Abstract
Background
In addition to regulating the antiviral response, increased expression of Toll-like receptor 3 (TLR3) in resident renal cells plays a role in developing some forms of glomerulonephritis. TLR3 activation leads to type I interferon (IFN) production, which induces the expression of IFN-stimulated genes (ISGs). However, the role of ISG20 expression in resident renal cells remains unclear.
Methods
Cultured normal human glomerular endothelial cells (GECs) were treated with polyinosinic-polycytidylic acid (poly IC), Escherichia coli lipopolysaccharide (LPS), R848, and CpG (TLR3, TLR4, TLR7, and TLR9 agonists, respectively). The mRNA levels of ISG20, CX3CL1/fractalkine, and CXCL10/IP-10 were measured by quantitative reverse transcription-polymerase chain reaction. ISG20 protein expression was assessed by Western blotting. RNA interference was used to knockdown IFN-β and ISG20 expression. CX3CL1 protein levels were assessed by enzyme-linked immunosorbent assay. We performed immunofluorescence to examine endothelial ISG20 expression in biopsy specimens from patients with lupus nephritis (LN).
Results
In GECs, the expression of ISG20 mRNA and protein was increased by polyIC, not by LPS, R848, or CpG treatment. Moreover, ISG20 knockdown prevented poly IC-induced CX3CL1 expression but had no effect on CXCL10 expression. Intense endothelial ISG20 immunoreactivity was observed in biopsy specimens obtained from patients with proliferative LN.
Conclusion
In GECs, ISG20 was regulated via TLR3 but not via TLR4, TLR7, or TLR9 signaling. Moreover, ISG20 was involved in regulating CX3CL1 production. In addition to regulating antiviral innate immunity, ISG20 may act as a mediator of CX3CL1 production, thereby inducing glomerular inflammation, particularly in patients with LN.
Introduction
In some forms of glomerulonephritis, innate immunity in resident renal cells is thought to play a role in the pathogenesis of kidney inflammation, in addition to the antiviral response [Citation1]. In this process, activated Toll-like receptors (TLRs) on circulating immune cells and resident renal cells induce the production of type I interferons (IFNs) and subsequently that of pro-inflammatory cytokines/chemokines [Citation2,Citation3]. Among the pro-inflammatory chemokines, CX3CL1/fractalkine and CXCL10/IFN-γ-induced protein 10 are representative chemokines that could initiate activated leukocyte infiltration into glomerular cells [Citation4–6]. Although Toll-like receptor 3 (TLR3)/type I IFN signaling plays a major role in antiviral innate immunity [Citation7], it can be induced by noninfectious stimuli such as endogenous ligands, including damage-associated molecular patterns (DAMPs). Therefore, it is postulated that TLR3/type I IFN signaling may be involved in the pathogenesis of some forms of glomerulonephritis, particularly lupus nephritis (LN) [Citation8–11]. The TLR3, TLR4, TLR7, and TLR9 signaling pathways play important roles in LN pathogenesis [Citation8–13]. Regarding the TLR3, a unique concept of the ‘pseudoviral’ immunity that focuses on the excess activation of regional TLR3 signaling has been proposed as additional pathogenetic factors of LN [Citation8–11,Citation14–16], although this remains controversial. As TLR3 signaling is unique [Citation7,Citation8], we previously determined the detailed mechanisms of TLR3 signaling in resident renal cells [Citation9–11]. However, the role of TLR3 activation in LN remains largely unknown [Citation9].
