Abstract
Purpose
The risk of thromboembolic events is elevated in patients with nephrotic syndrome, and warfarin use has been associated with an increased risk of bleeding. Indobufen, a selective cyclooxygenase-1 inhibitor, is currently being evaluated for the prevention of thromboembolic events in nephrotic syndrome. This study aimed to compare the efficacy and safety of indobufen with that of warfarin in patients with nephrotic syndrome.
Materials and methods
This multicenter, randomized, three-arm, open-label, parallel controlled trial involved a total of 180 adult patients with nephrotic syndrome from four centers in China. Patients were randomly assigned to receive 100 mg indobufen (bid), 200 mg indobufen (bid), and 3 mg warfarin (qd) daily for 12 weeks. The primary endpoints included thromboembolic and bleeding events, while laboratory results and adverse events constituted secondary endpoints.
Results
No thromboembolic events occurred in the high-/low-dose indobufen and warfarin groups. Moreover, the use of a low dose of indobufen significantly reduced the risk of minor bleeding events compared with warfarin use (2% versus 18%, p < .05). Finally, adverse events were more frequent in warfarin-treated patients.
Conclusions
This study found that indobufen therapy provided equivalent effects in preventing thromboembolic events compared with warfarin therapy, while low dose of indobufen was associated with a reduced risk of bleeding events, thus it should be recommended for the prevention of thromboembolic events in clinical practice in patients with nephrotic syndrome.
Trial registration number
ChiCTR-IPR-17013428.
Introduction
Nephrotic syndrome is characterized by a urine protein loss > 3.5 g/24 h, accompanied by hypoalbuminemia and edema, with an annual incidence of 1–3 per 100,000 adults [Citation1]. Nephrotic syndrome has been associated with an increased risk of thromboembolic events, predominantly occurring in the veins, including deep venous thrombosis, renal venous thrombosis, and pulmonary embolism, with prevalence of 15%, 27.9%, and 12.8%, respectively [Citation2]. Platelet activity and cohesion aggregation play important roles in the risk of thromboembolic events in patients with nephrotic syndrome. In patients with nephrotic syndrome, thrombocytosis potentially causes a decrease in red blood cell variability [Citation3] and an increase in the von Willebrand factor level, which potentially induces platelet aggregation to the vascular wall and increases platelet adhesion [Citation4]. In addition, platelet aggregation is increased in patients with nephrotic syndrome in vitro [Citation5].
Currently, warfarin is widely used as a prophylactic anticoagulant; however, its risk of bleeding events is significantly high [Citation6,Citation7]. Indobufen, a selective and reversible cyclooxygenase-1 inhibitor, effectively inhibits platelet activation, adhesion, and aggregation [Citation8]. Studies have demonstrated that indobufen reduces platelet factors 3 and 4, thereby reducing the activation of coagulation factors II and X, which play important roles in blood coagulation [Citation9]. Moreover, the use of indobufen versus placebo showed reduced risk of ischemic events in patients with heart disease, and indobufen versus acetylsalicylic acid plus dipyridamole was associated with the higher one-year cumulative patency rate for patients after femoropopliteal bypass [Citation10,Citation11]. A prior study found that thrombotic recanalization or disappearance occurred in patients with deep venous thrombosis treated with indobufen 600 mg/day, thus suggesting that indobufen use potentially reduces the risk of deep venous thrombosis [Citation12]. Our previous study on a rat model of adenine-induced chronic kidney disease revealed that indobufen was associated with lower degree of inflammatory infiltration and fibrosis of the renal tissue compared with warfarin [Citation13]. Moreover, indobufen may be associated with lower prothrombin time (PT) and activated partial thromboplastin time (APTT) levels. However, although indobufen may be useful for thromboembolic events in patients with nephrotic syndrome, no randomized controlled trial has investigated the efficacy and safety outcomes of indobufen in patients with nephrotic syndrome. Therefore, we performed this study to compare the efficacy and safety of indobufen with that of warfarin in patients with nephrotic syndrome.
Methods
Trial design and oversight
This prospective, multicenter, randomized clinical trial was conducted at four centers in China. All patients provided written informed consent before participation, and the institutional review board of each center approved the protocol before the initiation of the study. The coauthors contributed to the first draft of the original manuscript and revision of the original manuscript. This study was registered at http://www.chictr.org.cn (ChiCTR-IPR-17013428), and the current trial was performed following the consolidated standards of reporting trial CONSORT guidelines [Citation14].
