Abstract
Background
The relationship between cognitive impairment (CI) and arterial stiffness in peritoneal dialysis (PD) patients has not been clearly clarified. The aim of this study was to examine the relationship between CI and arterial stiffness in PD patients.
Methods
This cross-sectional study enrolled PD patients who performed a vascular profiler test at a single PD center in China between January 2014 and June 2016. The cognitive function was evaluated using the Montreal cognitive assessment (MoCA). A noninvasive vascular screening device was used to assess arterial stiffness relevant indicators.
Results
A total of 643 PD patients with median age 45 (37–57.4) years and median duration of PD 27.8 (8.7–56.4) months were enrolled. The rate of CI was 49.9%. The mean brachial-ankle pulse wave velocity (baPWV) was 17.2 ± 5.6 m/s. Compared with normal cognitive function group, patients with CI had higher baPWV (18.6 ± 7.0 vs. 15.8 ± 3.2 m/s), systolic blood pressure (150.3 ± 21.5 vs. 144.2 ± 20.2 mmHg), and pulse pressure (59.7 ± 14.7 vs. 52.5 ± 11.6 mmHg), and lower ankle-brachial index (ABI, 1.12 ± 0.12 vs. 1.15 ± 0.09) (all p<.05). Compared with systolic blood pressure, pulse pressure, and ABI in receiver operating characteristic (ROC) analysis, baPWV had better performance in predicting CI (area under curve: 0.68, 95% confidence interval: 0.64–0.72). BaPWV was independently associated with MoCA score (B per SD, −0.42 [95% confidence interval, −0.71 to −0.12]; p = .006) and CI (OR per SD, 1.55 [95% confidence interval, 1.11–2.17]; p = .011) in PD patients after adjustment for confounders.
Conclusions
Higher baPWV was independently associated with CI in PD patients.
Introduction
The end-stage renal disease (ESRD) patients with peritoneal dialysis (PD) are prone to suffer from cognitive impairment (CI). The prevalence of CI in this population was reported as 3.3–86.8% [Citation1–3]. The occurrence of CI may influence the ability of safe dialysate exchange and complex medication compliance [Citation3]. Previous studies have shown that dialysis patients with CI have higher risk for PD-related peritonitis [Citation4] and mortality [Citation5]. However, the etiology of CI in this population has not been clearly elucidated. Some studies have demonstrated that older age, female, diabetes, low educational level, anemia, malnutrition, inflammation, and inadequate dialysis were risk factors for CI in dialysis patients [Citation3,Citation6–10]. But a few longitudinal studies showed inconsistent results [Citation11–13]. Therefore, it is necessary to further explore the risk factors of CI in order to identify possible intervention measures and improve the prognosis of PD patients.
Arterial stiffness is a marker of functional and structural changes in arteries. Arterial stiffness has been appeared in the early stages of chronic kidney disease (CKD), and might be increased in ESRD patients due to deterioration of renal function, the deposit of abnormal product derived from proteins or lipids, the reduction of advanced glycation end products (AGEs), alteration of calcium and phosphate metabolism, low serum albumin levels, and high C-reactive protein serum levels [Citation14,Citation15]. As one of exact vascular risk factors, arterial stiffness has been demonstrated to be associated with CI in elderly population [Citation16,Citation17] and patients with chronic diseases such as hypertension [Citation18–20] and diabetes mellitus [Citation21,Citation22]. However, there is a lack of related studies focuses on PD patients. So the purpose of this cross-sectional study was to explore the potential relationship between CI and arterial stiffness in PD patients.
Materials and methods
Study population and design
This cross-sectional study enrolled PD patients who performed a vascular profiler test at a single PD center in China between January 2014 and June 2016. All participants were 18 years or older and accepting continuous ambulatory PD treatment at least one month. The exclusion criteria were dementia, or transfer from permanent hemodialysis (HD), or failed renal transplantation, or lack of complete consent. The study protocol was approved by the hospital’s Human Ethics Committees. Written informed consents were obtained from all participants.
