Abstract
Purpose
The purpose of this study was to investigate the association between serum proprotein convertase subtilisin/kexin type 9 (PCSK9) levels and renal function impairment in type 2 diabetes mellitus (T2DM) patients.
Methods
PCSK9 levels were measured in T2DM patients, streptozotocin plus high-fat diet (STZ + HFD) mice, human proximal tubular epithelial (HK-2) cells treated with high glucose plus palmitic acid (HGPA) and the corresponding control groups. The T2DM patients were further divided into three groups according to serum PCSK9 levels. An analysis of clinical data was conducted, and a binary logistic regression model was used to test the relationship between potential predictors and urine albumin/urine creatinine ratio (UACR) and estimated glomerular filtration rate (eGFR).
Results
PCSK9 levels were higher in the DM group than in the control group in humans, mice and HK-2 cells. The systolic blood pressure (SBP), serum creatinine (Scr), blood urea nitrogen (BUN), triglyceride (TG), and urine α1-MG/urine creatinine ratio (UαCR) values in PCSK9 tertile 3 were significantly higher than those in PCSK9 tertile 1 (p < 0.05). The DBP and UACR values were significantly higher in PCSK9 tertile 3 than in PCSK9 tertile 1 and PCSK9 tertile 2 (both p < 0.05). In addition, URCR values were significantly higher in PCSK9 tertile 3 and PCSK9 tertile 2 than in PCSK9 tertile 1 (both p < 0.05). Serum PCSK9 levels were positively correlated with SBP, Scr, BUN, TG, URCR, UαCR and UACR but inversely correlated with eGFR. In STZ + HFD mice, serum PCSK9 levels were positively correlated with Scr, BUN and UACR, which was consistent with the findings in the patients. A logistic regression model revealed that serum PCSK9 is an independent risk factor for UACR ≥30 mg/g and eGFR <60 mL/min/1.73 m2. The ROC curve showed that 170.53 ng/mL and 337.26 ng/mL PCSK9 were the best cutoff values for UACR ≥30 mg/g and eGFR <60 mL/min/1.73 m2, respectively.
Conclusion
Serum PCSK9 levels are associated with renal function impairment in T2DM patients and in some patients lower PCSK9 may be helpful to decrease chronic kidney disease.
Introduction
Diabetes mellitus (DM) is a chronic metabolic disorder. In 2021, there were approximately 536.6 million people with DM in the world, accounting for 10.5% of the adult population. China has the highest number of DM patients in the world. The number of DM patients reached 140.9 million, which is expected to increase to 174.4 million in 2045. More than 90% of DM patients have type 2 diabetes mellitus (T2DM) [Citation1]. With the global prevalence of T2DM, diabetic kidney disease (DKD) has become the leading cause of end-stage renal disease (ESRD) and poses a great burden to diabetes patients and the health care system [Citation2,Citation3]. In diabetes-related renal function impairment, multifactorial pathological processes contribute, including advanced glycation end products (AGEs) and ectopic lipid deposition [Citation4,Citation5]. It remains necessary, however, to explore the risk factors for the progression of renal function impairment in T2DM patients as a means of promoting clinical diagnosis and treatment.
PCSK9 is a serine kinase that is mainly synthesized as zymogen in the endoplasmic reticulum of hepatocytes, and then secreted into plasma after autocatalytic shearing. It can also be synthesized in the intestine and kidney [Citation6–8]. A close relationship between PCSK9 and DM patients has been identified. An analysis by Cui et al. [Citation9] found a positive correlation between fasting blood glucose levels and PCSK9. In addition to fasting blood glucose levels, other studies indicated that plasma PCSK9 levels were positively correlated with insulin levels and the insulin resistance index [Citation10]. Plasma PCSK9 levels were also positively correlated with HbA1C levels in patients with T2DM [Citation11,Citation12]. Additionally, PCSK9 is closely related to macrovascular complications in DM. Patients with high PCSK9 levels were 2.294 times more likely to suffer cardiovascular events than those with low PCSK9 levels after adjusting for established cardiovascular and metabolic factors. There was a lower survival rate among DM patients with high PCSK9 levels over a median follow-up of 3.3 years[Citation12]. In basic research, the injection of AAV8-PCSK9 into db/db mice induced diabetic atherosclerosis and fatty liver in a time-dependent manner [Citation13]. The anti-PCSK9 vaccine improved glucose and insulin tolerance impairments and the lipid profile in STZ-induced DM [Citation14]. In prediabetic ovariectomized rats, PCSK9 inhibitors attenuated cardiometabolic impairment [Citation15]. Moreover, Elewa et al. reported that therapy with lipid-lowering drugs, especially the fibrate/statin combination, was independently associated with higher PCSK9 levels in DKD [Citation16].