The antiviral innate immune response is mediated by TLR3 activation, which results in the upregulation of IFNs and IFN-dependent transcripts or IFN-stimulated genes (ISGs). Although the specific antiviral functions of some ISGs have been characterized, their role of several ISGs remains unknown [Citation17]. ISG20, a DEDD 3′-5′ exonuclease with specificity toward single-stranded RNA, has been shown to suppress the replication of multiple viruses by degrading viral genes or genomes [Citation18,Citation19]. We have reported that the expression of ISGs, such as ISG15, ISG20, IFN-induced protein 35 (IFI35), IFN-induced transmembrane protein 1 (IFIT1), and DExD/H-Box Helicase 60 (DDX60), was regulated by TLR3 signaling in cultured human resident renal cells [Citation10,Citation14–16,Citation19,Citation20]. Moreover, we reported that ISGs could play a role in the regional regulation of pro-inflammatory cytokine/chemokine production, which results in pro- and anti-inflammatory reactions [Citation14–16,Citation19]. However, the implication of resident renal cell-specific ISG20 expression in the pathogenesis of glomerular inflammation remains undetermined [Citation20].
Since glomerular endothelial cells (GECs) are directly exposed to circulating viral particles in the glomerulus, endothelial viral and DAMPs antigens can activate the regional TLR3 signaling cascade [Citation15,Citation16]. Therefore, in this study, we evaluated ISG20 expression and its postulated role in GECs treated with TLR3, TLR4, TLR7, and TLR9 agonists.
Materials and methods
Reagents
Polyinosinic-polycytidylic acid (poly IC), lipopolysaccharide (LPS) from Escherichia coli, and an actin antibody were obtained from Sigma-Aldrich (St Louis, MO, USA). The TLR7 ligand R848 and TLR9 ligand CpG were purchased from InVivoGen (San Diego, CA, USA) and Novus Biologicals (Centennial, CO, USA), respectively. The Illustra RNAspin kit was obtained from GE Healthcare (Buckinghamshire, UK). The rabbit antibodies against ISG20 and IFIT1 were purchased from GeneTex (Irvine, CA, USA). Lipofectamine RNAiMAX reagent, Moloney murine leukemia virus (MMLV) reverse transcriptase, dNTP mix, and small interfering RNA (siRNA) against IFN-β were obtained from Thermo Fisher Scientific (Waltham, MA, USA). The siRNAs against ISG20 and a non-targeting negative control siRNA were obtained from Qiagen (Hilden, Germany). The SsoAdvanced Universal SYBR Green Supermix was obtained from Bio-Rad (Hercules, CA, USA). Horseradish peroxidase (HRP)-conjugated anti-rabbit antibodies were obtained from Medical and Biological Laboratories (Nagoya, Japan). Polyvinylidene difluoride (PVDF) membranes and Luminata Crescendo Western HRP substrates were obtained from Merck Millipore (Darmstadt, Germany). The enzyme-linked immunosorbent assay (ELISA) kit for CX3CL1 was obtained from R&D Systems (Minneapolis, MN, USA).
Cells
Normal human GECs were purchased from ScienCell Research Laboratories (Carlsbad, CA, USA). Cells were cultured in endothelial growth medium-2 (EGM-2; Lonza, Walkersville, MD, USA) on gelatin-coated plates, as previously described [Citation15,Citation16]. Cells were treated with 30 μg/mL of poly IC, 1 μg/mL of LPS, 5 μg/mL of R848, or 100 μg/mL of CpG for up to 24 h. To examine the concentration-dependent effects of poly IC, cells were treated with 0.5-50 μg/mL of poly IC for 16 h. Regarding siRNA treatment, cells were cultured in a medium without antibiotics for 24 h and then transfected with specific siRNA against IFN-β, ISG20, or a nontargeting negative control siRNA using the Lipofectamine RNAiMAX reagent according to the supplier’s protocol. After 48 h of incubation, the cells were treated with 30 µg/mL of poly IC.
Quantitative reverse transcription-polymerase chain reaction (qRT-PCR)
Total RNA was extracted from the cells using the Illustra RNAspin kit. Single-stranded complementary DNA (cDNA) was synthesized from the total RNA using oligo(dT)18 and MMLV reverse transcriptase. ISG20, CX3CL1, and CXCL10 expression was quantified using specific primers and SsoAdvanced Universal SYBR Green Supermix. 18S ribosomal RNA (18SrRNA) was used as the internal control.