Demographic and clinical data collection
Demographic data were obtained by medical history, which included age, gender, educational level, primary renal disease, duration of PD, and smoking status. Comorbidities were assessed by Charlson comorbidity index [Citation23]. Clinical data were collected at enrollment, which included body mass index, hemoglobin, blood platelet count, high-sensitivity C-reactive protein, serum albumin, serum calcium, serum phosphorus, intact parathyroid hormone, total cholesterol, triglycerides, blood urea nitrogen, serum creatinine, residual renal function (measured glomerular filtration rate [mGFR]), and clearance index of urea (Kt/V).
Measurements of cognitive function and arterial stiffness relevant indicators
Cognitive function of PD patients was assessed by the Montreal Cognitive Assessment (mandarin version) (MoCA) [Citation24]. Two PD nurses were trained on the evaluation method of questionnaire before the study began. Then, the nurses used uniform language of instruction to assess the total score of MoCA of PD patients. The assessment content of MoCA included visuospatial and executive functions, attention, short-term memory, language, and orientation. The Cronbach’s alpha of the Chinese-Language MoCA was 0.78 for Mandarin population and 0.79 for Cantonese population [Citation25]. The total score of MoCA is ranged from 0 to 30 points after adjustment for education. The previous study indicated that a cut off score of less than 26 points yielded a sensitivity of 90% and specificity of 78% for mild CI in older adults [Citation24]. So CI was defined as the total score of MoCA less than 26 points in this study.
Within 1 year of the cognitive function assessment, the PD patients were tested for the arterial stiffness relevant indicators using a noninvasive vascular screening device (BP-203RPE III, Omron, Japan) when two liters of dialysate were injected into their abdominal cavity [Citation26]. The validation and reproducibility of this device have been demonstrated by previous study [Citation27]. Brachial-ankle pulse wave velocity (baPWV) is the velocity of the pulse wave that travels from the upper arm to the ankle joint. The formula of baPWV was as following: baPWV=(La-Lb)/Tba. The transmission distance from the brachium to ankle was calculated according to height of the patient. The path length from the suprasternal notch to the brachium (Lb) was obtained using the following equation: Lb = 0.2195 × height (cm)−2.0734. The path length from the suprasternal notch to the ankle (La) was obtained using the following equation: La = 0.8129 × height (cm)+12.328. Tba was the time interval between the initial increase in brachial and ankle waveforms. A higher PWV indicates poorer vessel wall elasticity and compliance, which contribute to increase in blood vessel stiffness [Citation28]. Ankle-brachial index (ABI) was defined as ankle systolic blood pressure divided by brachial systolic blood pressure. As the blood flows from the central artery to the periphery, it encounters resistance and forms a reflection wave that builds up augmentation pressure at the end of contraction. Augmentation index (AI) refers to augmentation pressure divided by the height of entire systolic pressure wave (pulse pressure). Heart rate, systolic and diastolic blood pressure, and pulse pressure were also obtained by this device.
Statistical analyses
All enrolled participants were divided into two groups according to whether the total score of MoCA was less than 26 points. Demographic, clinical data, and arterial stiffness relevant indicators between the two groups were compared. Normally distributed continuous variables were expressed as means and standard deviations. Continuous variables not normally distributed were expressed as medians and interquartile ranges. Categorical variables were expressed as frequencies and percentages. Independent-samples t-test, Mann–Whitney U test, and chi-squared test were used to test for differences in continuous or categoricalfactors between the two groups. Pearson correlation test was used to assess the correlations between total score of MoCA and arterial stiffness relevant indicators. Spearman rank correlation test was used to assess the correlations between CI and arterial stiffness relevant indicators. Receiver operating characteristic (ROC) analysis was used to calculate the sensitivity and specificity of arterial stiffness relevant indicators as diagnostic tool for CI. Multivariate linear regression analysis and binary logistic regression analysis were used to identify independent risk factors of cognitive function. According to presenting clinical relevance and avoiding multicollinearity, covariates with p < .05 in the univariate analysis were included in multivariate analysis. A two-tailed p < .05 was considered statistically significant. Statistical analysis was performed using SPSS version 16.0 (SPSS Inc., Chicago, IL).
