Abstract
Objectives
Data on angiotensin receptor-neprilysin inhibitor (ARNI) sacubitril-valsartan (SV) in patients undergoing maintenance dialysis is scarce. Our study aimed to investigate the effect of SV on patients undergoing dialysis.
Methods
We retrospectively reviewed the data of end-stage kidney disease (ESRD) patients undergoing either peritoneal dialysis (PD) or hemodialysis (HD) in our center. A total of 51 patients receiving SV treatment were enrolled in the SV group. Another 51 age and sex-matched patients on dialysis without SV treatment were selected as the control group. All the patients were regularly followed up in the dialysis clinic. Their clinical, biochemical, and echocardiographic parameters were all recorded at baseline and during follow-up. The effect and safety of SV were further analyzed.
Results
A total of 102 ESRD patients on dialysis (51 patients in the SV group and 51 patients in the control group) were finally enrolled. The median follow-up time was 349 days (interquartile range [IQR]: 217–535 days). The level of B-type natriuretic peptide (BNP) (median [IQR] before and after SV treatment: 596.35 pg/ml [190.6–1714.85] vs. 188.7 pg/ml [83.34–600.35], p < 0.001) or N-terminal pro-B-type natriuretic peptide (NT-proBNP) (median [IQR]: 6316.00 pg/ml [4552.00–28598.00] vs. 5074.00 pg/ml [2229.00–9851.00], p = 0.022) were significantly decreased after treatment with SV. The variant rate of left ventricular ejection fraction (LVEF) was significantly higher in the SV group compared to the control group, especially in the PD subgroup. No significant difference was found in other echocardiographic parameters between SV and control group. Subgroup analysis of the PD group showed an increase in daily PD ultrafiltration (median [IQR]: 400 ml/d [200-500] vs. 500 ml/d [200–850], p = 0.114) after SV treatment. Variant rate of overhydration (OH) measured by the body composition monitor (BCM) of the SV group were significantly different from the control group (median [IQR]: −13.13% [−42.85%–27.84%] vs. 0% [−17.95%–53.85%], p = 0.049). The rate of hyperkalemia was slightly higher but without significant difference before and after the introduction of SV (19.6% vs. 27.5%, p = 0.350). No event of hypotension and angioedema were observed.
Conclusions
SV might have a cardio-protective role in ESRD patients undergoing dialysis, especially in PD patients. Serum potassium should be monitored during the treatment.
Introduction
Cardiovascular disease is the leading cause of death in patients with advanced chronic kidney disease (CKD). The incidence of cardiovascular events like heart failure, coronary artery diseases, arrhythmias, and sudden cardiac death increases with the deterioration of kidney function [Citation1]. Up to 58% of patients on CKD grade 5 or renal replacement treatment died of cardiovascular diseases, among which heart failure and coronary artery disease represented the two major ones [Citation2,Citation3]. The presence of heart failure is associated independently with poor prognosis in patients undergoing either peritoneal dialysis (PD) or hemodialysis (HD) [Citation4]. Thus, exploring effective measures to prevent or improve heart failure is of great importance in patients on dialysis.