The sequences of the primers used are as follows:
ISG20-F: 5′- ATCTCTGAGGGTCCCCAAGGA -3′,
ISG20-R: 5′- TTCAGTCTGACACAGCCAGGCG -3′,
CX3CL1-F: 5′- GACCCCTAAGGCTGAGGAAC -3′,
CX3CL1-R: 5′- CTCTCCTGCCATCTTTCGAG -3′,
CXCL10-F: 5′- TTCAAGGAGTACCTCTCTCTAG -3′,
CXCL10-R: 5′- CTGGATTCAGACATCTCTTCTC -3′,
18S-F: 5′- ACTCAACACGGGAAACCTCA -3′,
18S-R: 5′- AACCAGACAAATCGCTCCAC -3′.
Western blotting
After incubation, the cells were lysed using the Laemmli reducing sample buffer. The lysates were subjected to 5–20% polyacrylamide gel electrophoresis. The separated proteins were transferred to a PVDF membrane, which was subsequently blocked with nonfat dry milk for 2 h at room temperature and incubated with antibodies against ISG20 (1:1,000), IFIT1 (1:5000), or actin (1:5000) for 18 h at 4 °C. After washing, the membranes were incubated with a horseradish peroxidase (HRP)-conjugated anti-rabbit IgG antibody. The Immobilon Crescendo Western HRP chemiluminescence substrate was used for detection.
ELISA
The concentration of CX3CL1 protein in the cell-conditioned medium was measured using a commercially available ELISA kit, according to the manufacturer’s recommended protocol.
Immunofluorescence staining of ISG20
Renal specimens were biopsied during routine diagnostic procedures in clinical practice and written informed consent for immunostaining was obtained from each patient and their parents. Renal biopsy was performed before initiating therapeutic intervention. For this pilot study, we used snap-frozen sections stored at −80 °C in good condition, from patients with diffuse proliferative LN [n = 3, Class IV-G (A) in accordance with the International Society of Nephrology/Renal Pathology Society (ISN/RPS) 2003 classification for LN] were stained for ISG20. Sections from patients with non-proliferative LN (n = 3, Class II in accordance with the ISN/RPS 2003 classification for LN) were also stained for ISG20. Moreover, sections from patients with proteinuric IgA nephropathy (n = 3, IgAN; defined as urinary protein/creatinine ratio, >1.0 associated with moderately mesangial proliferative lesion), and minimal change nephrotic syndrome in remission (n = 2, MCNS, as a non-inflammatory control) were stained for ISG20. Optimal cutting temperature compound (OCT)-embedded specimens were cut into 5-μm-thick sections using a cryostat, fixed in ice-cold acetone, and air-dried. Sections were transferred onto slides and washed in phosphate buffered saline (PBS) pH 7.4 immediately before immunohistochemistry. Anti-rabbit ISG20 antibody (GeneTex, Irvine, CA, USA) was added at a dilution of 1:200. After incubation for 40 min, at room temperature and several washes with PBS, the slides were sequentially incubated with fluorescein isothiocyanate-conjugated secondary antibodies at a dilution of 1:20 for 40 min at room temperature. The identity of ISG20-expressing cells was determined by dual labeling with ISG20 and an anti-rabbit CD34 antibody (Nichirei Bioscience Inc., Tokyo, Japan, undiluted) as a marker of endothelial cells, and an Alexa fluor 594-conjugated anti-rabbit IgG secondary antibody (1:20). This immunohistochemical study was conducted as a part of our research project for TLR3 signaling-mediated inflammatory signaling in GECs and was approved by the Ethics committee of Hirosaki University Graduate School of Medicine (2018-098).
Statistical analysis
Data are presented as mean ± standard deviation (SD). The Mann–Whitney U test was used to assess significant differences. Statistical significance was set at p < 0.05.