The majority of studies on PCSK9 and DM focus on macrovascular complications, such as glucose and lipid metabolism and cardiovascular disease. Few studies have been conducted on DKD as a microvascular complication of DM and PCSK9. In this study, based on T2DM patients and HFD-fed STZ mice, we investigated the correlation between PCSK9 and renal injury in T2DM and its influencing factors.
Methods
Subjects
Thirty-seven healthy participants were recruited from the health examination center of Third Xiangya Hospital, Central South University. There were no metabolic disorders, kidney injuries, or other diseases among the healthy participants. A total of 145 T2DM patients were recruited from the Third Xiangya Hospital, Central South University, from June 2019 to January 2020. All participants (18-80 years of age) with T2DM satisfied the 2020 Guidelines for the Prevention and Treatment of Type 2 Diabetes in China when diagnosed [Citation17] (). This study was approved by the medical research ethics committee of the Third Xiangya Hospital, Central South University and carried out under the principles of the Declaration of Helsinki (approval number: 22156). All participants signed informed consent voluntarily.
Clinical data collection
All data were collected from patient record files. Detailed medical history and physical examination data, including sex, age, height, weight, duration of diabetes, SBP and diastolic blood pressure (DBP), were obtained upon admission. All of the biochemical and serological markers, including BUN, SCr, serum uric acid (sUA), aspartate aminotransferase (AST), alanine aminotransferase (ALT), glycosylated hemoglobin (HbA1c), fasting blood glucose (FBG), albumin (ALB), hemoglobin (Hb), TG, total cholesterol (TC), high-density lipoprotein (HDL-C), and low-density lipoprotein (LDL-C), were measured. We calculated eGFR using Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) [Citation18].
Grouping
Subjects were grouped by tertiles of the PCSK9 level as follows [Citation19]: (i) tertile 1 (n = 48) with PCSK9 < 138.99 ng/mL; (ii) tertile 2 (n = 48) with PCSK9 in the range of 138.99-226.26 ng/mL; and (iii) tertile 3 (n = 49) with PCSK9 > 226.26 ng/mL.
Animal experimental design
A total of 15 four-week-old C57BL/6 mice (∼20 g B. W) were purchased from HUNAN SJA Laboratory Animal Co. LDT (Hunan, China), and then, they were divided randomly into two groups. The first group (n = 6) was fed a normal diet sustainably for 16 weeks. The second group (n = 9) was fed HFD. After 4 weeks of HFD feeding, mice in Group 2 were intraperitoneally injected with STZ for 5 consecutive days (40 mg/kg body weight). One week after the final injection, blood glucose levels were measured, and mice with random blood glucose levels above 16.7 mmol/l were considered to be diabetic mice. Mice were sacrificed 12 weeks after diabetes induction, and their blood, serum and kidneys were harvested. The animal experiments were approved by the Ethics Review Committee of Central South University (approval number: No. 2021sydw0279).
Cell lines, antibodies, and reagents
HK-2 cells were stored at the Institute of Kidney Disease, Central South University, in a liquid nitrogen container. STZ was purchased from Sigma–Aldrich (USA). Fetal bovine serum was obtained from Gibco (FBS-CBT). The following antibody was used in the experiments: anti-PCSK9 (Abcam, ab31762).
Cell culture
HK-2 cells were cultured in DMEM with F12 (1:1) supplemented with 10% fetal bovine serum. Subsequently, cells were stimulated with HGPA at a final concentration of 30 mmol/L glucose and 100 μmol/L palmitic acid for 24 h and then harvested. The control group used low glucose, mannitol and fatty acid-free BSA (Solarbio, China) as the isotonic solvent control group. The molar ratio between PA and BSA is 1:6. The final concentration of BSA is 16.67 μmol/L.