Sacubitril-valsartan (SV) is a crystal complex of a neprilysin inhibitor and an angiotensin receptor blocker (ARB), where the two components could act in synergy to enhance the diuresis, dilate blood vessels and prevent the maladaptive remodeling [Citation5,Citation6]. A prospective comparison of angiotensin receptor-neprilysin inhibitor with angiotensin converting enzyme inhibitor to determine the impact on global mortality and morbidity in heart failure (PARADIGM-HF) clinical trial demonstrated that SV was more effective in reducing the risks of death and hospitalization in patients with heart failure and reduced ejection fraction than angiotensin-converting enzyme inhibitor (ACEI) [Citation7]. The PARAGON-HF clinical trial (efficacy and safety of LCZ696 compared with valsartan, on morbidity and mortality in heart failure patients with preserved ejection fraction) also showed a decreasing trend of incidence of death from cardiovascular causes and total hospitalizations for heart failure, although failed to result in significance [Citation8]. Interestingly, the subgroup analysis of the PARADIGM-HF trial showed that SV led to improved cardiovascular outcomes, even in CKD patients [Citation9]. The UK HARP-III trial also confirmed the additional cardio-protective effect of SV in patients with moderate to severe CKD compared to irbesartan [Citation10]. However, patients undergoing dialysis, a high-risk population at cardiovascular risk with an unmet need, were enrolled in none of the aforementioned trials. Moreover, SV was proven to have a good anti-hypertensive effect and be more effective than ARB alone [Citation11,Citation12]. Hypertension is the most common complication in CKD patients and is often refractory to common medical treatment [Citation13]. Better control of blood pressure was able to reduce cardiovascular mortality [Citation14]. And again, patients in dialysis were not included in these studies.
In recent years, several preliminary studies have investigated the application of SV in patients with end-stage of renal disease (ESRD) and heart failure. Lee S et al. found that SV could safely reduce the high-sensitive troponin T levels and improve LVEF in patients with ESRD on HD [Citation15]. Another study conducted on 21 PD patients with heart failure with preserved ejection fraction (HFpEF) also demonstrated the effectiveness and safety of SV [Citation16]. However, Hsiao FC et al. found that in patients on dialysis, the ARNI users had a higher risk of HF hospitalization [Citation17].
The results of previous studies were not quite consistent. Moreover, whether it existed differential effect of SV in HD and PD patients remained to be elucidated. Herein, we undertook this study to investigate the effects of SV in patients undergoing dialysis and analyze its use in patients undergoing different dialysis modalities.
Methods
Study design and patients
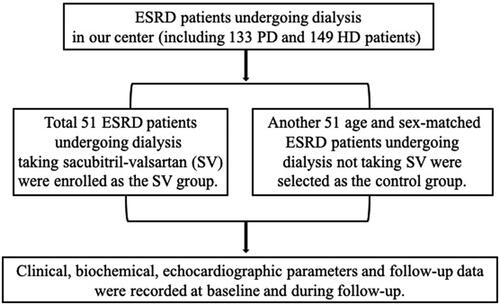
We retrospectively reviewed the data of ESRD patients undergoing HD or PD for at least 3 months in the Department of Nephrology, Peking University International Hospital from January 2015 to April 2022. All patients were regularly followed in the dialysis clinics. Patients with anuria were also included in the study. Those who received SV treatment for more than two weeks were enrolled in the SV group. Age and sex-matched patients who did not receive SV were selected from the rest as the control group (flow-chart presented as ). PD patients were treated with continuous ambulatory PD (CAPD) or automated PD (APD). HD patients were treated with thrice weekly intermittent HD (IHD). Patients prior prescribed ACEI or ARB were stopped 36 h before switching to SV treatment. SV was initiated by a small dose (50–100mg daily) and gradually titrated to the maximum tolerated dose. Other treatments did not change. The research complied with the Declaration of Helsinki, and the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) checklist. The study was approved by the China Ethics Committee of Registering Clinical Trials (Ethics number: ChiECRCT20200463) and all participants provided written informed consent.