Results
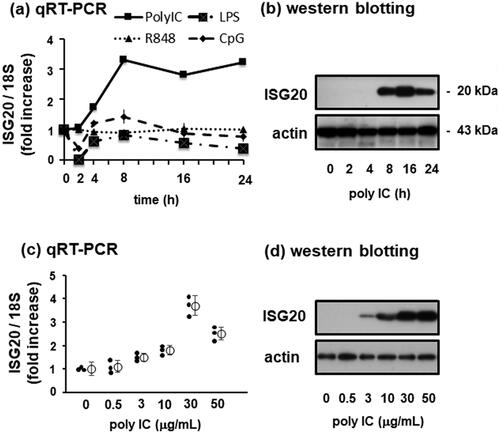
Poly IC treatment increases ISG20 expression in cultured human GECs
In unstimulated normal human GECs, mRNA and protein levels of ISG20 were low. Treatment of GECs with 30 µg/mL of poly IC, a TLR3 agonist, increased the expression of ISG20 mRNA in a time-dependent manner; ISG20 levels began to increase 4 h after stimulation and almost plateaued after 8 h (). In contrast, no significant change was observed in ISG20 mRNA expression when the cells were treated with LPS, R848, or CpG (). Concomitantly, the ISG20 protein levels increased after poly IC treatment. A marked increase in ISG20 protein levels was observed during 8–24 h, and maximum expression was observed 16 h after poly IC treatment (). Moreover, the increase in ISG20 mRNA () and protein () levels upon poly IC treatment was dose-dependent. Since ISG20 mRNA expression was not stimulated by LPS, R848, or CpG, the protein levels of ISG20 protein levels in response to these stimuli were not examined.
Figure 1. Polyinosinic-polycytidylic acid (poly IC) induces ISG20 expression in cultured human glomerular endothelial cells (GECs) in a time- and concentration-dependent manner. (a) GECs were treated with 30 µg/mL poly IC, 1 μg/mL LPS, 5 μg/mL R848, or 100 μg/mL CpG for up to 24 h. RNA was extracted from cells after incubation, and reverse-transcribed to cDNA using oligo (dT)18 and M-MLV reverse transcriptase. cDNA was used as a template for qPCR quantify ISG20 and 18S RNA expression. (b) Cells were treated with 30 µg/mL poly IC as described in (a) and lysed using the Laemmli sample buffer. Protein levels of ISG20 and actin protein were assessed by western blotting. (c and d) Cells were treated with 0.5-50 µg/mL poly IC for 16 h. RNA and protein were extracted from the cells and subjected to qRT-PCR analysis (c) and western blotting (d), respectively. Data from (a) and (c) represent the mean ± standard deviation (SD) (n = 3).
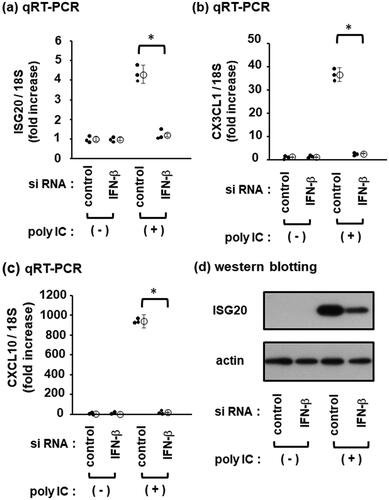
IFN-β is involved in ISG20 induction by poly IC
Next, we examined whether ISG20 upregulation by poly IC could be mediated by IFN-β. The chemokines CX3CL1 and CXCL10, which are induced by poly IC via IFN-β [Citation5,Citation21] were also examined as positive controls. IFN-β knockdown markedly inhibited poly IC-induced ISG20 (), CX3CL1 (), and CXCL10 expression (). Consistently, IFN-β knockdown inhibited poly IC-induced expression of ISG20 protein ().
