Nauta ST, van Domburg RT, Nuis RJ, et al. Decline in 20-year mortality after myocardial infarction in patients with chronic kidney disease: evolution from the prethrombolysis to the percutaneous coronary intervention era. Kidney Int. 2013;84(2):353–358.
- Widimsky P, Rychlik I. Renal disease and acute coronary syndrome. Heart. 2010;96(1):86–92.
- Tonelli M, Wiebe N, Culleton B, et al. Chronic kidney disease and mortality risk: a systematic review. JASN. 2006;17(7):2034–2047.
- Ibanez B, James S, Agewall S, ESC Scientific Document Group, et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2018;39(2):119–177.
- Faggioni M, Mehran R. Preventing contrast-induced renal failure: a guide. Interv Cardiol. 2016;11(2):98–104.
- Hillege HL, van Gilst WH, van Veldhuisen DJ, et al. Accelerated decline and prognostic impact of renal function after myocardial infarction and the benefits of ACE inhibition: the CATS randomized trial. Eur Heart J. 2003;24(5):412–420.
- Beddhu S, Allen-Brady K, Cheung AK, et al. Impact of renal failure on the risk of myocardial infarction and death. Kidney Int. 2002;62(5):1776–1783.
- McCullough PA, Choi JP, Feghali GA, et al. Contrast-induced acute kidney injury. J Am Coll Cardiol. 2016;68(13):1465–1473.
- McCullough PA, Adam A, Becker CR, CIN Consensus Working Panel, et al. Epidemiology and prognostic implications of contrast-induced nephropathy. Am J Cardiol. 2006;98(6A):5K–13K.
- Lucreziotti S, Centola M, Salerno-Uriarte D, et al. Female gender and contrast-induced nephropathy in primary percutaneous intervention for ST-segment elevation myocardial infarction. Int J Cardiol. 2014;1174(1):37–42.
- James MT, Samuel SM, Manning MA, et al. Contrast-induced acute kidney injury and risk of adverse clinical outcomes after coronary angiography: a systematic review and meta-analysis. Circ Cardiovasc Interv. 2013;6(1):37–43.
- Sadeghi HM, Stone GW, Grines CL, et al. Impact of renal insufficiency in patients undergoing primary angioplasty for acute myocardial infarction. Circulation. 2003;108(22):2769–2775.
- Ozkok S, Ozkok A. Contrast-induced acute kidney injury: a review of practical points. World J Nephrol. 2017;6(3):86–99.
- Shacham Y, Gal-Oz A, Leshem-Rubinow E, et al. admission glucose levels and the risk of acute kidney injury in nondiabetic ST segment elevation myocardial infarction patients undergoing primary percutaneous coronary intervention. Cardiorenal Med. 2015;5(3):191–198.
- Torres IR, Mercader IS, Rumbeu CC, et al. Long-term prognosis of chronic kidney disease in non-ST elevation acute coronary syndrome treated with invasive strategy. Nefrologia. 2017;37(3):276–284.
- Szummer K, Lundman P, Jacobson SH, for SWEDEHEART, et al. Influence of renal function on the effects of early revascularization in non-ST-elevation myocardial infarction: data from the Swedish Web-System for Enhancement and Development of Evidence-Based Care in Heart Disease Evaluated According to Recommended Therapies (SWEDEHEART). Circulation. 2009;120(10):851–858.
- Melloni C, Cornel JH, Hafley G, et al. Impact of chronic kidney disease on long-term ischemic and bleeding outcomes in medically managed patients with acute coronary syndromes: Insights from the TRILOGY ACS Trial. Eur Heart J Acute Cardiovasc Care. 2016;5(6):443–454.
- Wickenbrock I, Perings C, Maagh P, et al. Contrast medium induced nephropathy in patients undergoing percutaneous coronary intervention for acute coronary syndrome: differences in STEMI and NSTEMI. Clin Res Cardiol. 2009;98(12):765–772.
- Narula A, Mehran R, Weisz G, et al. Contrast-induced acute kidney injury after primary percutaneous coronary intervention: results from the HORIZONS-AMI substudy. Eur Heart J. 2014;35(23):1533–1540.
- Brown JR, Malenka DJ, DeVries JT, et al. Transient and persistent renal dysfunction are predictors of survival after percutaneous coronary intervention: insights from the Dartmouth dynamic registry. Cathet Cardiovasc Intervent. 2008;72(3):347–354.
- James MT, Ghali WA, Tonelli M, et al. Acute kidney injury following coronary angiography is associated with a long-term decline in kidney function. Kidney Int. 2010;78(8):803–809.