Serum measurement
Fresh blood samples were incubated at room temperature (22 °C) for 30 min and then centrifuged at 3000 rpm for 10 min. The supernatants were collected as serum samples and stored at −80 °C. Serum PCSK9 values were determined by human (ZC-34391) and rat enzyme-linked immunosorbent assay (ELISA) kits (ZC-55156). Scr, BUN, urine albumin and urine creatinine of mice were measured on the biochemical analyzer at The Third Xiangya Hospital, Central South University.
Western blot analysis
Western blot analysis was performed as described previously[Citation20]. Protein concentrations were measured using a BCA protein assay kit (Pierce, 23227). The protein expression levels were measured using chemiluminescent staining reagent kits (SuperSignal West Femto, 34095), and images of stained proteins were captured using Image Scanner. The intensities of the bands on the images were quantified with ImageJ software.
Real-time quantitative PCR
Total RNA was extracted from the renal cortex using TRIzol reagent (Invitrogen, 15596–018). cDNA was synthesized using a reverse transcription kit (ReverTra Ace qPCR RT Kit; Thermo Scientific, AB1453B) according to the manufacturer’s instructions. Real-time quantitative PCR was performed using SYBR Green PCR Master Mix (Thermo Scientific, K0251) on an Applied Biosystems 7300 Sequence Detection System. The corresponding primer sequences were as follows:
mouse PCSK9 forward, 5′-TTGAACAAACTGCCCATCGC-3′;
mouse PCSK9 reverse, 5′-CCCAACAGGTCACTGCTCAT-3′;
human PCSK9 forward, 5′-AGACCCACCTCTCGCAGTC -3′;
human PCSK9 reverse, 5′-GGAGTCCTCCTCGATGTAGTC-3′. Using GAPDH
as the internal control, the relative amounts of the mRNAs were expressed as 2−ΔΔCT.
Statistical analysis
SPSS 23.0 software was used for data analysis. Kolmogorov-Smirnov (K-S) test was employed to determine whether the data followed a normal distribution. Categorical variables are expressed as frequencies (percentages), and continuous variables are expressed as the mean ± SD or as medians with interquartile ranges. Fisher’s exact test or the χ2 test was used to determine differences between patient groups for categorical data, and Student’s t test or the Mann–Whitney U test was used for continuous variables. For univariate correlation analyses, we used Pearson correlation if we observed a bivariate normal distribution and Spearman correlation otherwise. Logistic regression was applied for multivariate analysis. A ROC curve was used to determine the optimal cutoff value for albuminuria and renal insufficiency in patients with T2DM. p < 0.05 was considered significant.
Results
PCSK9 levels are elevated in DM
As shown in , compared to that in the healthy control individuals, serum PCSK9 levels in T2DM patients were significantly increased (p < 0.05). More details of the serum PCSK9 expression levels of the control group and T2DM subjects can be seen in Supplemental Table 1. Serum PCSK9 levels were significantly higher in STZ + HFD mice than in the control mice (, p < 0.05). The PCSK9 mRNA level in the kidney tissue of STZ + HFD mice was higher than that in the control mice (< 0.05). Furthermore, immunohistochemical staining and Western blot analysis revealed that STZ + HFD greatly increased the expression of PCSK9 in kidney tissues (, both p < 0.05). The uncropped western blots were detailed in Supplemental Figure 1(A and B). Since we found that PCSK9 colocalized with tubular epithelial cells in the kidneys of STZ + HFD mice, we treated HK-2 cells with high levels of glucose and palmitic acid. As shown in , the expression of PCSK9 at the mRNA and protein levels was increased under high glucose and palmitic acid conditions (both p < 0.05). The uncropped western blot was listed in Supplemental Figure 1C.
Figure 2. PCSK9 levels are elevated in DM. (A) Serum PCSK9 levels in T2DM patients and healthy control individuals. (B) Serum PCSK9 levels in STZ + HFD mice and control mice. (C) PCSK9 mRNA levels in kidney tissue of STZ + HFD mice and control mice. (D and E) Representative Western blot (left panel) and bar graph analysis (right panel) of PCSK9 levels in kidney tissue of STZ + HFD mice and control mice. (F and G) Representative immunohistochemical staining (left panel) and bar graph analysis (right panel) of PCSK9 levels in kidney tissue of STZ + HFD mice and control mice. Scale bar: 100 μm. (H) PCSK9 mRNA levels in HK-2 cells with HGPA and the control cells. (I and J) Representative Western blot and bar graph analysis of PCSK9 levels in HK-2 cells with HGPA and the control cells. *p < 0.05, **p < 0.01.