Figure 2. IFN-β is involved in poly IC-induced CXCL10, CX3CL1, and ISG20 expression. The cells were transfected with a nontargeting negative control siRNA or an IFN-β siRNA and incubated for 48 h. Subsequently, the cells were incubated with 30 µg/mL of poly IC for 16 h. (a, b, and c) RNA was extracted from cells and qRT-PCR for ISG20 (a), CX3CL1 (b), and CXCL10 (c) was performed. Data are presented as mean ± SD (n = 3, *P < 0.01). (d) Cells were lysed, and western blotting was performed for ISG20 and actin.
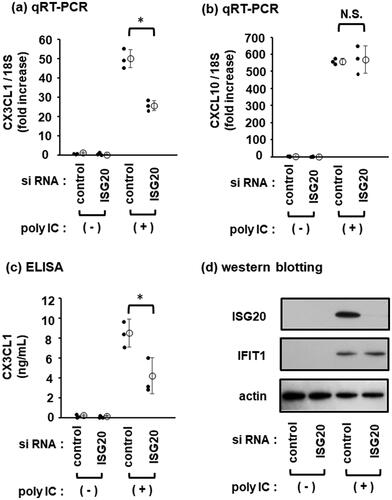
ISG20 is involved in CX3CL1 induction by poly IC
To determine whether ISG20 is involved in the regulation of CX3CL1 and CXCL10 expression, knockdown experiments were performed. ISG20 knockdown partially decreased poly IC-induced CX3CL1 expression () but had no effect on the CXCL10 expression (). The concentration of CX3CL1 protein in the conditioned medium of poly IC-treated cells also partially decreased upon ISG20 knockdown (). However, ISG20 knockdown, as confirmed by western blotting, had no effect on the poly IC-induced expression of IFIT1 protein (which served as a positive control) ().
Figure 3. ISG20 is partially involved in poly IC-induced CX3CL1 expression. Cells were transfected with the negative control siRNA or ISG20 siRNA and incubated for 48 h. Then, 30 µg/mL of poly IC was added to the cultures and incubated for additional 24 h. (a and b) RNA was extracted from the cells, and qRT-PCR for CX3CL1 (a) and CXCL10 (b) was performed. (c) The culture medium was collected, and the concentration of CX3CL1 protein in the culture medium was estimated by ELISA. Data (a), (b), and (c) are presented as mean ± SD (n = 3, *P < 0.01; N.S., not significant). (d) Cells were lysed and western blotting was performed for ISG20, IFIT1, and actin.
ISG20 is expressed in GECs in the biopsy specimen of proliferative lupus nephritis
Significant positive staining of ISG20 in the endothelial area was observed in the biopsy specimens from patients with class IV LN, whereas ISG20 expression in the specimens from patients with Class II LN was weak. However, in other specimens, ISG20 expression was negligible or undetectable (). Representative dual-labeling staining with anti-ISG20 and anti-CD34, markers of GECs, for biopsy specimen from a patient with class IV LN showed that ISG20 expression was mainly concentrated in the endothelial area (, a specimen of class IV LN showing merged staining of ISG20 and CD34).
Figure 4. Immunofluorescence staining of ISG20 in renal biopsy specimens obtained from patients with non-proliferative LN, proliferative LN, IgA nephropathy, and minimal-change nephrotic syndrome. Dual immunostaining of ISG20 (green) and CD34 (red) in renal biopsy specimens. (a) Upper left, non-proliferative lupus nephritis (LN) [Class II in accordance with the International Society of Nephrology/Renal Pathology Society (ISN/RPS) 2003 classification for LN]; upper right, proliferative LN [Class IV-G (A) in accordance with the ISN/RPS 2003 classification for LN]; lower left, IgA nephropathy; and lower right, minimal-change nephrotic syndrome (served as a non-inflammatory control). A significant increase in ISG20 immunoreactivity was observed in proliferative LN specimens, whereas immunoreactivity was weak or negligible (upper right corner) in the other specimens (×400 magnification). (b) In proliferative lupus nephritis, positive staining for ISG20 (green) was detected mainly in the endothelial area (×400 magnification).