Data collection
Baseline demographic and clinical data were collected within two weeks before or after the initiation of SV including age, sex, body mass index, primary renal disease, and past medical histories like hypertension, diabetes, hyperlipidemia, and cardiovascular disease, were recorded. Clinical and laboratory parameters including weekly fractional clearance index for urea (Kt/V), ultrafiltration (UF), urine volume, blood pressure, serum albumin, serum potassium, serum calcium, and serum phosphorus were collected within two weeks before or after the use of SV and within two weeks at the end of follow-up. The bioimpedance machine Body Composition Monitor (BCM) was used to measure the overhydration (OH) and relative OH to evaluate patients’ fluid status [Citation18]. A two-dimensional echocardiography was performed by an experienced ultrasonographer and regularly reviewed by a senior physician within two weeks before or after the use of SV and within two weeks at the end of the follow-up to evaluate cardiac structure and function. The measurement techniques for echocardiographic parameters were as follows: patients were placed in the left lateral position, connected to the ECG, and the measured parameters were collected after 3 consecutive cardiac cycles for sinus rhythm or 5 consecutive cardiac cycles for arrhythmias and were averaged. The anteroposterior diameter of the left atrium (LAd), the end-diastolic internal diameter of the left ventricle (LVEDd), and the interventricular septum diastolic thickness (IVSd) were measured in a parasternal left ventricular long-axis view. Apical four-chamber cardiac views were taken to measure right heart internal diameter parameters including right atrium diameter (RAd), and right ventricular diastolic diameter (RVDd). The left ventricular ejection fraction (LVEF) was measured by Simpson’s method. Cardiac biomarkers B-type natriuretic peptide (BNP) or N-terminal pro-B-type natriuretic peptide (NT-proBNP) were noted before and after the use of SV.
Adverse effects of SV were defined as one of the following: hypotension (systolic blood pressure < 90mmHg), hyperkalemia (serum potassium > 5.0 mmol/L) [Citation19], and angioedema. The composite endpoints were defined by death and major adverse cardiovascular events (MACE), which were defined as a composite of cardiovascular death, nonfatal myocardial infarction, and stroke [Citation20].
Statistical analysis
Statistical software SPSS 21.0 (SPSS, Chicago, IL, USA) and GraphPad Prism 9.0 (GraphPad, San Diego, CA, USA) were used for statistical analysis. Quantitative data were expressed as mean ± SD or as median with range (minimum and maximum)/interquartile range (IQR). Categorical data were expressed as percentages and numbers. The variant rate was calculated as: (parameters at follow-up - parameters at initiation)/parameters at initiation*100% (e.g.: Variant rate of LVEF in the SV group= [LVEF after SV application- LVEF before SV application]/LVEF before SV application *100%). Paired sample t-test or Wilcoxon paired signed rank test were used to compare the self-matching data for parametric and non-parametric data respectively. The chi-square test was applicated for the analysis of qualitative data. Differences in semiquantitative data were tested with the Kruskal–Wallis test and the Mann–Whitney U-test. Kaplan–Meier curves were used to analyze patients’ prognoses. Cox proportional hazards regression model was performed to identify prognostic risk factors related to the composite outcomes. Results were expressed as hazard ratios with 95% confidence intervals. Statistical significance was considered at p < 0.05.
Results
Baseline data of the patients
A total of 51 patients on maintenance dialysis who received SV were enrolled from the ESRD patients in our dialysis center as the SV group. Another 51 age and sex-matched patients on maintenance dialysis who did not take SV with comparable dialysis duration were selected as the control group (). In the SV group, the average dose of SV used was 133.0 ± 70.97 mg per day. Among them, 32 patients were on PD and 19 patients were on HD. Baseline data of the HD and PD patients in the SV group were presented in Supplementary Table 1. The baseline data of the patients recruited in the SV group and control group were listed in . Diabetes-related kidney disease was the leading cause of ESRD in both SV and control groups, followed by glomerulonephritis, hypertensive nephropathy, polycystic kidney disease, and others. Demographic, clinical, and laboratory data were comparable between the two groups. ACEI/ARB was used in 90.2% of the patients in the control group. The median (IQR) follow-up time was 349 (217-535) days.
Table 1. Baseline data of the sacubitril-valsartan (SV) group and control group patients.
