Abstract
Background
Acute kidney injury (AKI) in COVID-19 patients is associated with poor prognosis. Characterization of AKI by timing and trajectory and early prediction of AKI progression is required for better preventive management and the prediction of patient outcomes.
Methods
A total of 858 patients who were hospitalized due to coronavirus disease 2019 (COVID-19) were retrospectively enrolled from December 2020 to August 2021. The occurrence of AKI was evaluated throughout hospitalization. The hazard ratios (HRs) of mortality outcomes according to the trajectory of AKI were measured using Cox regression models after adjustment for multiple variables.
Results
Among 858 patients, 226 (26.3%) presented AKI at admission, and 44 (5.1%) developed AKI during hospitalization. Patients with AKI at admission or hospital-acquired AKI had a higher risk of mortality than those without AKI, with HRs of 9.87 (2.81–34.67) and 13.74 (3.57–52.84), respectively. Of 226 patients with AKI at admission, 104 (46.0%) recovered within 48 hr, 83 (36.7%) had AKI beyond 48 hr and recovered in 7 days, and 39 (17.3%) showed no recovery from AKI on Day 7. Delayed recovery and persistent AKI were significantly associated with an increased risk of mortality, with HRs of 4.39 (1.06–18.24) and 24.33 (7.10–83.36), respectively.
Conclusions
The onset and progression of AKI was significantly associated with in-hospital mortality in patients with COVID-19. A thorough observation of the recovery trajectory of early AKI after infection is necessary.
Introduction
Coronavirus disease 2019 (COVID-19) is primarily known to affect respiratory systems, however, the kidney has also been recognized as a vulnerable target organ for infection. The reported prevalence of acute kidney injury (AKI) has been shown to be elevated among individuals diagnosed with COVID-19 in comparison to those without the disease [Citation1]. The occurrence of AKI constitutes a hallmark of COVID-19 and represents a substantial risk factor with the potential to induce unfavorable outcomes among hospitalized patients [Citation2–4].
The pathogenesis behind the impact of COVID-19 on kidney injury is understood to be multifaceted. One possible mechanism of kidney involvement could be similar to that observed during severe acute respiratory syndrome coronavirus (SARS-CoV) infection in the past, including cytokine release syndrome, inter-organ communication, and systemic effects [Citation5–7]. The development of AKI is believed to be the result of both local and systemic inflammatory and immune responses, along with endothelial damage and activation of coagulation pathways and the renin-angiotensin system [Citation8,Citation9]. Although direct kidney infection by SARS-CoV-2 is still a matter of debate, a study largely based on autopsies revealed increased tubulointerstitial fibrosis in COVID-19 patients and suggested a direct kidney infection [Citation10].
Most previous studies of the trajectory after AKI in the pre-COVID era show favorable outcomes after renal recovery compared to nonrecovery, including a lower risk of progression to CKD or mortality [Citation11–14]. However, even in the presence of renal recovery, AKI remains associated with long-term mortality [Citation13,Citation15,Citation16]. Characterization of AKI by timing and trajectory and early prediction of AKI progression might help with risk stratification and allow better preventive management, hospital resource allocation, and patient prognostication in this pandemic. Accordingly, we aimed to investigate patient outcomes according to the time and trajectory of AKI following hospitalization with COVID-19 and to identify risk factors associated with impaired renal recovery.
Method
Patient and data collection
The study was retrospective in nature and included a cohort of 1402 patients who were hospitalized with COVID-19 at a single secondary hospital from December 2020 to August 2021. Among them, patients who were under 18 years old (n = 100) and those who had been on dialysis because of end-stage kidney disease (n = 82) were excluded. Baseline information at the time of admission was not available for 362 patients; thus, a total of 858 patients were ultimately analyzed. The study design was approved by the institutional review board of the Seoul National University Boramae Medical Center (no. 20-2021-88) and complied with the Declaration of Helsinki. The requirement for informed consent was waived by the review board.