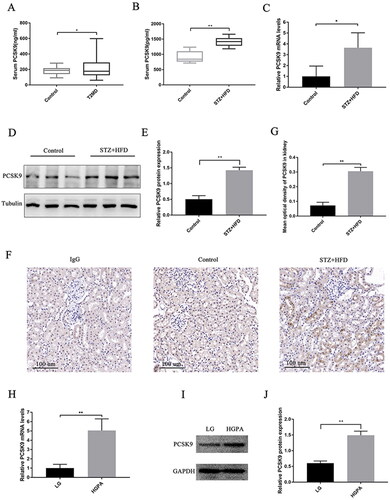
Additionally, we assessed body weight and glucose and lipid levels. The blood glucose, LDL-C, total cholesterol (TC) and triglyceride (TG) levels in diabetic mice were remarkably higher than those in the control mice, while body weight showed the opposite trend (Supplemental Table 2).
Baseline characteristics in T2DM patients with different PCSK9 levels
Baseline characteristics in T2DM patients according to the PCSK9 tertiles are shown in . There was a significant increase in SBP, sCr, BUN, TG, and UαCR levels in PCSK9 tertile 3 compared with PCSK9 tertile 1 (p < 0.05). DBP and UACR values were significantly higher in PCSK9 tertile 3 than in PCSK9 tertile 1 and PCSK9 tertile 2 (both p < 0.05). In addition, URCR values were significantly higher in PCSK9 tertile 3 and PCSK9 tertile 2 than in PCSK9 tertile 1 (both p < 0.05). The other characteristics were similar between the groups (all p > 0.05) ().
Table 1. Comparison of baseline characteristics in T2DM patients according to PCSK9 tertile.
Serum PCSK9 levels were significantly associated with renal function indicators
As shown in , in T2DM patients, serum PCSK9 levels were positively correlated with SBP (r = 0.216, p = 0.009), Scr (r = 0.189, p = 0.024), BUN (r = 0.213, p = 0.010), and TG (r = 0.183, p = 0.028) levels, URCR (r = 0.322, p < 0.001), UαCR (r = 0.201, p = 0.015) and UACR (r = 0.365, p < 0.001) but inversely correlated with eGFR (r=-0.173, p = 0.037). Although partial correlation confirmed that PCSK9 levels were significantly associated with several renal function indicators (), no significant correlation between serum PCSK9 and UβCR (p > 0.05) was observed. Inaddition, in diabetic mice, serum PCSK9 levels were positively correlated with Scr (r = 0.789, p = 0.011) and BUN (r = 0.838, p = 0.005) levels and UACR (r = 0.748, p = 0.021), which was consistent with the findings in the patients (Supplemental Figure 2(A–C)).
Figure 3. Serum PCSK9 levels were significantly associated with several renal function indicators in T2DM patients. Serum PCSK9 levels were positively correlated with Scr (A), BUN (B), URCR (C), UαCR (D) and UACR (E) but inversely correlated with eGFR (F).
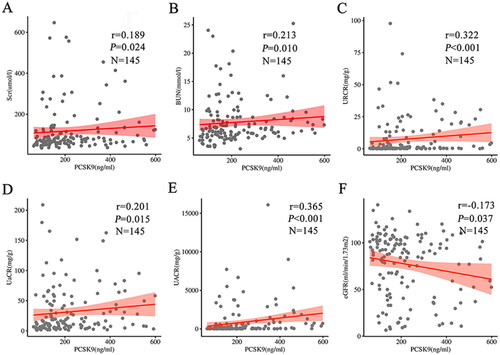
Table 2. The correlation of serum PCSK9 with clinical indicators in T2DM patients.