Comparison of cardiac parameters before and after the initiation of SV
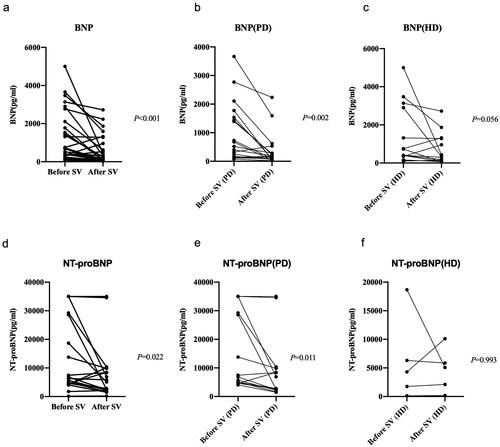
Cardiac biomarkers BNP or NT-proBNP were available in 32 patients and 19 patients respectively before and after the initiation of SV. Compared with the baseline levels, both BNP (median [IQR]: 596.35 pg/ml [190.6–1714.85] vs. 188.7 pg/ml [83.34–600.35], p < 0.001) and NT-proBNP (median [IQR]: 6316.00 pg/ml [4552.00–28598.00] vs. 5074.00 pg/ml [2229.00–9851.00], p = 0.022) had markedly decreased after the SV treatment. Subgroup analysis showed a more significant decline in the parameters in PD patients than in HD patients ().
Figure 2. Levels of BNP or NT-proBNP before and after sacubitril-valsartan (SV) introduction in SV group. BNP, B-type natriuretic peptide; NT-proBNP, pro-B-type natriuretic peptide; PD, peritoneal dialysis; HD, hemodialysis.
In the before-and-after self-comparison of the SV group, parameters of echocardiography including LVEF, LVEDd, LAd, RVDd, RAd, and IVSd were not found to be of significant difference before and after the application of SV (all p > 0.05). When compared to the control group, the improvement rate of LVEF in the SV group was significantly higher (2.2% vs. −3.01%, p = 0.033). While the other parameters did not show significant differences between groups (). In the subgroup analysis of PD patients, LVEF improved significantly after SV application (62.5% vs. 61.0%, p = 0.007). The variant rate of LVEF in the SV group increased significantly compared to the control group (4.4% vs. −4.8%, p = 0.004) (). The rate of patients with pericardial effusion decreased pronouncedly after the treatment by SV (25.00% vs 6.25%, p = 0.039) in the PD patients. The rate of improvement of LVEF was significantly higher in the PD group compared to the HD group (4.4% VS −4.6%, p = 0.02). No significant difference in echocardiography parameters was noted in the HD subgroup analysis (all p > 0.05) (Supplementary Table 2).
Table 2. Comparison of echocardiography parameters between SV and control group.
Table 3. Comparison of echocardiography parameters between PD patients and the control group.
The relevance of the echocardiographic parameters and values of atrial peptides were further compared. The level of BNP was negatively correlated with LVEF (r= −0.580, p = 0.001) and positively correlated with LVEDd (r = 0.445, p = 0.011), LAd (r = 0.556, p = 0.001) and RAd (r = 0.659, p = 0.010) at baseline and similar results were found in the follow-up. NT-proBNP values were positively correlated with LAd at baseline and with IVSd at follow-up (Supplementary Table 3).
The effect of SV on fluid status, blood pressure, and peritoneal ultrafiltration
Overhydration (OH) and relative OH measured by the bioimpedance method were used to evaluate the fluid status. The variant rate of OH in the SV group was significantly higher than the control group [median variant rate, IQR: −13.13% (−42.85%−27.84%) vs. 0% (−17.95%–53.85%), p = 0.049]. Variant rate of relative OH in the SV group was significantly different from that of the control group (p = 0.021) (). In the subgroup of PD, the median (IQR) OH significantly decreased from 6.8 L (3.7–7.5) to 4.3 L (2.8–6.2L) after SV treatment (p = 0.024). Similar results were found for relative OH (32.00% [20.50-36.60%] before SV and 23.30% [15.10–32.20%] after SV treatment, p = 0.014). In the HD group, neither OH and relative OH decreased significantly after SV introduction (p = 0.306 for OH, p = 0.523 for relative OH).
Table 4. Fluid statue and urine of SV and control group.