Results
Demographic and clinical characteristics
Among 789 PD patients who performed vascular profile test, five patients were younger than 18 years, 13 patients were tested within the first month of PD, five patients presented dementia, 15 patients transferred from HD, seven failed renal transplantation, and 101 patients were not informed consent. Finally, 643 PD patients were enrolled in this study (). The median age of participants was 45 (37–57.4) years, and the median duration of PD at enrollment was 27.8 (8.7–56.4) months. Among them, 42.1% of patients were female, and 16.3% with diabetes mellitus. The prevalence of CI is 49.9% (n = 321) in the study PD population. The mean total MoCA score was 24.1 ± 4.3 points in all patients, 20.8 ± 3.8 points in CI patients, and 27.5 ± 1.2 points in normal cognitive function patients, respectively. The mean baPWV was 17.2 ± 5.6 m/s in all patients, 18.6 ± 7.0 m/s in patients with CI, and 15.8 ± 3.2 m/s in patients with normal cognitive function, respectively.
Comparison of clinical characteristics according to different cognitive function
Compared with normal cognitive function group, patients with CI had older age; longer duration of PD; higher proportion of female, diabetes mellitus and cardiovascular disease; lower educational level; higher Charlson comorbidity index score, body mass index, blood platelet count, high-sensitivity C-reactive protein, total cholesterol, triglyceride, baPWV, systolic blood pressure, and pulse pressure; lower level of serum albumin, serum calcium, serum creatinine, ABI, and total MoCA score (all p < .05) ().
Table 1. Comparison of demographic and clinical data.
Correlation between arterial stiffness relevant indicators and cognitive function
Pearson correlation analysis showed that total MoCA score was correlated with baPWV, heart rate, systolic blood pressure, pulse pressure, and ABI (all p < .05) (). Spearman’s correlation analysis showed that baPWV, heart rate, systolic blood pressure, and pulse pressure were positively correlated with CI, while ABI was negatively correlated with CI (all p < .05) (). The ROC analysis was used for evaluating the performance of different arterial stiffness relevant indicators in predicting CI. showed that baPWV had a best discrimination and calibration for predicting CI compared with systolic blood pressure, pulse pressure, and ABI, with a higher area under the curve of 0.68 (95% confidence interval: 0.64–0.72).
Figure 2. Performance of different measurement of arterial stiffness in predicting cognitive impairment in the receiver operating characteristic analysis.
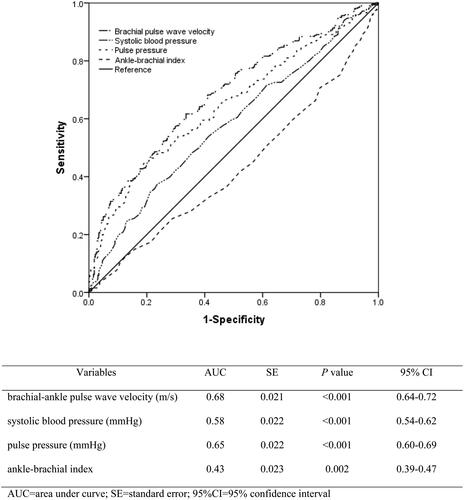
Table 2. Correlation between Arterial stiffness relevant indicators and cognitive function.
Association of baPWV with cognitive function
Univariate linear regression analysis showed that older age, female, lower educational level, diabetes mellitus, duration of PD, blood platelet count, high-sensitivity C-reactive protein, serum albumin, total cholesterol, serum creatinine and baPWV were associated with total score of MoCA in PD patients (all p < .05) (). These factors and smoking status were included into multivariate linear regression analysis. The results showed that baPWV was independently associated with total score of MoCA in PD patients after adjustment for the underlying cofounders (B per SD, −0.42 [95% confidence interval, −0.71 to −0.12]; p = .006) ().
Table 3. Associations between brachial pulse wave velocity and score of Montreal cognitive assessment by linear regression analysis.