![Figure 4. Immunofluorescence staining of ISG20 in renal biopsy specimens obtained from patients with non-proliferative LN, proliferative LN, IgA nephropathy, and minimal-change nephrotic syndrome. Dual immunostaining of ISG20 (green) and CD34 (red) in renal biopsy specimens. (a) Upper left, non-proliferative lupus nephritis (LN) [Class II in accordance with the International Society of Nephrology/Renal Pathology Society (ISN/RPS) 2003 classification for LN]; upper right, proliferative LN [Class IV-G (A) in accordance with the ISN/RPS 2003 classification for LN]; lower left, IgA nephropathy; and lower right, minimal-change nephrotic syndrome (served as a non-inflammatory control). A significant increase in ISG20 immunoreactivity was observed in proliferative LN specimens, whereas immunoreactivity was weak or negligible (upper right corner) in the other specimens (×400 magnification). (b) In proliferative lupus nephritis, positive staining for ISG20 (green) was detected mainly in the endothelial area (×400 magnification).](/cms/asset/5de55978-bec9-4d0e-8844-e7d645bc9d31/irnf_a_2224890_f0004_c.jpg)
Discussion
In terms of glomerulonephritis, CX3CL1/fractalkine could induce chemotaxis, leading to the activation of circulating CX3CL1 receptor-positive immune cells [Citation22]. Mesangial CX3CL1 expression correlates with histopathological activity and severity in patients with LN [Citation4]. Thus, the induction of CX3CL1 in resident glomerular cells and the subsequent recruitment of circulating inflammatory cells are involved in the initiation and progression of LN [Citation5]. Given the importance of TLR3 signaling in resident glomerular cells [Citation8,Citation9], we examined its role in regulating CX3CL1 expression in cultured human mesangial cells (MCs) and GECs [Citation5,Citation21]. We previously reported that the TLR3/IFN regulatory factor 3 (IRF3) axis, but not the TLR3/nuclear factor κB (NF-κB) axis, was involved in regulating CX3CL1 expression in MCs [Citation21]. However, the TLR3/IFR3 and TLR3/NF-κB axes were equally involved in regulating CX3CL1 expression in GECs [Citation5]. Although this discrepancy might be due to differences between the two cell types, this issue remains to be resolved [Citation5]. To better understand the pathogenesis of glomerular inflammation, it is important to decipher the role of signaling cascades involved in regulating ISG20 expression in resident renal cells, as ISG20 is involved in the glomerular endothelial expression of CX3CL1, a strong chemoattractant of activated leukocytes.
ISGs could induce early innate immune responses [Citation17]. ISG20 interferes with the replication of RNA viruses by directly degrading viral RNA [Citation23]. Furthermore, the expression of ISG20 in nerve cells is reportedly associated with tumorigenesis, suggesting that ISG20 could also regulate cellular processes [Citation24]. Although we previously reported that treatment with poly IC, a TLR3 agonist, increased ISG20 expression in human MCs, its implications remain unknown [Citation20]. In the present study, we found that similar to MCs, poly IC treatment induced ISG20 expression in a time- and concentration-dependent manner in GECs. Notably, ISG20 expression was induced only by the TLR3 agonist and not by the TLR4, TLR7, or TLR9 agonists. Thus, ISG20 expression in GECs was TLR3-dependent. Our previous studies using cultured human MCs and GECs demonstrated that IFN-β, but not IFN-α, was synthesized de novo following TLR3 activation and IFN-β was crucial for regional inflammatory cascades [Citation10,Citation14–16,Citation19,Citation20]. IFN-β knockdown markedly inhibited poly IC-induced ISG20 expression, indicating that endothelial ISG20 expression was regulated via the TLR3/IFN-β axis. Furthermore, ISG20 knockdown led to a decrease in CX3CL1 expression but not CXCL10 production. Thus, we conclude that endothelial CX3CL1 expression can be regulated by the TLR3/IFN-β/ISG20 axis. The production of CX3CL1 via ISG20 by resident renal cells may be involved in glomerular inflammation and glomerulonephritis initiation. In this study, we found intense ISG20 immunoreactivity in the glomerular endothelial area of specimens from patients with proliferative LN. Thus, our present observation suggests that marked type I IFN activation leading to ISG20 expression could occur in the glomerular lesions of patients with proliferative LN. In this context, it has been reported that ISGs enhanced innate immune signaling [Citation17]. Thus, endothelial expression of ISG20 functions enhances innate immune reactions in resident renal cells through CX3CL1 production, although further studies are required to obtain more conclusive evidence.