Baseline information at the time of admission was collected from electronic medical records and included age, sex, clinical symptoms, comorbid conditions, laboratory data, and ICU admission. The laboratory dataset included complete blood counts, liver function tests, renal function tests, inflammatory markers, and cycle threshold (Ct) values of real-time reverse transcription-polymerase chain reaction (RT–PCR) assays, which are a confirmatory test for COVID-19. Estimated glomerular filtration rate (eGFR) was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation. Underlying chronic kidney disease (CKD) was identified using previous medical records, laboratory data, and patient reports. The study outcome was in-hospital mortality until discharge.
Definitions
The baseline serum creatinine level was defined as a preadmission outpatient creatinine measurement within the 6 months before admission (n = 27), and if not available, the minimum serum creatinine value during hospitalization (n = 831). According to the timing of AKI, patients were divided into two groups: those with AKI at admission and those with hospital-acquired AKI that developed after hospitalization.
The classification of hospital-acquired AKI was defined according to the Kidney Disease: Improving Global Outcomes (KDIGO) criteria as: Stage 1, an increase in serum creatinine level by 0.3 mg/dL within 48 h or a 1.5–1.9-fold increase from baseline within 7 days; Stage 2, a 2.9-fold increase in serum creatinine level within 7 days; and Stage 3, a 3-fold or greater increase in serum creatinine level within 7 days or initiation of renal replacement therapy.
AKI at admission was defined using the retrospective diagnostic criteria proposed in the previous literature as follows: Stage 1, 0.66–0.49 times the reference value; Stage 2, 0.5–0.32 times the reference value; and Stage 3, ≤0.33 times the reference value [Citation17]. The reference value was the creatinine value at admission. In addition, patients with AKI at admission were classified according to the rate of AKI recovery, which was adjudicated based on the serum creatinine level at 7 days after admission. Early recovery was defined as a period of reversal to the baseline creatinine level within 48 h, and delayed recovery was defined as reversal occurring between 48 h and 7 days. Persistent AKI was defined as no recovery to baseline level within 7 days after admission.
Statistical analysis
The expressions of categorical and continuous variables were reported as proportions and means with standard deviations, respectively, if they were found to have a normal distribution based on the results of the Kolmogorov–Smirnov test. For non-normally distributed variables, medians with interquartile ranges were used instead. The chi-square test or Fisher’s exact test was applied to compare categorical variables. In the case of continuous variables, the Student’s t test or Mann–Whitney U test was applied depending on the normality.
Kaplan–Meier survival curves were drawn and compared between groups using the log-rank test. The hazard ratios (HRs) and confidence intervals for hospital mortality were calculated using a Cox proportional hazards regression model in which multiple variables were adjusted. The odds ratios (ORs) and confidence intervals for delayed recovery or persistent AKI in the AKI at admission group were calculated using logistic regression analysis. All statistical analyses were performed using SPSS software (version 27; IBM, Armonk, NY, USA) and R software (version 3.5.1; R core team, Vienna, Austria).
Results
Trajectory of AKI
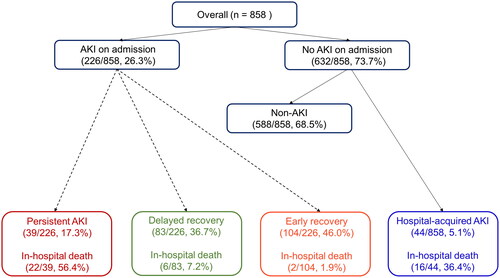
shows the flow chart of all patients according to the course of AKI. Among 858 patients, AKI was present in 226 (26.3%) patients at the time of admission, and 44 (5.1%) patients developed AKI during hospitalization. We classified the 226 patients with AKI at the time of admission into three groups according to renal recovery. Kidney function recovered to baseline within 48 h in 104 patients, and for 83 patients, recovery took more than 48 h. Thirty-three patients did not recover by the 7th day.
Patient characteristics by timing of AKI
The mean age of the patients was 56 ± 20 years, and 37.1% of the patients were male. Patients who developed AKI were more likely to be older and have comorbid diseases such as hypertension, diabetes, and CKD. The difference was more pronounced in the hospital-acquired AKI group. Laboratory findings such as hemoglobin, platelet count, serum albumin, C-reactive protein (CRP) and brain natriuretic peptide (BNP) also showed significant differences between groups. Other baseline characteristics are presented in .
Table 1. Baseline characteristics of the patients by onset time of AKI.
Timing of AKI and mortality outcome
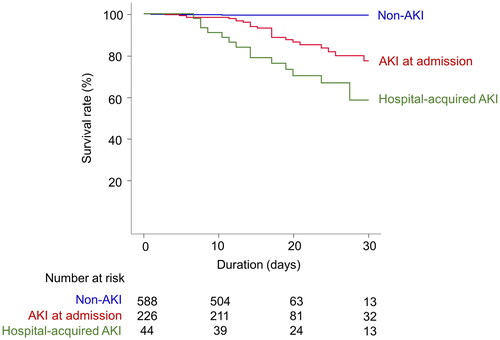
Among a total of 858 patients, 50 patients (5.8%) died. The mortality incidence was 3.8 deaths per 1,000 person-days. In the Kaplan–Meier survival curves, the survival rates differed according to the timing of AKI (long-rank p < 0.001, ). In the Cox proportional hazards regression, the development of AKI was independently associated with mortality outcome after adjustment for multiple variables, and the hazard for mortality was higher in the hospital-acquired AKI group than in the AKI at admission group ().
Table 2. Risks of mortality outcomes according to onset time of AKI.
Patient characteristics by recovery from AKI at admission
Of 226 patients with AKI at admission, 104 (46.0%) recovered within 48 h, 83 (36.7%) had AKI beyond 48 h and recovered in 7 days, and 39 (17.3%) showed no recovery from AKI on Day 7. No recurrence of AKI was observed within the first 7 days. Patients in the delayed recovery or persistent AKI group were more likely to be older and male and have underlying CKD than those in the early recovery group. Laboratory findings such as hemoglobin, platelet count, serum albumin, CRP and BNP also showed significant differences between groups. Other baseline characteristics are presented in .
Table 3. Baseline characteristics of the patients by recovery of AKI at admission.
Recovery from AKI at admission and mortality outcome
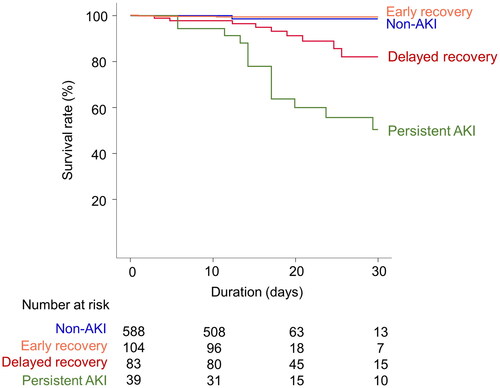
shows the Kaplan–Meier survival curves according to recovery from AKI at admission. There was no significant difference in the survival curves between the patients in the early recovery and non-AKI groups (p = 0.852). However, the delayed recovery group and the persistent AKI group had significantly higher mortality than the non-AKI group (p = 0.005). In the Cox proportional hazards regression model, delayed recovery or persistent AKI significantly increased the risk of hospital mortality ().
Table 4. Hazard ratios for hospital mortality according to recovery from AKI at admission.
Predictive factors for delayed recovery or persistent AKI
shows a logistic regression analysis to identify the predictive factors for either delayed recovery or persistent AKI. Old age, low hemoglobin level, and low platelet count were potential predictive factors of a longer duration of AKI.
Table 5. Odds ratios for delayed recovery or persistent AKI.
Discussion
The present study assessed the trajectory of AKI as a predictive factor for mortality in hospitalized patients with COVID-19. The occurrence of AKI increased the risk of mortality regardless of the time of onset; however, hospital-acquired AKI conferred a higher risk than AKI at admission. Importantly, the duration of AKI at admission was also significantly associated with the risk of mortality.
The incidence of AKI has been reported by previous studies, ranging from 0.5 to 80% among hospitalized patients with COVID-19 [Citation18], and these discrepancies in the reported incidence likely reflect differences in sample sizes and cohort characteristics according to patterns of identifying AKI or the disease severity of COVID-19. Few studies have explored the temporal relationship between SARS-CoV-2 infection and the development of AKI. Some studies reported that AKI developed throughout the hospital stay, at an average of 5 to 9 days after admission [Citation19,Citation20], and other studies reported AKI at hospital admission, with an incidence ranging from 1 to 29% [Citation21,Citation22]. Our data reported that one in four COVID-19 patients presented with AKI at admission, and an additional 6% developed AKI throughout hospitalization. Given that the time from confirmation of COVID-19 to hospitalization was less than a day in this cohort, we found that a considerable proportion of patients developed early AKI after infection. As in many previous studies, although discrepancies exist in the timing of identifying AKI, we found that early AKI after COVID-19 infection is associated with poor short-term survival in COVID-19 patients [Citation23–25].
Additionally, our data demonstrate differences in demographics, comorbidity profiles, and survival outcomes according to the onset time of AKI. Compared with the AKI at admission group, the hospital-acquired AKI group had significantly higher levels of hs-CRP, which represents a systemic inflammatory response, higher levels of BNP, disproportionately severe AKI, and higher mortality. This result was congruent with a previous retrospective study that defined early AKI as AKI onset occurring before the onset date of any other organ dysfunction and late AKI as all other circumstances [Citation26]. In our study, it could be assumed that the difference in mortality rate was attributed to the cause of AKI; AKI at admission was predominantly caused by dehydration or direct viral infection of the kidney, and hospital-acquired AKI occurred with secondary infection or multiorgan failure following acute respiratory distress syndrome (ARDS).
Renal recovery was significantly associated with a low risk of mortality in both non-COVID-19 and COVID-19 patients [Citation13,Citation27–30]. On the other hand, the development of AKI increases the risk of long-term mortality even in the presence of renal recovery [Citation16]. Our finding that persistent AKI at 7 days after admission was associated with a high risk of hospital mortality is consistent with a previous prospective multicenter cohort study [Citation25]; however, we added an analysis by duration of AKI. The duration of AKI intersects with the period of renal recovery. Patients experiencing transient AKI generally exhibit prompt renal recovery, whereas those with prolonged AKI are considered to have a delayed renal function restoration. The Acute Disease Quality Initiative Workgroup, in a consensus report, has defined transient AKI as lasting less than 48 h, while persistent AKI is characterized by a duration that extends beyond 48 h. Furthermore, they have introduced a new term to categorize AKI cases that persist between 7 to 90 days, which is referred to as acute kidney disease [Citation31]. A meta-analysis of 19 global studies has revealed that the duration of AKI significantly impacts long-term mortality, cardiovascular outcomes, and the development of CKD [Citation32]. In the present study, despite the brief follow-up period, it appears that the duration of early AKI provides information on the risk of mortality in patients with COVID-19. Additionally, our results indicate a significant association of old age and anemia with increased adjusted odds of persistent AKI. This suggests that AKI may partially contribute to the elevated mortality risk previously observed among these subgroups in the context of COVID-19 [Citation33–36].
This study provides valuable information, however, it is important to acknowledge the existence of several limitations that warrant attention. The retrospective design of the study may have introduced unmeasured biases and confounders that could have influenced the results of our analysis. Furthermore, the potential impact of differences in practice on mortality outcomes was not evaluated in this study. A history of other diseases or the use of medications, such as treatments for COVID-19, were not included. The cause of death could not be ascertained in the present dataset. Long-term outcomes after discharge according to the trajectory of hospital-acquired AKI were not available in this cohort. Future research on this issue will provide us with more evidence for the risk stratification of COVID-19 survivors.
Conclusions
The occurrence of AKI in patients hospitalized with COVID-19 is prevalent and has a significant correlation with short-term mortality. The progression of early AKI offers significant information for evaluating short-term patient outcomes. Hence, it is recommended to closely monitor renal function shortly after infection in order to classify the risk and effectively prevent the adverse outcomes in COVID-19 patients.
Ethical approval
This study was approved by the institutional review board of Seoul National University Boramae Medical Center (no. 20-2021-88) and complied with the Declaration of Helsinki. The requirement for informed consent was waived by the review board.
Author contributions
SGK and JPL designed this study. SGK, SK, and YK analyzed the data. CHH, SBY, HL, and BGK collected the data. JL, YKO, DKK, CSL and YSK provided a critical reading of the manuscript. SGK and JPL wrote the manuscript. All authors approved the final manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data are available from the authors upon request.
Additional information
Funding
References
- Fisher M, Neugarten J, Bellin E, et al. AKI in hospitalized patients with and without COVID-19: a comparison study. J Am Soc Nephrol. 2020;31(9):2145–2157.
- Cheng Y, Luo R, Wang K, et al. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int. 2020;97(5):829–838.
- Hirsch J, Ng J, Ross D, Northwell Nephrology COVID-19 Research Consortium, et al. Northwell COVID-19 research consortium; northwell nephrology COVID-19 research consortium. Acute kidney injury in patients hospitalized with COVID-19. Kidney Int. 2020;98(1):209–218.
- Xu H, Garcia-Ptacek S, Annetorp M, et al. Acute kidney injury and mortality risk in older adults with COVID-19. J Nephrol. 2021;34(2):295–304.
- Ronco C, Reis T. Kidney involvement in COVID-19 and rationale for extracorporeal therapies. Nat Rev Nephrol. 2020;16(6):308–310.
- Bhatia M, Moochhala S. Role of inflammatory mediators in the pathophysiology of acute respiratory distress syndrome. J Pathol. 2004;202(2):145–156.
- Legrand M, Bell S, Forni L, et al. Pathophysiology of COVID-19-associated acute kidney injury. Nat Rev Nephrol. 2021;17(11):751–764.
- Golmai P, Larsen CP, DeVita MV, et al. Histopathologic and ultrastructural findings in postmortem kidney biopsy material in 12 patients with AKI and COVID-19. J Am Soc Nephrol. 2020;31(9):1944–1947.
- Sharma P, Uppal NN, Wanchoo R, Northwell Nephrology COVID-19 Research Consortium, et al. COVID-19–associated kidney injury: a case series of kidney biopsy findings. J Am Soc Nephrol. 2020;31(9):1948–1958.
- Jansen J, Reimer KC, Nagai JS, et al. SARS-CoV-2 infects the human kidney and drives fibrosis in kidney organoids. Cell Stem Cell. 2022;29(2):217–231.e8.
- Fiorentino M, Tohme FA, Wang S, et al. Long-term survival in patients with septic acute kidney injury is strongly influenced by renal recovery. PLOS One. 2018;13(6):e0198269.
- Bagshaw SM, Laupland KB, Doig CJ, et al. Prognosis for long-term survival and renal recovery in critically ill patients with severe acute renal failure: a population-based study. Critical Care. 2005;9(6):1–10.
- Pannu N, James M, Hemmelgarn B, Network AKD., et al. Association between AKI, recovery of renal function, and long-term outcomes after hospital discharge. Clin J Am Soc Nephrol. 2013;8(2):194–202.
- Kellum JA, Chawla LS, Keener C, ProCESS and ProGReSS-AKI Investigators, et al. The effects of alternative resuscitation strategies on acute kidney injury in patients with septic shock. Am J Respir Crit Care Med. 2016;193(3):281–287.
- Hobson CE, Yavas S, Segal MS, et al. Acute kidney injury is associated with increased long-term mortality after cardiothoracic surgery. Circulation. 2009;119(18):2444–2453.
- Ozrazgat-Baslanti T, Loftus TJ, Ren Y, et al. Association of persistent acute kidney injury and renal recovery with mortality in hospitalised patients. BMJ Health Care Informat. 2021;28(1):e100458.
- Duff S, Murray PT. Defining early recovery of acute kidney injury. Clin J Am Soc Nephrol. 2020;15(9):1358–1360.
- Robbins-Juarez SY, Qian L, King KL, et al. Outcomes for patients with COVID-19 and acute kidney injury: a systematic review and meta-analysis. Kidney Int Rep. 2020;5(8):1149–1160.
- Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062.
- Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8(5):475–481.
- Du Y, Tu L, Zhu P, et al. Clinical features of 85 fatal cases of COVID-19 from Wuhan. A retrospective observational study. Am J Resp Crit Care Med. 2020;201(11):1372–1379.
- Guan W-J, Ni Z-y, Hu Y, China Medical Treatment Expert Group for Covid-19, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720.
- Nadim MK, Forni LG, Mehta RL, et al. COVID-19-associated acute kidney injury: consensus report of the 25th acute disease quality initiative (ADQI) workgroup. Nat Rev Nephrol. 2020;16(12):747–764.
- Qian J-Y, Wang B, Liu B-C. Acute kidney injury in the 2019 novel coronavirus disease. Kidney Diseases. 2020;6(5):318–323.
- Wan YI, Bien Z, Apea VJ, et al. Acute kidney injury in COVID-19: multicentre prospective analysis of registry data.Clin Kidney J. 2021;14(11):2356–2364.
- Peng S, Wang H-Y, Sun X, et al. Early versus late acute kidney injury among patients with COVID-19—a multicenter study from Wuhan, China.Nephrol Dial Transplant. 2020;35(12):2095–2102.
- Sun S, Annadi RR, Chaudhri I, et al. Short-and Long-Term recovery after moderate/severe AKI in patients with and without COVID-19. Kidney360. 2022;3(2):242–257.
- Thongprayoon C, Cheungpasitporn W, Srivali N, et al. The association between renal recovery after acute kidney injury and long-term mortality after transcatheter aortic valve replacement. PLoS One. 2017;12(8):e0183350.
- Mehta RH, Honeycutt E, Patel UD, et al. Impact of recovery of renal function on long-term mortality after coronary artery bypass grafting. Am J Cardiol. 2010;106(12):1728–1734.
- Pei G, Zhang Z, Peng J, et al. Renal involvement and early prognosis in patients with COVID-19 pneumonia. J Am Soc Nephrol. 2020;31(6):1157–1165.
- Chawla LS, Bellomo R, Bihorac A, Acute Disease Quality Initiative Workgroup 16, et al. Acute kidney disease and renal recovery: consensus report of the acute disease quality initiative (ADQI) 16 workgroup. Nat Rev Nephrol. 2017;13(4):241–257.
- Mehta S, Chauhan K, Patel A, et al. The prognostic importance of duration of AKI: a systematic review and meta-analysis. BMC Nephrology. 2018;19(1):10.
- Zhang J, Cao Y, Tan G, et al. Clinical, radiological, and laboratory characteristics and risk factors for severity and mortality of 289 hospitalized COVID‐19 patients. Allergy. 2021;76(2):533–550.
- Wolff D, Nee S, Hickey NS, et al. Risk factors for covid-19 severity and fatality: a structured literature review. Infection. 2021;49(1):15–28.
- Zhang J, Wang X, Jia X, et al. Risk factors for disease severity, unimprovement, and mortality in COVID-19 patients in Wuhan, China. Clin Microbiol Infect. 2020;26(6):767–772.
- Tao Z, Xu J, Chen W, et al. Anemia is associated with severe illness in COVID‐19: a retrospective cohort study. J Med Virol. 2021;93(3):1478–1488.