Univariate binary logistic regression analysis showed that older age, female, lower educational level, diabetes mellitus, duration of dialysis, body mass index, blood platelet count, high-sensitivity C-reactive protein, serum albumin, serum calcium, total cholesterol, serum creatinine, baPWV, systolic blood pressure, pulse pressure, and ABI were associated with CI in PD patients (all p < .05) (). These factors and smoking status were included into multivariate binary logistic regression analysis. And the results showed that baPWV, systolic blood pressure, and pulse pressure was independently associated with CI in PD patients after adjustment for the underlying cofounders (all p < .05) ().
Table 4. Univariate binary logistic regression analysis for the influence factors of cognitive impairment in peritoneal dialysis patients.
Table 5. Associations between arterial stiffness relevant indicators and cognitive impairment by binary logistic regression analysis.
Discussion
This cross-sectional study demonstrated that the prevalence of CI in the study PD population was 49.9% assessed by the MoCA. The mean baPWV was 17.2 ± 5.6 m/s. Compared with systolic blood pressure, pulse pressure, and ABI, baPWV had better performance in predicting CI. After adjustment for the underlying confounders, it was found that higher baPWV was independently associated with CI in PD patients.
In this study, the prevalence of CI in PD patients was 49.9% through MoCA test. A systematic review and meta analysis showed that the prevalence of CI in PD patients ranged from 3.3% to 74.5% and the overall pooled prevalence of CI was 28.7%, which analyzed the relevant data from 1736 PD patients in eight studies [Citation29]. The different results of these studies may be due to different cognitive function assessment tools and different demographic characteristics of patients. Xu et al. [Citation30] applied the modified mini–mental state examination, trail making tests A and B, subtests of Repeatable Battery for the Assessment of Neuropsychological Status to assessed the cognitive function of 476 PD patients from 5 PD units in China, and found that the prevalence of CI was 28.4% in PD patients with the mean age 51.9 ± 14.3 years and the proportion of diabetes mellitus 23.7%. Zheng et al. [Citation2] enrolled 72 PD patients with the mean age 56.2 ± 16.0 years and the proportion of diabetes mellitus 31.9%, and found that 25% and 86.8% of PD patients could be diagnosed as CI, according to the MMSE and MoCA test, respectively. But overall, these findings suggested that CI was common in patients with PD.
Our results showed that the mean baPWV in PD patients was higher than that in healthy adults, which was similar to other literature reports [Citation31,Citation32]. The relationship between arterial stiffness and CKD is complex. CKD is associated with accelerated vascular aging. Activation of renin–angiotensin system, aortic inflammation, and vascular metalloproteinase activity lead to changes in the extracellular matrix and endothelial dysfunction, which thereby increase arterial stiffness [Citation33]. Through both a reduction in baroreflex sensitivity and the passive effect of loss of arterial wall elastic properties, arterial stiffness increases the variability of blood pressure, which in turn might contribute to the development and progression of kidney damage [Citation34]. In the process from CKD to ESRD, premature aging of the vascular system leads to extreme increases in arterial stiffness [Citation35]. Hypertension, disorder of lipid metabolism and calcium and phosphate metabolism, accumulation of uremic toxins such as AGEs, and chronic inflammation associated with peritoneal fluid biocompatibility are common in PD patients. These risk factors also contribute to the progression of arterial stiffness [Citation14].
Through noninvasive vascular screening device, arterial stiffness can be assessed using various indicators such as PWV, ABI, AI, cardio-ankle vascular index, etc. Carotid-femoral PWV (cfPWV) is considered the gold standard method for assessing aortic stiffness [Citation36]. However, measuring cfPWV with tonometer or doppler requires specialized training and exposure to the groin area, and is thought to be not easy to operate in clinical practice [Citation37]. Sharing the same theoretical background with cfPWV, baPWV is easier to use clinically because it just requires wrapping of a pressure cuff in each of the exposed extremities [Citation27]. Although baPWV reflects the stiffness of small arteries (especially the muscular arteries of the arms and legs) and does not fully reflect the stiffness of the central artery [Citation38], it is closely correlated with the directly measured aortic PWV and cfPWV [Citation39]. As the ROC curves of this study showed, baPWV had higher area under the curve for predicting CI compared with systolic blood pressure, pulse pressure, and ABI.