We have reported on the mesangial expression of myxovirus resistance protein 1 and IFI35, endothelial expression of IFIT1 and DDX60, enhanced glomerular inflammation, and that the expression of these ISGs in renal biopsy specimens was positively related to the severity of proliferative LN [Citation10,Citation14,Citation15]. In contrast, mesangial ISG15 was involved in the negative feedback loop of activated innate immune reactions, and its expression in renal biopsy specimens is negatively correlated with the severity of proliferative LN [Citation19]. Therefore, ISGs may play either protective or deleterious roles in the pathogenesis of glomerular inflammation.
Conclusion
Based on our experimental results, we suggest that the dynamic regional expression of ISG20 may be associated with the activation of innate immune reactions in GECs. Thus, altered levels of ISG20 and other components of the TLR3/IFN-β/ISG axis in resident renal cells might be a characteristic in patients with glomerular diseases, particularly LN [Citation14–16,Citation19]. Further detailed studies focusing on the interactions between these ISGs and the downstream expression of chemokines/cytokines are necessary. We also consider that the overexpression of ISG20 and its downstream inflammatory mediators, including CX3CL1, may be involved in the pathogenesis of glomerular inflammation. Thus, the pharmacological modulation of these signaling pathways might be a novel therapeutic approach for the treatment of glomerular diseases, particularly LN.
Ethics statement
This article does not contain any studies involving human participants or animals performed by any of the authors that otherwise requires ethical approval.
Patient consent
All authors read and approved the final manuscript.
Author contributions
T.I. and H.T. designed the study; T.K., R.S., M.F., T.A., K.T., S.K., K.S. and T.I. performed the experiments; T.K. and H.T. wrote the manuscript; and D.M. and K.T. provided technical support. K.J. performed immunostaining for ISG20 in renal biopsy specimens. T.I. and H.T. gave conceptual advice.
Acknowledgement
A preprint version of the core tip of this MS has been published previously (Karasawa T, Sato R, Imaizumi T, et al. Implication of glomerular endothelial expression of interferon-stimulated gene 20, an antiviral effector protein, in human kidney inflammation. https://www.researchsquare.com/article/rs-1770007/v1.).
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
All data generated or analyzed during this study are included in this article.
Additional information
Funding
References
- Lai AS, Lai KN. Viral nephropathy. Nat Clin Pract Nephrol. 2006;2(5):1–8. doi: 10.1038/ncpneph0166.
- Anders HJ, Banas B, Schlöndorff D. Signaling danger: toll-like receptors and their potential roles in kidney disease. J Am Soc Nephrol. 2004;15(4):854–867. doi: 10.1097/01.asn.0000121781.89599.16.
- Sepe V, Libetta C, Gregorini M, et al. The innate immune system in human kidney inflammaging. J Nephrol. 2022;35(2):381–395. doi: 10.1007/s40620-021-01153-4.
- Yoshimoto S, Nakatani K, Iwano M, et al. Elevated levels of fractalkine expression and accumulation of CD16+ monocytes in glomeruli of active lupus nephritis. Am J Kidney Dis. 2007;50(1):47–58. doi: 10.1053/j.ajkd.2007.04.012.
- Hirono K, