Abstract
Purpose
This cohort study was designed to explore whether roxadustat or erythropoietin could affect thyroid function in patients with renal anemia.
Methods
The study involved 110 patients with renal anemia. Thyroid profile and baseline investigations were carried out for each patient. The patients were divided into two groups: 60 patients taking erythropoietin served as the control group (rHuEPO group) and 50 patients using roxadustat served as the experimental group (roxadustat group).
Results
The results indicated that there were no significant differences in serum total thyroxine (TT4), total triiodothyronine (TT3), free triiodothyronine (FT3), free thyroxine (FT4) or thyroid stimulating hormone (TSH) between the two groups at baseline. After treatment, TSH, FT3, and FT4 were significantly lower in the roxadustat group than in the rHuEPO group (p < 0.05). After adjusting for age, sex, dialysis modality, thyroid nodules and causes of kidney disease, Cox regression showed that roxadustat was an independent influencing factor on thyroid dysfunction (HR 3.37; 95% CI 1.94–5.87; p < 0.001). After 12 months of follow-up, the incidence of thyroid dysfunction was higher in the roxadustat group than in the rHuEPO group (log-rank p < 0.001).
Conclusion
Roxadustat may lead to a higher risk of thyroid dysfunction, including low TSH, FT3 and FT4, than rHuEPO in patients with renal anemia.
Introduction
Chronic kidney disease (CKD) is a serious public health challenge wordwide. In China, approximately 10.8% of patients with CKD are affected by renal anemia [Citation1]. Renal anemia is most commonly associated with CKD and may affect the quality of life of patients with CKD, increasing the morbidity and mortality associated with cardiovascular disease and the risk of hospitalization [Citation2]. Abnormal iron metabolism in the kidney and impaired kidney erythropoietin are the main causes of renal anemia [Citation3]. The current standard of care for renal anemia focuses on the above two causes including the use of iron, recombinant human EPO (rHuEPO), and their combinations [Citation3]. Nevertheless, the use of rHuEPO requires intravenous or subcutaneous injections, which may increase pain for the patient and needle sticks for the nurse. Hypoxia inducible factor (HIF) increases endogenous erythropoietin in CKD patients [Citation4] and is rapidly degraded by HIF proline dehydrogenase (HIF-PH). Therefore, HIF-PH inhibition can stabilize HIF, which is considered a new strategy for the treatment of renal anemia. Clinical trials have shown that roxadustat, a novel orally administered hypoxia-inducible factor prolyl hydroxylase inhibitor, can be used to treat anemia in dialysis or nondialysis patients and is noninferior to EPO [Citation5,Citation6].
The association between thyroid function and kidney disease has been an important concern in recent decades. On the one hand, thyroid hormones play a major role in kidney development, such as regulating the major functions of glomeruli and kidney tubules. On the other hand, the kidneys are important for the metabolism and elimination of thyroid hormones [Citation7]. Clinically, end-stage kidney disease (ESKD) is a risk factor for thyroid disorders, including hypothyroidism and euthyroid sick syndrome (ESS) [Citation8]. All these efforts to assess thyroid dysfunction were because it might lead to high mortality [Citation9,Citation10], cardiovascular disease [Citation9,Citation10], and impaired health-related quality of life (HRQOL) [Citation11,Citation12] in CKD patients. Cardiovascular disease-related mortality in dialysis patients is approximately 45%, and hypothyroidism leads to accelerated atherosclerosis, which is considered a possible cardiovascular risk factor in this population [Citation9].
At present, roxadustat is available for the treatment of renal anemia in China. Two early clinical studies published in the New England Journal of Medicine showed that compared with the placebo group, the roxadustat group had a higher frequency of hyperkalemia and metabolic acidosis, but these studies did not evaluate thyroid related adverse reactions [Citation5,Citation6]. Recently, it was noticed that roxadustat might affect thyroid function in dialysis patients, which might be related to interference with the homeostasis of the hypothalamus-pituitary-thyroid axis [Citation13,Citation14]. Thyroid hormone is controlled by the strict feedback regulation of the hypothalamus-pituitary-thyroid axis. Thyroid hormone receptor (THR) plays an important role in the biological activity of thyroid hormone. THR and retinoid X receptor (RXR) form a heterodimer in the gene response element of thyroid hormone, and jointly activate promoter and gene transcription after binding with T3. Roxadustat has a molecular structure similar to that of T3, and the affinity between roxadustat and THR is higher than that between T3 and THR. Therefore, it is speculated that roxadustat, as a THR β agonist, can inhibit the secretion of TSH, resulting in a reduction in serum FT4 and FT3 levels [Citation15]. It is well known that there is a correlation between CKD and thyroid dysfunction related to thyroid hormone and eGFR, age, sex, and immune nephropathy in CKD patients, but substantial data are still lacking. Therefore, we tried to explore the factors influencing the relationship between CKD and thyroid function, specifically, we tried to illustrate the relationship between roxadustat or erythropoietin and thyroid function.
Materials and methods
Participants
We analyzed data from patients treated at Ningbo First Hospital from 2020 to 2021. The inclusion criteria were as follows: (I) patients over 18 years old admitted due to renal anemia; (II)the presence of follow-up and laboratory examination data at baseline and within 1–12 months after treatment; and (III)patients who started treatment with standard doses of roxadustat or rHuEPO. Patients were excluded for the following reasons: (I) medications that are known to affect thyroid function such as thyroxine, antithyroid drugs, amiodarone or lithium; (II) untreated adrenal insufficiency or adrenal diseases; and (III) hypothyroidism or hyperthyroidism.
Study design
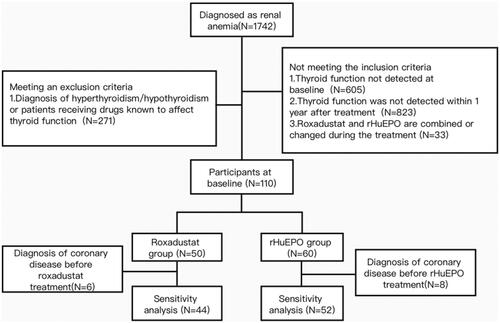
A total of 110 patients with renal anemia were included in the analysis. The flow chart is shown in . The basic information and clinical data for each patient were obtained from their medical documents. Patients receiving roxadustat were the experimental group (n = 50), and those receiving rHuEPO were the control group (n = 60). The changes in TSH, TT3, TT4, FT3 and FT4 at baseline and after 12 months were evaluated in each group. The study protocol was approved and supervised by the Ethics Committee of Ningbo First Hospital (2022-011RS), and the study was performed in accordance with the guidelines of the Declaration of Helsinki. Each participant was followed up until the first corresponding outcome, or follow-up failure, which occurred first prior to October 31, 2022.
Laboratory assays
Blood samples were collected, and thyroid function was tested, by evaluating the levels of thyroid stimulating hormone (TSH), total triiodothyronine (TT3), serum total thyroxine (TT4), total triiodothyronine (TT3), free triiodothyronine (FT3) and free T4(FT4). Serum levels of TSH, TT3, TT4, FT3 and FT4 were determined by automated chemiluminescent immunoassays (Abbott i2000, Wiesbaden, Germany). Laboratory reference ranges were as follows: TSH, 0.56–5.91 μIU/ml; TT3, 1.01–2.48 nmol/l; TT4, 69.97–152.52 nmol/l; FT3, 3.28–6.47 pmol/l; FT4, 7.64–16.03 pmol/l. Thyroid dysfunction was defined as a decrease in TSH, FT3 or FT4.
Statistical analysis
Baseline characteristics were compared among participants with different treatments for renal anemia. The end date was defined as the date of a reduction in TSH/FT3/FT4 events or 12 months of follow-up, whichever occurred first. Cox proportional hazard models were used to assess the associations between treatment modality (roxadustat or rHuEPO) and thyroid dysfunction. Hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated. Multivariable regression models were constructed with adjustment for (1) age and sex; and (2) age, sex, dialysis mode, thyroid nodule, and causes of kidney disease.
We performed the analyses by excluding patients with coronary disease for sensitivity analysis. Subgroup analyses were also performed to assess the potential impact on the association of treatments (Roxadustat or rHuEPO) with thyroid dysfunction, including age (<65 years, ≥65 years), sex, dialysis mode (nondialysis, peritoneal dialysis, hemodialysis), thyroid nodule (no, yes), and causes of kidney disease (nonimmune, immune).
For continuous variables, normally distributed data are presented as the mean ± SD. The comparison between the two groups was performed with an independent sample t test or corrected t test. Skewed distribution data are presented as M (P25, P75). The Mann-Whitney U test was used for the comparison between the two groups. Categorical variables are presented as total numbers (proportions). The chi-square test or Fisher’s exact test was used for intergroup comparisons. Kaplan-Meier survival curves were used to describe the incidence of thyroid dysfunction, and the log-rank test was used for intergroup comparisons. All statistical analyses were performed using SPSS 22.0 software. Differences were considered statistically significant when p < 0.05.
Results
Baseline characteristics of the study population
A total of 110 patients were included in this observational study, 60 treated with rHuEPO and 50 treated with roxadustat. We reviewed the last examination data before starting medication as the baseline data. The general characteristics of the participants are listed in . There were no significant differences in the distribution of the rHuEPO and roxadustat groups in terms of age, sex, dialysis mode, thyroid nodules, and causes of kidney disease. Thyroid stimulating hormone was not significantly different between patients treated with rHuEPO and those treated with roxadustat (2.18(1.56,3.83) vs. 2.21(1.55,3.44) μIU/ml, p = 0.962), and TT3, TT4, FT3 and FT4 were also comparable between the two groups (). The mean baseline hemoglobin was 8.19 g/dl and 8.18 g/dl for the rHuEPO and roxadustat groups, respectively, and after 3 months of treatment, the mean hemoglobin was increased in the rHuEPO and roxadustat groups (9.93 ± 1.28 vs. 10.06 ± 1.47 g/dl, p = 0.634) (Table S1).
Table 1. Comparison of baseline characteristics in subjects with rHuEPO and roxadustat treatment in patients with renal anemia.
Comparison of serum TSH, TT3, TT4, FT3 and FT4 between the rHuEPO and roxadustat groups
There were no significant differences in TSH, FT3 and FT4 between the rHuEPO and roxadustat groups baseline, but the differences were significant after treatment (TSH: z=-3.32, p = 0.001; FT3: t = 2.56, p = 0.012; FT4: t = 2.51, p = 0.014) (). The delta changes in individual patients from baseline to the end of follow-up also showed that TSH, FT3 and FT4 were significantly reduced in most patients at the end of follow-up in the roxadustat group, but were not obvious in the rHuEPO group (Figure S2).
Table 2. Comparison of serum TSH, TT3, TT4, FT3 and FT4 between the rHuEPO and roxadustat groups.
Association between roxadustat and the occurrence of thyroid dysfunction
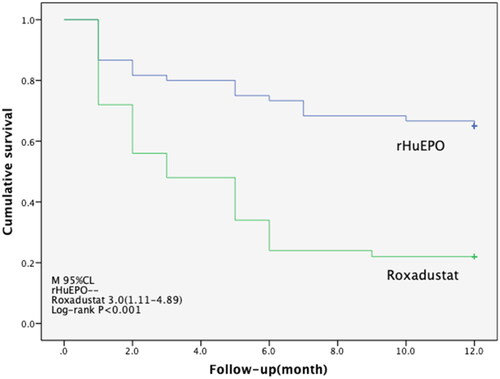
During a median follow-up of 3 months, 36 cases of thyroid dysfunction were identified in the roxadustat group (95% Cl 1.11–4.89), but 21cases were identified in the rHuEPO group. The difference in the cumulative incidence curves between the two groups was statistically significant, as shown in (log-rank p < 0.001). In the time-dependent Cox proportional hazards model, after adjusting for age, sex, dialysis mode, thyroid nodules, and causes of kidney disease, it was found that there was a statistically significant association between treatment methods and the incidence of thyroid dysfunction (HR 3.37; 95% CI 1.94–5.87; p < 0.001) ().
Table 3. Association between roxadustat and the occurrence of thyroid dysfunction.
Sensitivity analyses
The correlations between roxadustat and thyroid dysfunction remained when participants with coronary disease were excluded. Compared with the rHuEPO group, participants in the roxadustat group had a 2.66 (95% CI: 1.47–4.83; p = 0.001) times increased risk of thyroid dysfunction. The association between roxadustat and thyroid dysfunction did not differ significantly by age (<65 years, ≥65 years), sex, dialysis mode (nondialysis, peritoneal dialysis, hemodialysis), thyroid nodule (no, yes), or causes of kidney disease (nonimmune, immune) (). If the dialysis mode was divided into dialysis and nondialysis, the relationship between roxadustat and thyroid dysfunction remained unchanged, and the effect of dialysis on hypothyroidism was still not statistically significant in our study (Table S2).
Table 4. Interaction between the treatments of renal anemia and basic information on the incidence of thyroid dysfunction.
Discussion
In our cohort study, we found that roxadustat use in the treatment of renal anemia was positively associated with a higher risk of thyroid dysfunction, which was independent of age, sex, dialysis modality, etc. There was a 2.37-fold increase in the incidence of thyroid dysfunction in the roxadustat group compared to the rHuEPO group, including a decrease in TSH, FT3 and FT4. However, for FT3, we found decreasing levers in the rHuEPO group as well (t = -3.30, p = 0.002).
Roxadustat was the first HIF-PHI drug approved for the treatment of renal anemia in China. Its advantage was probably related to hepcidin, including effectively improving iron metabolism and treatment was not affected by the inflammatory state [Citation16]. Hepcidin is an important factor for iron homeostasis and the inflammatory response. Inflammatory factors such as TNF- α, IFN- γ and IL-6 increase the expression of hepcidin, and roxadustat may regulate anemia due to inflammation by downregulating the level of hepcidin [Citation17,Citation18]. Several recent clinical trials have shown that roxadustat can increase hemoglobin concentrations in patients on or off dialysis [Citation5,Citation6,Citation19], and roxadustat may be associated with hyperkalemia or metabolic acidosis in participants. Nevertheless, no clinical trials have focused on the changes in thyroid function with roxadustat. The kidneys are essential for thyroid hormone metabolism, and our study found that roxadustat may be a risk factor for thyroid dysfunction in patients with renal anemia, which was in accordance with some previous reports [Citation13–15]. Two recent cases reported that roxadustat was significantly associated with thyroid-stimulating hormone suppression [Citation13,Citation14].
In our study, the level of FT3 was also decreased in the rHuEPO group, and overt hypothyroidism that is clinically relevant was not present. A similar result was found in Pan’s study of the relationships between CKD and thyroid dysfunction [Citation20]. We speculate that FT3 reduction may occur in patients with CKD who are treated with roxadustat or rHuEPO. Underlying mechanisms of reduced FT3 levels include multiple pathways, such as metabolic acidosis, protein losses and dietary factors [Citation21,Citation22]. However, perhaps the most essential is the decrease in the peripheral synthesis of T3 from T4. On the other hand, we found that FT3 was lower in the roxadustat group than in the rHuEPO group.
Controversies still exist regarding whether the basic characteristics of patients might be associated with abnormal thyroid function. Some studies have shown a higher rate of thyroid abnormalities in females [Citation8], while in this study, the results were insignificant [Citation23,Citation24]. The prevalence of hypothyroidism was higher in dialysis patients, and no significant difference was found between peritoneal dialysis and hemodialysis patients [Citation24]. However, our subgroup analysis showed that dialysis was not related to thyroid dysfunction. Jain et al. reported that the prevalence of hypothyroidism in nephrotic syndrome patients was greater than that in other populations [Citation25]. However, in our study, we did not find a significant correlation between them.
To our knowledge, this is the first report of an association of roxadustat with thyroid dysfunction in anemia patients. We provided strong evidence through a 1-year follow-up in patients with renal anemia. This association can be explained by the following mechanisms. First, roxadustat has a structure similar to triiodothyronine (T3), but notably, a unique structural feature of roxadustat different from T3 is its extended hydrophobic phenyl group, suggesting that a larger hydrophobic cavity is needed for effective ligand binding (Figure S1). Interestingly, helix 10 of THRb shifts outward to make extra space for the phenyl extension. Therefore, like FT3, roxadustat occupies a binding site in the thyroid hormone receptor β (THRβ) pocket, and is stabilized by a combination of hydrogen bonds and hydrophobic interactions [Citation15]. Therefore, similar to competitive inhibition, roxadustat acts as an agonist of THR β and produces an allosteric effect in the pituitary gland and/or hypothalamus, possibly suppressing TSH secretion and resulting in decreased serum FT3 and FT4 levels. Second, roxadustat is effective for increasing the expression of HIF-1a, which could suppress metabolism. Metabolism is closely related to thyroid function [Citation26].
The strengths of our study include the breadth of the population design, including dialysis and nondialysis patients, and the completeness of participant information, including a wide range of potential confounding factors. Therefore, the results of the present study might be useful for further studies of roxadustat in the treatment of renal anemia, focusing on the effect of roxadustat on thyroid function.
Limitations of cohort studies should be considered. In this study, we observed a correlation between roxadustat and thyroid function in patients with renal anemia, but it was difficult to verify a causal relationship between them. Selection bias could not be completely avoided. Moreover, the small number of participants in this study could have reduced the statistical power to detect differences between the two groups. Therefore, caution is needed in interpreting the study results, including the association between roxadustat and thyroid dysfunction. Further prospective, long-term, randomized clinical studies need to be performed.
Conclusion
This cohort study of renal anemia found that roxadustat was associated with a risk of thyroid dysfunction. This correlation was not related to age, sex, dialysis mode, thyroid nodule, or causes of kidney disease.
Supplemental Material
Download PDF (365.3 KB)Acknowledgement
The technical support by Hong-bin Xu is greatly appreciated.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Zhang L, Wang F, Wang L, et al. Prevalence of chronic kidney disease in China: a cross-sectional survey. Lancet. 2012;379(9818):1–7.
- Drozdz M, Weigert A, Silva F, et al. Achievement of renal anemia KDIGO targets by two different clinical strategies-a european hemodialysis multicenter analysis. BMC Nephrol. 2019;20(1):5.
- Mikhail A, Brown C, Williams JA, et al. Renal association clinical practice guideline on anaemia of chronic kidney disease. BMC Nephrol. 2017;18(1):345.
- Gupta N, Wish JB. Hypoxia-Inducible factor prolyl hydroxylase inhibitors: a potential new treatment for anemia in patients with CKD. Am J Kidney Dis. 2017;69(6):815–826.
- Chen N, Hao C, Liu BC, et al. Roxadustat treatment for anemia in patients undergoing Long-Term dialysis. N Engl J Med. 2019;381(11):1011–1022.
- Chen N, Hao C, Peng X, et al. Roxadustat for anemia in patients with kidney disease not receiving dialysis. N Engl J Med. 2019;381(11):1001–1010.
- Rhee CM. The interaction between thyroid and kidney disease: an overview of the evidence. Curr Opin Endocrinol Diabetes Obes. 2016;23(5):407–415.
- Naseem F, Mannan A, Dhrolia MF, et al. Prevalence of subclinical hypothyroid- dism in patients with chronic kidney disease on maintenance hemodialysis. Saudi J Kidney Dis Transpl. 2018;29(4):846–851.
- Rhee CM, Kim S, Gillen DL, et al. Association of thyroid functional disease with mortality in a national cohort of incident hemodialysis patients. J Clin Endocrinol Metab. 2015;100(4):1386–1395.
- Xu H, Brusselaers N, Lindholm B, et al. Thyroid function test derangements and mortality in dialysis patients: a systematic review and meta-analysis. Am J Kidney Dis. 2016;68(6):923–932.
- Rhee CM, Chen Y, You AS, et al. Thyroid status, quality of life, and mental health in patients on hemodialysis. Clin J Am Soc Nephrol. 2017;12(8):1274–1283.
- Kalantar SS, You AS, Norris KC, et al. The impact of race and ethnicity upon Health-Related quality of life and mortality in dialysis patients. Kidney Med. 2019;1(5):253–262.
- Tokuyama A, Kadoya H, Obata A, et al. Roxadustat and thyroid-stimulating hormone suppression. Clin Kidney J. 2021;14(5):1472–1474.
- Ichii M, Mori K, Miyaoka D, et al. Suppression of thyrotropin secretion during roxadustat treatment for renal anemia in a patient undergoing hemodialysis. BMC Nephrol. 2021;22(1):104.
- Yao B, Wei Y, Zhang S, et al. Revealing a Mutant-Induced receptor allosteric mechanism for the thyroid hormone resistance. iScience. 2019;20:489–496.
- Maxwell PH. A new approach to treating renal anaemia. Nat Rev Nephrol. 2019;15(12):731–732.
- Mima A. Hypoxia-inducible factor-prolyl hydroxylase inhibitors for renal anemia in chronic kidney disease: advantages and disadvantages. Eur J Pharmacol. 2021;912:174583.
- Weiss G, Ganz T, Goodnough LT. Anemia of inflammation. Blood. 2019;133(1):40–50.
- Mima A, Horii Y. Treatment of renal anemia in patients with hemodialysis using hypoxia-inducible factor (HIF) stabilizer, roxadustat: a short-term clinical study. In Vivo. 2022;36(4):1785–1789.
- Pakfetrat M, Dabbaghmanesh MH, Karimi Z, et al. Prevalence of hypothyroid -dism and thyroid nodule in chronic hemodialysis iranian patients. Hemodial Int. 2017;21(1):84–89.
- Sanai T, Okamura K, Rikitake S, et al. The high prevalence of reversible subclinical hypothyroidism with elevated serum thyroglobulin levels in chronic kidney disease patients. CN. 2017;87(05):237–244.
- Narasaki Y, Sohn P, Rhee CM. The interplay between thyroid dysfunction and kidney disease. Semin Nephrol. 2021;41(2):133–143.
- Pan B, Du X, Zhang H, et al. Relationships of chronic kidney disease and thyroid dysfunction in Non-Dialysis patients: a pilot study. Kidney Blood Press Res. 2019;44(2):170–178.
- Nazzal ZA, Khazneh EN, Rabi RA, et al. Prevalence of hypothyroidism among dialysis patients in palestine: a Cross-Sectional study. Int J Nephrol. 2020;2020:2683123.
- Jain D, Aggarwal HK, Pavan Kumar YM, et al. Evaluation of thyroid dysfunc-tion in patients with nephrotic syndrome. Med Pharm Rep. 2019;92(2):139–144.
- Varghese T, Rejish Kumar VJ, Anand G, et al. Dietary GABA enhances hypoxia tolerance of a bottom-dwelling carp, cirrhinus mrigala by modulating HIF-1α, thyroid hormones and metabolic responses. Fish Physiol Biochem. 2020;46(1):199–212.