Serum PCSK9 was a risk factor for abnormal UACR and decreased eGFR
We employed binary logistic regression analysis to analyze the association between serum PCSK9 and UACR ≥30 mg/g. SBP, DBP, renal function (Scr, BUN, sUA), ALT, ALB, Hb,/TC and PCSK9 were selected as explanatory variables through single factor screening, and the forward (LR) method was selected for logistic regression analysis (). We found that serum PCSK9 (p = 0.029) was significant in predicting UACR ≥30 mg/g (). We further analyzed the correlation between serum PCSK9 and eGFR <60 mL/min/1.73 m2. Explanatory variables were age, diabetes duration, SBP, FPG, HbA1C, ALT, ALB, Hb and PCSK9 (). PCSK9 (p = 0.034) also showed a significant positive relationship with eGFR < 60 mL/min/1.73 m2 ().
Table 3. The correlation of UACR, eGFR with clinical indicators in T2DM patients.
Table 4. Logistic regression analysis of risk factors for renal dysfunction in patients with T2DM.
PCSK9 cutoff values for evaluating renal dysfunction in T2DM patients
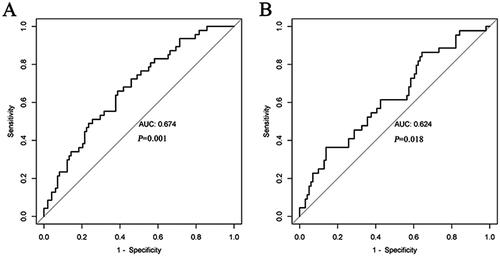
The most effective PCSK9 cutoff value for evaluating UACR in T2DM patients was 170.53 ng/mL (AUC = 0.674; sensitivity 61.2%; specificity 66.0%). Additionally, the PCSK9 cutoff value for eGFR in T2DM patients was 337.26 ng/mL (AUC = 0.624; sensitivity 36.4%; specificity 86.1%; ).
Discussion
In previous studies, increased levels of PCSK9 have been observed in DM patients compared to healthy control individuals [Citation21–23]. The hepatic levels of PCSK9 were increased in the livers of the db/db mouse model of diabetes [Citation24]. In CKD patients, PCSK9 levels were nearly twice as high as those in non-CKD control individuals [Citation25]. Nephrotic syndrome (NS) patients had significantly higher plasma PCSK9 levels than healthy control individuals [Citation26]. Moreover, a 7-fold and 24-fold increase in plasma PCSK9 was observed in podocyte knockout and nephrotoxic serum-induced NS mouse models, respectively [Citation27]. Furthermore, our study demonstrated that in patients with T2DM and STZ + HFD mice, the level of serum PCSK9 was markedly elevated, and the same trend was observed in the kidneys of STZ + HFD mice and HK-2 cells treated with HGPA. As a result of podocyte injury and protein loss in the urine, PCSK9 was likely to be secreted in increased amounts by the renal system. Furthermore, podocyte injury could lead to increased PCSK9 expression in the liver and reduced PCSK9 clearance from the blood [Citation27].
As of now, there is still no clear understanding of the mechanisms underlying T2DM-induced renal dysfunction, and DKD prediction strategies remain limited [Citation28]. In this study, we determined whether serum PCSK9 was associated with diabetes-related renal dysfunction in 145 patients with established T2DM. The results showed that serum PCSK9 was significantly correlated with markers of SBP, lipid metabolism (TG), and renal glomerular and tubular function. PCSK9 could affect blood pressure regulation by reducing the amount of sodium channel proteins and thereby regulating sodium absorption by the kidneys [Citation29]. As a regulator of LDL-c, PCSK9 has not been found to be correlated with TC and LDL-c. This is speculated to be because most of the subjects in our study took statins and lipid-lowering drugs, which decrease TC and LDL-c levels and increase serum PCSK9 levels [Citation30]. Consistent with our findings, Cai et al. [Citation31] showed that PCSK9 was positively correlated with TG, suggesting that PCSK9 might be involved in the regulation of other lipids besides LDL-c. Moreover, the renal cortical collecting duct could secrete PCSK9 to increase circulating PCSK9 levels, regulate liver LDLR and lead to dyslipidemia[Citation27,Citation32]. These findings indicate that PCSK9 levels are closely related to renal tubular function. In CKD patients, PCSK9 was negatively correlated with eGFR [Citation25] and positively correlated with proteinuria [Citation33]. This was consistent with the trend in T2DM patients. In addition, a logistic regression model revealed that serum PCSK9 is an independent risk factor for UACR ≥30 mg/g and eGFR <60 mL/min/1.73 m2. According to the ROC curve, 170.53 ng/mL and 337.26 ng/mL PCSK9 were the best cutoff values for UACR ≥30 mg/g and eGFR <60 mL/min/1.73 m2, indicating that patients with T2DM may develop albuminuria and progress to renal insufficiency with a gradual increase in serum PCSK9 levels.