Trial population
Adult patients with nephrotic syndrome were eligible for inclusion in this study, and the inclusion criteria were as follows: (1) age between 18 and 75 years; (2) 24-h urinary protein excretion > 3.5 g and/or serum albumin < 30 g/L; and (3) exposure to thrombosis risk factors, such as recent abdominal surgery, prolonged immobilization, heart failure, and body mass index (BMI) > 35 kg/m2. Patients were excluded if they had (1) a history of allergy to indobufen or warfarin and (2) evidence or suspicion of thrombosis at enrollment.
Randomization and treatment protocol
After enrollment, patients were randomly assigned to three groups in a 1:1:1 ratio, and an observer-blinded approach was followed. Randomization was performed using a random number table with a central computerized system. Group A was administered an oral dose of 100 mg of indobufen twice daily. Group B was administered an oral dose of 200 mg of indobufen twice daily. Group C received 3 mg of warfarin orally once a day. Dose adjustment was based on the PT. The international normalized ratio (INR) was maintained between 1.5 and 3.0.
Patients were followed-up for 12 weeks, and study visits occurred at baseline and at weeks 1, 4, 8, and 12. Patients or researchers had the option of discontinuing treatment early because of unacceptable serious adverse events (SAEs), any change in the patient’s condition that justified discontinuation (decided by the researcher; included the requirement for any drug that treats thrombosis, which was an exclusion criterion), withdrawal of consent (decided by the patient), and complete remission of nephrotic syndrome (24-h urinary protein < 0.3 g, serum albumin > 35 g/L).
Collected variables
Baseline data included age, sex, weight, BMI, and pathological diagnosis from kidney biopsy samples. BMI is the weight in kilograms divided by the square of the height in meters. Baseline laboratory data obtained from the central laboratory included the following: hemoglobin (Hb), platelet count, serum albumin (Salb), blood urea nitrogen, serum creatinine, APTT, PT, thrombin time (TT), fibrinogen (FIB), and D-dimer. All laboratory examinations were performed at the central hospital laboratory.
Endpoints
The primary outcomes were the incidence rates of thrombosis and bleeding. Thromboembolic events were confirmed by ultrasound (for deep vein thrombosis), renal magnetic resonance venography (for renal vein thrombosis), or computed tomography pulmonary ventilation-perfusion scan (for pulmonary embolism). Major bleeding referred to any of the following: fatal bleeding, two symptomatic bleeding events at a critical site, bleeding causing the Hb level to drop by ≥ 20 g/L (1.24 mmol/L), or bleeding requiring the transfusion of two or more units of whole blood or red blood cells [Citation15]. Minor bleeding events were defined as bleeding not meeting the criteria for major bleeding but associated with medical intervention; contact with a physician; interruption of the study drug; or discomfort/impairment in performing daily activities, including gastrointestinal bleeding, nasal bleeding, subcutaneous hemorrhage, fundus bleeding, and non-glomerular origin hematuria. The secondary outcome was coagulation function (APTT, PT, TT, and D-dimer). In group C, INR values were also collected. The incidences of AEs and SAEs were recorded from the initial treatment until the final follow-up visit. AEs were summarized using the MedDRA system organ class and the preferred term. An independent data monitoring committee reviewed the cumulative safety data.
Statistical analysis
Patient demographic, clinical, and laboratory data are described using (1) the mean (±SD) or median (range), depending on the underlying distribution, for continuous variables and (2) frequencies (percentages) for categorical variables. Continuous variables were compared using analysis of variance, while categorical variables were compared using the chi-square test. A mixed linear regression model was applied to assess the potential changes in laboratory data among the groups and various visit time points, and the intercept was random. The reported p values were two-sided, and the inspection level was .05. The IBM Statistical Package for the Social Sciences for Windows (version 19.0; IBM, Armonk, NY, USA) was used to perform all statistical analyses.
Results
Patients
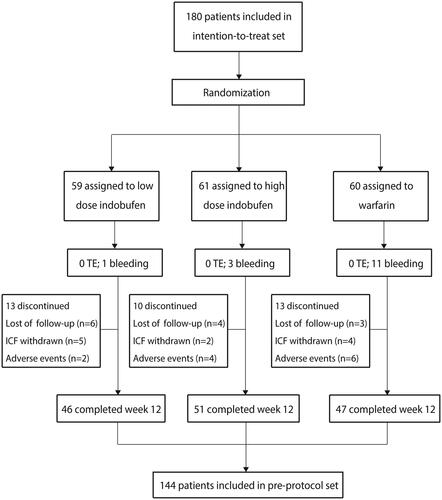
Between December 2017 and December 2020, 180 patients from four different centers were randomly assigned (59 in group A, 61 in group B, and 60 in group C) and followed up until February 2021. Nine patients discontinued treatment early during the COVID-19 lockdown. Of the 180 patients, 36 (20%) had missing primary-outcome data. Therefore, the baseline characteristics and primary-outcome analysis were based on the intention-to-treat set, which included 180 patients, while the pre-protocol analysis population included 144 patients (46 in group A, 51 in group B, and 47 in group C) ().
Baseline characteristics
The baseline characteristics of the enrolled patients are shown in . We noted that the baseline characteristics of the groups were well balanced. The mean adherence was 78% in group A (95% confidence interval [CI]: 67%–89%), 84% in group B (95% CI: 74%–93%), and 78% in group C (95% CI: 68%–89%). No statistically significant differences were noted among the three groups (p = .686). Premature discontinuation of the study drug occurred in 13, 10, and 13 patients in groups A, B, and C, respectively.
Table 1. Baseline characteristics of enrolled patients.a
Primary endpoints
The median follow-up time was 10.3 weeks. After 12 weeks of intervention, no thromboembolic and major-bleeding events occurred in the three groups. Minor bleeding occurred in 1 of 59 patients in group A (2%; 95% CI:0%–5%), 3 of 61 patients in group B (5%; 95% CI:0%–11%), and 11 of 60 patients in group C (18%, 95% CI:6%–30%). We noted that patients in group A were associated with a reduced risk of minor bleeding events compared with those in group C (p < .05) ().
Table 2. Clinical outcomes (n = 180).a
Secondary endpoints
The changes in coagulation function in the groups and at various visits are shown in . Significant differences in APTT and PT were observed among the groups at weeks 4, 8, and 12 (p < .05). Furthermore, the TT level at four weeks among the groups was statistically significant (p = .008). The results of the mixed linear regression model are presented in the Supplementary File. First, we noted that the APTT in group C was higher than that in group A (p < .001), and the changes in APTT at weeks 4 and 8 were statistically significant. Second, patients in group C had a higher PT than those in group A (p < .001), while the changes in PT at weeks 1, 4, 8, and 12 were statistically significant. Third, D-dimer levels at weeks 4, 8, and 12 were significantly reduced. Finally, although the APTT and PT levels among the three groups were similar and within the normal range at baseline, we noted that these levels were reduced in groups A and B and remained high in group C after the intervention.
Table 3. Laboratory analysis.
The AEs in the groups are shown in . There was one case of cough and another of new-onset tumor in patients treated with a low dose of indobufen, while one case each of cough, hand twitch, nausea, and new-onset tumor was reported among patients treated with a high dose of indobufen. Moreover, one case each of erythra, headache, dry mouth, abdominal distension, diarrhea, and sudden cardiac death was reported among patients treated with warfarin.
Table 4. Adverse events (n = 180).
Serum albumin was considered an indicator of predisposition for thromboembolic events. At baseline, 35 (76.1%), 41 (80.4%), and 36 (76.6%) patients exhibited serum albumin levels < 28 g/L in groups A, B, and C, respectively. After 12 weeks, the number of patients with serum albumin levels < 28 g/L in groups A, B, and C decreased to 11 (23.9%), 11 (21.6%), and 13 (27.7%), respectively. Moreover, the mean serum albumin levels improved in groups A, B, and C (11.3 [8.9], 11.7, and 10.8 [10.1] g/L), and no significant differences were observed among the three groups (p = .678).
At baseline, the INR for patients in group C was 0.93 (0.79–1.01), and it increased to 1.44 (0.95–2.04) and 2.03 (1.44–2.92) on days 3 and 7, respectively. Moreover, the mean INR remained high during follow-up, and a total of 15 patients showed INR > 1.5 after 12 weeks. Furthermore, the mean INRs for patients with and without minor bleeding events were 3.37 (2.11–4.81) and 1.67 (1.22–2.37), respectively, and the difference between patients with and without bleeding events was statistically significant (p < .01).
Discussion
Hypercoagulability, the main pathological change in nephrotic syndrome, is possibly caused by reduced anticoagulants (antithrombin III), increased liver procoagulant synthesis (FIB, factor V, and factor VIII), increased platelet activation and aggregability, and decreased fibrinolytic activity in the kidney [Citation15]. Moreover, the Kidney Disease Improving Global Outcomes clinical practice guidelines indicate that full-dose prophylactic anticoagulants should be administered to patients presenting with hypoproteinemia and high thromboembolism risk [Citation1]. Several prophylactic anticoagulants have already been used in clinical practice, including low-molecular-weight heparin, warfarin, aspirin, and direct oral anticoagulants. However, these prophylactic anticoagulants have several limitations, including poor patient compliance with low-molecular-weight heparin owing to cold storage and intravenous administration [Citation16], the high cost of direct oral anticoagulants [Citation17], various warfarin-related complications [Citation18,Citation19], and aspirin’s limited efficacy [Citation20,Citation21].
The current study was designed as a prospective, multicenter, randomized clinical trial, and it recruited 180 patients with nephrotic syndrome. This study found no thromboembolic events in the three groups, while low doses of indobufen were associated with a effectively reduced risk of minor bleeding events compared with warfarin. Moreover, APTT and PT levels in the warfarin group were higher than those in the low-dose indobufen group. Finally, one death occurred in the warfarin group; the patient died of sudden cardiac arrest between visits 3 and 4. Finally, other nonspecific AEs predominantly occurred in the warfarin group.
This study did not observe any thromboembolic events in the three groups, suggesting that indobufen has an antithrombotic effect equivalent to that of warfarin, and it may be superior to warfarin owing to the significantly lower incidence of minor bleeding events. The trends in serum albumin and creatinine levels in the three groups were similar, suggesting that indobufen did not affect the efficacy of immunosuppressants in the treatment of nephrotic syndrome compared with warfarin. Moreover, the mean APTT and PT values were higher than normal (APTT:23–27 s; PT:11–15 s) after treatment with warfarin, suggesting that the warfarin dose was larger than expected, thus potentially increasing the risk of bleeding. Furthermore, D-dimer concentration decreased slightly in all three groups during the 12-week intervention. However, no statistically significant differences were observed among the three groups. Because D-dimer has a relatively high sensitivity in diagnosing thrombosis [Citation22,Citation23], these results demonstrated that the antithrombotic effects of indobufen and warfarin were similar in patients with nephrotic syndrome. Although a previous study found side effects of indobufen, including digestive complications such as nausea, diarrhea, and abdominal distension [Citation24], our study did not observe the risk of digestive complications in patients treated with indobufen. Moreover, the warfarin group experienced more AEs during the trial, including erythema, headache, dry mouth, abdominal distension, diarrhea, and sudden cardiac death. However, given the low prevalence of AEs, no significant differences in AEs were observed among the groups.
To the best of our knowledge, our study is the first to provide evidence corroborating indobufen’s effectiveness and safety in preventing thromboembolic events in patients with nephrotic syndrome. Although no major bleeding occurred in any of the three groups, we recorded a numerically higher rate of clinically relevant minor bleeding (11/60 vs. 4/120) in the warfarin group than in the indobufen group. Moreover, this study found that treatment with warfarin requires frequent surveillance of INR and poses a higher risk of bleeding, whereas indobufen potentially offers a less burdensome treatment.
Notwithstanding, this study has certain limitations. The sample size was relatively small and the duration of follow-up was 12 weeks. Considering the median number of days to the first venous thromboembolism in patients with nephrotic syndrome was 272 days [Citation25], the follow-up duration in our study was shorter than expected, which may be associated with unobserved thromboembolic events during follow-up. In our study, only 11 (23.9%), 11 (21.6%), and 13 (27.7%) patients’ SAlb levels remained below 28 g/L by the end of the trial in groups A, B, and C, respectively, suggesting that most patients had recovered from nephrotic syndrome after receiving treatment for three months. This indicates that the three-month intervention in this study was acceptable. A greater density of patients and longer follow-up period would be useful in defining the advantages and disadvantages of indobufen more definitively. Moreover, the use of an open-label design introduces the risk of ascertainment bias. Furthermore, 20% of the patients were lost to follow-up after 12 weeks, an occurrence predominantly caused by the COVID-19 pandemic. Finally, patients were given warfarin once daily while indobufen was given twice daily, which may introduce potential observer bias.
In conclusion, indobufen, an antiplatelet drug with an anticoagulation effect, provided equivalent effects to warfarin in preventing thromboembolic events and was associated with a reduced risk of bleeding events in adult patients with nephrotic syndrome. Therefore, indobufen is recommended for the prevention of thromboembolic events in patients with nephrotic syndrome. Nonetheless, further large-scale, randomized controlled trials should be performed to verify the efficacy and safety of indobufen in patients with nephrotic syndrome.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Author contributions
Research idea and study design: Li YW; data acquisition and centralized laboratory measurements: Zheng DN, Liu YM, Li H, Xiong XL, Chen HY, Wang H, Yu XY, Qu K; data analysis, statistical analysis, and manuscript drafting: Gao XY, Liu YM; supervision of the study or mentorship: Lin B, Jin J, He Q.
Supplemental Material
Download PDF (153.2 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Additional information
Funding
References
- Kidney Disease: Improving Global Outcomes (KDIGO) Glomerular Diseases Work Group. KDIGO 2021 clinical practice guideline for the management of glomerular diseases. Kidney Int. 2021;100(4S):S1–S276.
- Singhal R, Brimble KS. Thromboembolic complications in the nephrotic syndrome: pathophysiology and clinical management. Thromb Res. 2006;118(3):397–407.
- Llach F. Hypercoagulability,renal vein thrombosis,and other thrombotic complications of nephrotic syndrome. Kidney Int. 1985;28(3):429–439.
- Rabelink TJ, Zwaginga JJ, Koomans HA, et al. Thrombosis and hemostasis in renal disease. Kidney Int. 1994;46(2):287–296.
- Bennett A, Cameron JS. Platelet hyperaggregability in the nephrotic syndrome which is not dependent on arachidonic acid metabolism or on plasma albumin concentration. Clin Nephrol. 1987;27(4):182–188.
- Weber J, Olyaei A, Shatzel J. The efficacy and safety of direct oral anticoagulants in patients with chronic renal insufficiency: a review of the literature. Eur J Haematol. 2019;102(4):312–318.
- Robertson L, Yeoh SE, Ramli A. Secondary prevention of recurrent venous thromboembolism after initial oral anticoagulation therapy in patients with unprovoked venous thromboembolism. Cochrane Database Syst Rev. 2017;12(12):CD011088.
- Lee JY, Sung KC, Choi HI. Comparison of aspirin and indobufen in healthy volunteers. Platelets. 2016;27(2):105–109.
- Vinazzer H, Fuccella LM. Clinical pharmacology studies with indobufen (K 3920): inhibitor of platelet aggregation. J Clin Pharmacol. 1980;20(5-6 Pt 1):316–325.
- D'Addato M, Curti T, Bertini D, et al. Indobufen vs acetylsalicylic acid plus dipyridamole in long-term patency after femoropopliteal bypass. Int Angiol. 1992;11(2):106–112.
- Fornaro G, Rossi P, Mantica PG, et al. Indobufen in the prevention of thromboembolic complications in patients with heart disease. A randomized, placebo-controlled, double-blind study. Circulation. 1993;87(1):162–164.
- Huo J, Li M, Liu B, et al. A comparison of indobufen and low-molecular-weight heparin in the prevention of deep vein thrombosis in patients after total hip arthroplasty: a prospective randomized controlled study. Int J Clin Exp Med. 2019;12(3):2720–2728.
- Lou X, Jin J, Gong J, et al. Comparison of the effects of indobufen and warfarin in a rat model of adenine-induced chronic kidney disease. Med Sci Monit. 2019;25:3566–3572.
- Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. Trials. 2010;11(1):1–8.
- Gordon-Cappitelli J, Choi MJ. Prophylactic anticoagulation in adult patients with nephrotic syndrome. Clin J Am Soc Nephrol. 2020;15(1):123–125.
- Farooq V, Hegarty J, Chandrasekar T, et al. Serious adverse incidents with the usage of low molecular weight heparins in patients with chronic kidney disease. Am J Kidney Dis. 2004;43(3):531–537.
- Eriksson BI, Borris LC, Friedman RJ, et al. Rivaroxaban versus enoxaparin for thromboprophylaxis after hip arthroplasty. N Engl J Med. 2008;358(26):2765–2775.
- Pirmohamed M. Warfarin: the end or the end of one size fits all therapy? JPM. 2018;8(3):22.
- Mohan A, Wanat MA, Abughosh SM. Medication taking behaviors in patients taking warfarin versus direct oral anticoagulants: a systematic review. Expert Rev Cardiovasc Ther. 2019;17(6):427–434.
- Hofstra JM, Wetzels JFM. Should aspirin be used for primary prevention of thrombotic events in patients with membranous nephropathy? Kidney Int. 2016;89(5):981–983.
- Baigent C, Blackwell L, Collins R, et al. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet. 2009;373(9678):1849–1860.
- Lensing AW, Prandoni P, Prins MH, et al. Deep-vein thrombosis. Lancet. 1999;353(9151):479–485.
- Fowkes FJI, Price JF, Fowkes FGR. Incidence of DVT diagnosis deep vein thrombosis in the general population: systematic review. Eur J Vasc Endovasc Surg. 2003;25(1):1–5.
- Borghi B, Casati A. Thromboembolic complications after total hip replacement. Int Orthop. 2002;26(1):44–47.
- Barbour SJ, Greenwald A, Djurdjev O, et al. Disease-specific risk of venous thromboembolic events is increased in idiopathic glomerulonephritis. Kidney Int. 2012;81(2):190–195.