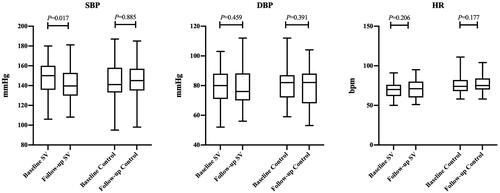
The median of systolic blood pressure (SBP) decreased from 150 mmHg to 139 mmHg (p = 0.017), and the median of diastolic blood pressure (DBP) decreased from 80 mmHg to 76 mmHg (p = 0.459). In the control group, the median of SBP or DBP has not much changed during the follow-up period ().
Figure 3. Baseline and follow-up data of blood pressure and heart rate in the SV group and control group. SBP, systolic blood pressure; DBP, diastolic blood pressure; HR, heart rate.
For PD patients, an increasing trend of the daily peritoneal ultrafiltration (UF) was observed after SV treatment [median, IQR: 400 (200–500) vs. 500 (200–850), p = 0.114] compared to the control group [median, IQR: 450 (128–762) vs. 475 (300–800), p = 0.477]. Residual urine volume declined in both the SV and control group during the follow-up period, but no significant difference in the decline rate was noted between the groups ().
Safety of SV
No angioedema or hypotensive events were detected in the follow-up period in both groups. Hyperkalemia tended to increase in the SV group, however, the rate of hyperkalemia did not show a significant difference before and after the introduction of SV (19.6% vs. 27.5%, p = 0.350).
Clinical outcomes
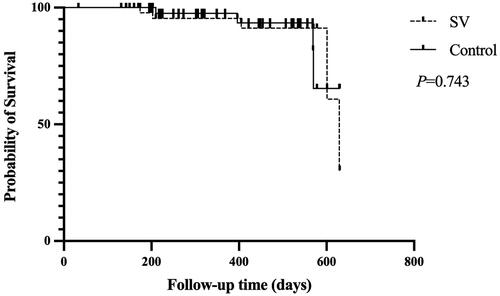
During the follow-up period, the number of hospitalizations due to cardiovascular events was 10/51 (19.6%) in the SV group and 12/51 (23.5%) in the control group (p = 0.630). The composite endpoints events (MACE or all-cause death) occurred in 5/51 (9.8%) in the SV group and 4/51(7.8%) in the control group. Five patients had MACE events in the SV group and 2 patients had all-cause death and 2 had MACE in the control group. No difference was detected in the survival rate between the two groups (p = 0.743) (). Univariate Cox regression survival analysis (unadjusted risk) failed to show a significant difference between SV and control group [hazard ratio (HR), 95% confidence interval (CI): 1.246, 0.335–4.640, p = 0.743]. Multivariate Cox proportional hazards regression showed similar results (modela: adjusted for age, sex and diuretic use, HR 0.683, 95% CI: 0.157–2.964, p = 0.611, modelb: adjusted for age, sex, and ultrafiltration, HR 0.675, 95% CI: 0.156–2.915, p = 0.675) (Supplementary Table 4).
Discussion
In this study, we investigated the effects of SV in patients on dialysis and initially compared the role of SV in PD and HD patients. We revealed a potential cardio-protective role of SV in patients undergoing dialysis, especially in patients on PD, evidenced by a decrease in cardio-markers like BNP or NT-proBNP level and amelioration in echocardiographic parameters like LVEF. Besides, both systolic and diastolic blood pressure ameliorated after the initiation of SV treatment.
SV has been recommended in clinical practice guidelines to reduce morbidity and mortality in patients with heart failure [Citation21] and proved to be superior to ACEIs or ARBs [Citation7,Citation10]. Heart failure is a major comorbidity in patients on dialysis [Citation3], while few studies have explored the application of SV in this part of patients. LEE S et al. found that SV was able to reduce the level of cardiac markers and improve LVEF in a total of 23 patients with ESRD on HD [Citation15]. Another study conducted on 21 PD patients showed that SV could alleviate signs and symptoms of heart failure and reduce NT-proBNP levels, but echocardiography parameters did not change significantly [Citation16]. Our results in cardio-makers were consistent with the above studies. Although echocardiographic parameters did not improve much after SV initiation in its own before-and-after comparison, the improving rate of LVEF was higher in the SV group when compared to the control group.
We further carried out a subgroup analysis. Interestingly, SV-PD (SV group-PD subgroup) group had a more pronounced decrease in cardio-markers compared to HD patients. A significant improvement in LVEF was found in PD when compared to its corresponding control group, which was not observed in the SV-HD (SV group-HD subgroup) group. As this phenomenon was not reported by previous studies, the possible mechanism remained to be elucidated. It was inferred that changes in body fluid might make a contribution. Fluid overload is one of the major determinants of mortality in advanced CKD patients which would act on the cardiovascular system, leading to hypertension, left ventricular hypertrophy, and finally heart failure [Citation22,Citation23]. Peritoneal ultrafiltration and residual renal function are the two main ways of removing excess water from PD patients. A loss of urine output was noted in both SV and the control groups, which indicated that SV was not able to retain the residual renal function. So, the alleviation of the fluid should attribute to the increasing peritoneal ultrafiltration after SV initiation. Numerous factors can influence PD ultrafiltration [Citation24, Citation25] which rendered the ultrafiltration more difficult to control compared to HD patients. Zhang F et al. conducted a short-term observation of the effect of SV on peritoneal ultrafiltration and found that SV increased ultrafiltration by 66.4 mL/24h in PD patients within 7 days after its use [Citation26]. Our study had similar results, and moreover, we had a much longer follow-up time which proved that the effect of SV on increasing peritoneal ultrafiltration was sustainable.
SV consists of sacubitril and valsartan, the former is a neprilysin inhibitor [Citation7]. Neprilysin is a zinc-dependent metallopeptidase with a wide spectrum of peptide substrates and promiscuous physiological functions such as vasoconstriction, and sodium retention. It is distributed ubiquitously in the tissue including epithelia, fibroblasts, and kidney and it could also be in the soluble form in the plasma [Citation27, Citation28]. Since the peritoneum is covered by epithelium and rich in the vasculature, the inhibition of neprilysin in PD patients might result in the dilation of blood vessels, which might help increase the peritoneal ultrafiltration [Citation26]. But the exact mechanism involved requires further exploration.
Moreover, SV plays an outstanding role in anti-hypertension and was superior to RASS inhibitors [Citation7,Citation29]. Hypertension is the most modifiable factor in the development of heart failure [Citation30] and long-term pressure overload would lead to cardiac remodeling [Citation31]. Forty-four patients in our study converted from ACEI or ARB to SV treatment, and a decrease in both the systolic and diastolic blood pressure was observed. Blood pressure control and reduction in volume load might contribute to improving cardiac function.
The side effects were also monitored during the follow-up. No serious adverse side effect was observed just as in the previous reports [Citation17, Citation32]. However, we observed a slight hyperkalemia tendency in SV group compared to the control group which should be monitored during the follow-up.
Our study was retrospective research composing patients with both PD and HD, which has not been done before. However, existed several limitations in our study. First, it is a small study and the ratio of patients on HD is low, so the results here might only provide some insight and future research direction. Second, since the data on NT-proBNP was not available in more than half of the patients of the SV group, so cardio-makers were not in uniform for all the patients. Third, fluid overload could not be completely excluded in the patients which might also contribute to the development of heart failure. Last, this was a retrospective study, there might be many confounding factors involved. Further research with larger sample sizes and more comprehensive study designs would be necessary to confirm the results and draw stronger conclusions.
Conclusion
SV might play a cardio-protective role in ESRD patients undergoing dialysis, especially for PD patients. Serum potassium should be monitored during the treatment.
Supplemental Material
Download PDF (270.5 KB)Disclosure steatement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Jankowski J, Floege J, Fliser D, et al. Cardiovascular disease in chronic kidney disease: pathophysiological insights and therapeutic options. Circulation. 2021;143(11):1–9. doi: 10.1161/CIRCULATIONAHA.120.050686.
- Gansevoort RT, Correa-Rotter R, Hemmelgarn BR, et al. Chronic kidney disease and cardiovascular risk: epidemiology, mechanisms, and prevention. Lancet. 2013;382(9889):339–352. doi: 10.1016/S0140-6736(13)60595-4.
- Rangaswami J, McCullough PA. Heart failure in end-stage kidney disease: pathophysiology, diagnosis, and therapeutic strategies. Semin Nephrol. 2018;38(6):600–617. doi: 10.1016/j.semnephrol.2018.08.005.
- Harnett JD, Foley RN, Kent GM, et al. Congestive heart failure in dialysis patients: prevalence, incidence, prognosis and risk factors. Kidney Int. 1995;47(3):884–890. doi: 10.1038/ki.1995.132.
- Kuhn M. Molecular physiology of natriuretic peptide signalling. Basic Res Cardiol. 2004;99(2):76–82. doi: 10.1007/s00395-004-0460-0.
- Iborra-Egea O, Gálvez-Montón C, Roura S, et al. Mechanisms of action of sacubitril/valsartan on cardiac remodeling: a systems biology approach. NPJ Syst Biol Appl. 2017;3:12. doi: 10.1038/s41540-017-0013-4.
- McMurray JJ, Packer M, Desai AS, et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371(11):993–1004. doi: 10.1056/NEJMoa1409077.
- Solomon SD, McMurray JJV, Anand IS, et al. Angiotensin-neprilysin inhibition in heart failure with preserved ejection fraction. N Engl J Med. 2019;381(17):1609–1620. doi: 10.1056/NEJMoa1908655.
- Damman K, Gori M, Claggett B, et al. Renal effects and associated outcomes during angiotensin-neprilysin inhibition in heart failure. JACC Heart Fail. 2018;6(6):489–498. doi: 10.1016/j.jchf.2018.02.004.
- Haynes R, Judge PK, Staplin N, et al. Effects of sacubitril/valsartan versus irbesartan in patients with chronic kidney disease. Circulation. 2018;138(15):1505–1514. doi: 10.1161/CIRCULATIONAHA.118.034818.
- Kario K, Sun N, Chiang FT, et al. Efficacy and safety of LCZ696, a first-in-class angiotensin receptor neprilysin inhibitor, in Asian patients with hypertension: a randomized, double-blind, placebo-controlled study. Hypertension. 2014;63(4):698–705. doi: 10.1161/HYPERTENSIONAHA.113.02002.
- Cheung DG, Aizenberg D, Gorbunov V, et al. Efficacy and safety of sacubitril/valsartan in patients with essential hypertension uncontrolled by olmesartan: a randomized, double-blind, 8-week study. J Clin Hypertens. 2018;20(1):150–158. doi: 10.1111/jch.13153.
- Ni X, Zhang J, Zhang P, et al. Effects of spironolactone on dialysis patients with refractory hypertension: a randomized controlled study. J Clin Hypertens. 2014;16(9):658–663. doi: 10.1111/jch.12374.
- Lewington S, Clarke R, Qizilbash N, et al. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360(9349):1903–1913.
- Lee S, Oh J, Kim H, et al. Sacubitril/valsartan in patients with heart failure with reduced ejection fraction with end-stage of renal disease. ESC Heart Fail. 2020;7(3):1125–1129. doi: 10.1002/ehf2.12659.
- Fu S, Xu Z, Lin B, et al. Effects of Sacubitril-Valsartan in heart failure with preserved ejection fraction in patients undergoing peritoneal dialysis. Front Med. 2021;8:657067. doi: 10.3389/fmed.2021.657067.
- Hsiao FC, Lin CP, Yu CC, et al. Angiotensin receptor-neprilysin inhibitors in patients with heart failure with reduced ejection fraction and advanced chronic kidney disease: a retrospective multi-institutional study. Front Cardiovasc Med. 2022;9:794707. doi: 10.3389/fcvm.2022.794707.
- Ekinci C, Karabork M, Siriopol D, et al. Effects of volume overload and current techniques for the assessment of fluid status in patients with renal disease. Blood Purif. 2018;46(1):34–47. doi: 10.1159/000487702.
- Bianchi S, Aucella F, De Nicola L, et al. Management of hyperkalemia in patients with kidney disease: a position paper endorsed by the Italian society of nephrology. J Nephrol. 2019;32(4):499–516. doi: 10.1007/s40620-019-00617-y.
- Valgimigli M, Gagnor A, Calabró P, et al. Radial versus femoral access in patients with acute coronary syndromes undergoing invasive management: a randomised multicentre trial. Lancet. 2015;385(9986):2465–2476. doi: 10.1016/S0140-6736(15)60292-6.
- Yancy CW, Jessup M, Bozkurt B, et al. ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American college of cardiology/American heart association task force on clinical practice guidelines and the heart failure society of america. Circulation. 2017;136(6):e137–e61. doi: 10.1161/CIR.0000000000000509.
- Ortiz A, Covic A, Fliser D, et al. Epidemiology, contributors to, and clinical trials of mortality risk in chronic kidney failure. Lancet. 2014;383(9931):1831–1843. doi: 10.1016/S0140-6736(14)60384-6.
- Karaaslan DC, Afsar B, Kanbay M. Is relative overhydration measurement by bioimpedance spectroscopy useful in reducing morbidity and mortality in chronic kidney disease? Clin Kidney J. 2018;11(3):370–371. doi: 10.1093/ckj/sfy021.
- Noh H, Kim JS, Han KH, et al. Oxidative stress during peritoneal dialysis: implications in functional and structural changes in the membrane. Kidney Int. 2006;69(11):2022–2028. doi: 10.1038/sj.ki.5001506.
- Fusshoeller A. Histomorphological and functional changes of the peritoneal membrane during long-term peritoneal dialysis. Pediatr Nephrol. 2008;23(1):19–25. doi: 10.1007/s00467-007-0541-z.
- Zhang F, Zhang T, Yang S, et al. Sacubitril-valsartan increases ultrafiltration in patients undergoing peritoneal dialysis: a short-term retrospective self-controlled study. Front Med. 2022;9:831541. doi: 10.3389/fmed.2022.831541.
- Bayes-Genis A, Barallat J, Richards AM. A test in context: neprilysin: function, inhibition, and biomarker. J Am Coll Cardiol. 2016;68(6):639–653. doi: 10.1016/j.jacc.2016.04.060.
- Nalivaeva NN, Zhuravin IA, Turner AJ. Neprilysin expression and functions in development, ageing and disease. Mech Ageing Dev. 2020;192:111363. doi: 10.1016/j.mad.2020.111363.
- Chang HY, Feng AN, Fong MC, et al. Sacubitril/valsartan in heart failure with reduced ejection fraction patients: real world experience on advanced chronic kidney disease, hypotension, and dose escalation. J Cardiol. 2019;74(4):372–380. doi: 10.1016/j.jjcc.2019.03.010.
- Raby K, Rocco M, Oparil S, et al. Heart failure primary prevention: what does SPRINT add?: recent advances in hypertension. Hypertension. 2021;77(6):1804–1814. doi: 10.1161/HYPERTENSIONAHA.121.16503.
- Messerli FH, Rimoldi SF, Bangalore S. The transition from hypertension to heart failure: contemporary update. JACC Heart Fail. 2017;5(8):543–551. doi: 10.1016/j.jchf.2017.04.012.
- Wen Y, Xia Y, Gong Y. Effects and safety of sacubitril/valsartan (SV) on heart function and blood pressure in maintenance hemodialysis (MHD) patients. Am J Transl Res. 2022;14(5):3439–3447.