This study had some limitations. First, this is a single-center cross-sectional study with a small sample size. In addition, only total serum PCSK9 levels were detected, but not free PCSK9 levels. We will conduct a large sample survey in the future to further clarify the changes in circulating PCSK9 levels in T2DM patients with renal injury and confirm the protective effects of PCSK9 inhibitors on renal injury by a multicenter prospective study.
Conclusion
In summary, our study found that serum PCSK9 levels were closely associated with urinary albumin, eGFR and renal tubular injury in T2DM patients. Moreover, PCSK9 was an independent risk factor for albuminuria and renal insufficiency. These new findings will provide potential management strategies that may improve renal outcomes in T2DM patients.
Consent for publication
All authors agreed on the submission and copyright policies of the journal.
Supplemental Material
Download JPEG Image (712.7 KB)Supplemental Material
Download JPEG Image (622.4 KB)Supplemental Material
Download PDF (119.5 KB)Supplemental Material
Download PDF (118.9 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The datasets in this study are available from the corresponding author upon reasonable request.
Additional information
Funding
References
- Sun H, Saeedi P, Karuranga S, et al. IDF diabetes atlas: global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract. 2022;183:1.
- Pereira PR, Carrageta DF, Oliveira PF, et al. Metabolomics as a tool for the early diagnosis and prognosis of diabetic kidney disease. Med Res Rev. 2022;42(4):1518–9.
- Pitt B, Filippatos G, Agarwal R, et al. Cardiovascular events with finerenone in kidney disease and type 2 diabetes. N Engl J Med. 2021;385(24):2252–2263.
- Jung CY, Yoo TH. Pathophysiologic mechanisms and potential biomarkers in diabetic kidney disease. Diabetes Metab J. 2022;46(2):181–197.
- Mitrofanova A, Burke G, Merscher S, et al. New insights into renal lipid dysmetabolism in diabetic kidney disease. World J Diabetes. 2021;12(5):524–540.
- Grefhorst A, McNutt MC, Lagace TA, et al. Plasma PCSK9 preferentially reduces liver LDL receptors in mice. J Lipid Res. 2008;49(6):1303–1311.
- Schulz R, Schlüter KD, Laufs U. Molecular and cellular function of the proprotein convertase subtilisin/kexin type 9 (PCSK9). Basic Res Cardiol. 2015;110(2):4.
- Shapiro MD, Tavori H, Fazio S. PCSK9: from basic science discoveries to clinical trials. Circ Res. 2018;122(10):1420–1438.
- Cui Q, Ju X, Yang T, et al. Serum PCSK9 is associated with multiple metabolic factors in a large han chinese population. Atherosclerosis. 2010;213(2):632–636.
- Lakoski SG, Lagace TA, Cohen JC, et al. Genetic and metabolic determinants of plasma PCSK9 levels. J Clin Endocrinol Metab. 2009;94(7):2537–2543.
- Yang SH, Li S, Zhang Y, et al. Positive correlation of plasma PCSK9 levels with HbA1c in patients with type 2 diabetes. Diabetes Metab Res Rev. 2016;32(2):193–199.
- Peng J, Liu MM, Jin JL, et al. Association of circulating PCSK9 concentration with cardiovascular metabolic markers and outcomes in stable coronary artery disease patients with or without diabetes: a prospective, observational cohort study. Cardiovasc Diabetol. 2020;19(1):167.
- Xu M, Wu X, Liu Z, et al. A novel mouse model of diabetes, atherosclerosis and fatty liver disease using an AAV8-PCSK9-D377Y injection and dietary manipulation in db/db mice. Biochem Biophys Res Commun. 2022;622:163–169.
- Momtazi-Borojeni AA, Jaafari MR, Abdollahi E, et al. Impact of PCSK9 immunization on glycemic indices in diabetic rats. J Diabetes Res. 2021;2021:4757170.