Some previous studies have demonstrated the association between arterial stiffness and CI with inconsistent results [Citation7,Citation40,Citation41]. However, this association has not been reported in PD patients. Our results found that higher baPWV was independently associated with CI in PD patients. Consistent with our results, Angermann et al. found that higher baPWV was independently associated with CI in 201 HD patients by MoCA test [Citation7]. Another study also showed the positive association of cfPWV with CI among HD patients [Citation40]. But a resent prospective study did not show significant associations between cfPWV and cognitive function in HD patients, mainly due to the relatively younger age of study population [Citation41]. The possible mechanisms of the association between arterial stiffness and CI were as following. First, greater arterial stiffness can lead to microcirculatory damage of the brain through increasing pulsatile pressure and flow load [Citation42]. Second, high pulse pressure may lead to structural changes in the cerebral vessels that may interfere with the transport of important nutrients to the brain and the removal of toxic byproducts from the brain [Citation43]. Finally, brain imaging studies have found arterial stiffness was associated with cerebral microvascular disease and changes of cerebral white matter lesions, which in turn were associated with CI [Citation44,Citation45].
One of strengths of this study was that it was the first to reveal the association between arterial stiffness and CI in PD patients. Another strength was large sample of study to reduce selection bias. However, there were some limitations of this study. First, the neuroimaging was not performed for participants, so it was possible that the rate of CI might be underestimated. Second, inter-rater and intra-rater reliability of MoCA were not measured in this study, which might cause bias. Finally, this study could not make the causal inferences because of a cross-sectional nature. Longitudinal studies are needed to determine if higher baPWV predicts cognitive decline in PD patients.
Conclusions
In this cohort of PD patients, we indicated that higher baPWV was independently associated with CI of PD patients. Further prospective studies need to confirm the association of baPWV and cognitive decline in PD patients.
Ethical approval
The study was conducted ethically in accordance with the World Medical Association Declaration of Helsinki and approved by the Human Ethics Committee of Sun Yat-sen University. All the participants have given their written informed consent.
Author contributions
All authors have contributed significantly and in keeping with the latest guidelines of the International Committee of Medical Journal Editors. Xiao Yang, Chunyan Yi and Wenbo Zhang conceived and designed the study. Chunyan Yi, Xuan Huang and Jianxiong Lin prepared and performed data collection. Chunyan Yi, Hongjian Ye and Haishan Wu analyzed the data. Chunyan Yi and Wenbo Zhang interpreted the results and drafted the manuscript. Xiao Yang coordinated the study and finally approved the version to be published.
Acknowledgments
We thank all staffs in our PD center for their patient care and data collection.
Disclosure statement
All authors have no conflicts of interest to declare.
Additional information
Funding
References
- Sithinamsuwan P, Niyasom S, Nidhinandana S, et al. Dementia and depression in end stage renal disease: comparison between hemodialysis and continuous ambulatory peritoneal dialysis. J Med Assoc Thai. 2005;88(3):S141–S147.
- Zheng K, Wang H, Hou B, et al. Malnutrition-inflammation is a risk factor for cerebral small vessel diseases and cognitive decline in peritoneal dialysis patients: a cross-sectional observational study. BMC Nephrol. 2017;18(1):366.
- Kalirao P, Pederson S, Foley RN, et al. Cognitive impairment in peritoneal dialysis patients. Am J Kidney Dis. 2011;57(4):612–620.
- Shea YF, Lee MC, Mok MM, et al. Self-care peritoneal dialysis patients with cognitive impairment have a higher risk of peritonitis in the second year. Perit Dial Int. 2019;39(1):51–58.
- Griva K, Stygall J, Hankins M, et al. Cognitive impairment and 7-year mortality in dialysis patients. Am J Kidney Dis. 2010;56(4):693–703.