- Amput P, Palee S, Arunsak B, et al. PCSK9 inhibitor and atorvastatin reduce cardiac impairment in ovariectomized prediabetic rats via improved mitochondrial function and Ca(2+) regulation. J Cell Mol Med. 2020;24(16):9189–9203.
- Elewa U, Fernández-Fernández B, Mahillo-Fernández I, et al. PCSK9 in diabetic kidney disease. Eur J Clin Invest. 2016;46(9):779–786.
- Diabetology branch of the Chinese medical association, guidelines for prevention and treatment of type 2 diabetes in China (2020 edition). Chin J Diab. 2021;13:315–409.
- Levey AS, Stevens LA, Schmid CH, 3rd, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612.
- Bucci T, Ames PR, Cammisotto V, et al. Proprotein convertase subtilisin/kexin type 9 (PCSK9) levels in primary antiphospholipid syndrome. The multicenter ATHERO-APS study. J Autoimmun. 2022;129:102832.
- Li A, Yi B, Han H, et al. Vitamin D-VDR (vitamin D receptor) regulates defective autophagy in renal tubular epithelial cell in streptozotocin-induced diabetic mice via the AMPK pathway. Autophagy. 2022;18(4):877–890.
- Arsenault BJ, Petrides F, Tabet F, et al. Effect of atorvastatin, cholesterol ester transfer protein inhibition, and diabetes mellitus on circulating proprotein subtilisin kexin type 9 and lipoprotein(a) levels in patients at high cardiovascular risk. J Clin Lipidol. 2018;12(1):130–136.
- Nekaies Y, Baudin B, Kelbousi S, et al. Plasma proprotein convertase subtilisin/kexin type 9 is associated with Lp(a) in type 2 diabetic patients. J Diab Compli. 2015;29(8):1165–1170.
- Peng J, Zhu CG, Li JJ. The predictive utility of circulating PCSK9 levels on diabetes mellitus. Cardiovasc Diabetol. 2021;20(1):45.
- Wang Y, Ye J, Li J, et al. Polydatin ameliorates lipid and glucose metabolism in type 2 diabetes mellitus by downregulating proprotein convertase subtilisin/kexin type 9 (PCSK9). Cardiovasc Diabetol. 2016;15:19.
- Konarzewski M, Szolkiewicz M, Sucajtys-Szulc E, et al. Elevated circulating PCSK-9 concentration in renal failure patients is corrected by renal replacement therapy. Am J Nephrol. 2014;40(2):157–163.
- Jin K, Park BS, Kim YW, et al. Plasma PCSK9 in nephrotic syndrome and in peritoneal dialysis: a cross-sectional study. Am J Kidney Dis. 2014;63(4):584–589.
- Haas ME, Levenson AE, Sun X, et al. The role of proprotein convertase subtilisin/kexin type 9 in nephrotic syndrome-associated hypercholesterolemia. Circulation. 2016;134(1):61–72.
- Sugahara M, Pak WLW, Tanaka T, et al. Update on diagnosis, pathophysiology, and management of diabetic kidney disease. Nephrology. 2021;26(6):491–500.
- Sharotri V, Collier DM, Olson DR, et al. Regulation of epithelial sodium channel trafficking by proprotein convertase subtilisin/kexin type 9 (PCSK9). J Biol Chem. 2012;287(23):19266–19274.
- Welder G, Zineh I, Pacanowski MA, et al. High-dose atorvastatin causes a rapid sustained increase in human serum PCSK9 and disrupts its correlation with LDL cholesterol. J Lipid Res. 2010;51(9):2714–2721.
- Cai G, Yu L, Huang Z, et al. Serum PCSK9 levels, but not PCSK9 polymorphisms, are associated with CAD risk and lipid profiles in southern Chinese Han population. Lipids Health Dis. 2018;17(1):213.
- Molina-Jijon E, Gambut S, Macé C, et al. Secretion of the epithelial sodium channel chaperone PCSK9 from the cortical collecting duct links sodium retention with hypercholesterolemia in nephrotic syndrome. Kidney Int. 2020;98(6):1449–1460.
- Kwakernaak AJ, Lambert G, Slagman MC, et al. Proprotein convertase subtilisin-kexin type 9 is elevated in proteinuric subjects: relationship with lipoprotein response to antiproteinuric treatment. Atherosclerosis. 2013;226(2):459–465.