- Lu R, Kiernan MC, Murray A, et al. Kidney-brain crosstalk in the acute and chronic setting. Nat Rev Nephrol. 2015;11(12):707–719.
- Angermann S, Baumann M, Wassertheurer S, et al. Pulse wave velocity is associated with cognitive impairment in hemodialysis patients. Clin Sci. 2017;131(13):1483–1493.
- Shea YF, Lam MF, Lee MS, et al. Prevalence of cognitive impairment among peritoneal dialysis patients, impact on peritonitis and role of assisted dialysis. Perit Dial Int. 2016;36(3):284–290.
- Sarnak MJ, Tighiouart H, Scott TM, et al. Frequency of and risk factors for poor cognitive performance in hemodialysis patients. Neurology. 2013;80(5):471–480.
- Khatri M, Nickolas T, Moon YP, et al. CKD associates with cognitive decline. J Am Soc Nephrol. 2009;20(11):2427–2432.
- Drew DA, Weiner DE, Tighiouart H, et al. Cognitive decline and its risk factors in prevalent hemodialysis patients. Am J Kidney Dis. 2017;69(6):780–787.
- Giang LM, Weiner DE, Agganis BT, et al. Cognitive function and dialysis adequacy: no clear relationship. Am J Nephrol. 2011;33(1):33–38.
- Kurella Tamura M, Unruh ML, Nissenson AR, et al. Effect of more frequent hemodialysis on cognitive function in the frequent hemodialysis network trials. Am J Kidney Dis. 2013;61(2):228–237.
- Fischer EC, Zocalo Y, Galli C, et al. Arterial stiffness and renal replacement therapy: a controversial topic. Int J Nephrol. 2015;2015:729609.
- Yang L, Lin Y, Ye C, et al. Effects of peritoneal dialysis and hemodialysis on arterial stiffness compared with predialysis patients. Clin Nephrol. 2011;75(03):188–194.
- Elias MF, Robbins MA, Budge MM, Abhayaratna WP, et al. Arterial pulse wave velocity and cognition with advancing age. Hypertension. 2009;53(4):668–673.
- Watson NL, Sutton-Tyrrell K, Rosano C, et al. Arterial stiffness and cognitive decline in well-functioning older adults. J Gerontol A Biol Sci Med Sci. 2011;66A(12):1336–1342.
- Muela HCS, Costa-Hong VA, Yassuda MS, et al. Higher arterial stiffness is associated with lower cognitive performance in patients with hypertension. J Clin Hypertens. 2018;20(1):22–30.
- Triantafyllidi H, Arvaniti C, Lekakis J, et al. Cognitive impairment is related to increased arterial stiffness and microvascular damage in patients with never-treated essential hypertension. Am J Hypertens. 2009;22(5):525–530.
- Kalaitzidis RG, Panagiotopoulou T, Stagikas D, et al. Arterial stiffness, cognitive dysfunction, and adherence to antihypertensive agents. Is there a link to hypertensive patients? Curr Vasc Pharmacol. 2019; 18:410–417.
- Geijselaers SL, Sep SJ, Schram MT, et al. Carotid stiffness is associated with impairment of cognitive performance in individuals with and without type 2 diabetes. The maastricht study. Atherosclerosis. 2016;253:186–193.
- Mehrabian S, Raycheva M, Gateva A, et al. Cognitive dysfunction profile and arterial stiffness in type 2 diabetes. J Neurol Sci. 2012;322(1–2):152–156.
- Hemmelgarn BR, Manns BJ, Quan H, et al. Adapting the charlson comorbidity index for use in patients with esrd. Am J Kidney Dis. 2003;42(1):125–132.
- Nasreddine ZS, Phillips NA, Bedirian V, et al. The montreal cognitive assessment, moca: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695–699.
- Zheng L, Teng EL, Varma R, et al. Chinese-language montreal cognitive assessment for cantonese or mandarin speakers: age, education, and gender effects. Int J Alzheimers Dis. 2012;2012:204623.
- Chen SC, Chang JM, Liu WC, et al. Brachial-ankle pulse wave velocity and rate of renal function decline and mortality in chronic kidney disease. Clin J Am Soc Nephrol. 2011;6(4):724–732.
- Yamashina A, Tomiyama H, Takeda K, et al. Validity, reproducibility, and clinical significance of noninvasive brachial-ankle pulse wave velocity measurement. Hypertens Res. 2002;25(3):359–364.
- Laurent S, Cockcroft J, Van Bortel L, et al. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J. 2006;27(21):2588–2605.
- Shea YF, Lee MC, Mok MM, Chan FH, et al. Prevalence of cognitive impairment among peritoneal dialysis patients: a systematic review and meta-analysis. Clin Exp Nephrol. 2019;23(10):1221–1234.
- Xu R, Pi HC, Xiong ZY, et al. Hyponatremia and cognitive impairment in patients treated with peritoneal dialysis. Clin J Am Soc Nephrol. 2015;10(10):1806–1813.
- Kuang DW, Li CL, Kuok UI, et al. Risk factors associated with brachial-ankle pulse wave velocity among peritoneal dialysis patients in macao. BMC Nephrol. 2012;13(1):143.
- Cai K, Luo Q, Zhu B, et al. Neutrophil-lymphocyte ratio is associated with arterial stiffness in patients with peritoneal dialysis. BMC Nephrol. 2016;17(1):191.
- Salvi P, Parati G. Chronic kidney disease: arterial stiffness and renal function-a complex relationship. Nat Rev Nephrol. 2015;11(1):11–13.
- Parati G, Ochoa JE, Bilo G. Blood pressure variability, cardiovascular risk, and risk for renal disease progression. Curr Hypertens Rep. 2012;14(5):421–431.
- Wang MC, Tsai WC, Chen JY, et al. Stepwise increase in arterial stiffness corresponding with the stages of chronic kidney disease. Am J Kidney Dis. 2005;45(3):494–501.
- Van Bortel LM, Laurent S, Boutouyrie P, et al. Expert consensus document on the measurement of aortic stiffness in daily practice using carotid-femoral pulse wave velocity. J Hypertens. 2012;30(3):445–448.
- Williams B, Mancia G, Spiering W, et al. 2018 ESC/ESH guidelines for the management of arterial hypertension: the task force for the management of arterial hypertension of the european society of cardiology and the European society of hypertension: the task force for the management of arterial hypertension of the European society of cardiology and the European society of hypertension. J Hypertens. 2018;36(10):1953–2041.
- Iulita MF, Noriega de la Colina A, Girouard H. Arterial stiffness, cognitive impairment and dementia: confounding factor or real risk? J Neurochem. 2018;144(5):527–548.
- Tanaka H, Munakata M, Kawano Y, et al. Comparison between carotid-femoral and brachial-ankle pulse wave velocity as measures of arterial stiffness. J Hypertens. 2009;27(10):2022–2027.
- Tasmoc A, Donciu MD, Veisa G, et al. Increased arterial stiffness predicts cognitive impairment in hemodialysis patients. Hemodial Int. 2016;20(3):463–472.
- Kim ED, Meoni LA, Jaar BG, et al. Association of arterial stiffness and central pressure with cognitive function in incident hemodialysis patients: the pace study. Kidney Int Rep. 2017;2(6):1149–1159.
- van Sloten TT, Protogerou AD, Henry RM, et al. Association between arterial stiffness, cerebral small vessel disease and cognitive impairment: a systematic review and meta-analysis. Neurosci Biobehav Rev. 2015;53:121–130.
- Iadecola C, Park L, Capone C. Threats to the mind: aging, amyloid, and hypertension. Stroke. 2009;40(3):S40–S4.
- Henskens LH, Kroon AA, van Oostenbrugge RJ, et al. Increased aortic pulse wave velocity is associated with silent cerebral small-vessel disease in hypertensive patients. Hypertension. 2008;52(6):1120–1126.
- Ohmine T, Miwa Y, Yao H, et al. Association between arterial stiffness and cerebral white matter lesions in community-dwelling elderly subjects. Hypertens Res. 2008;31(1):75–81.

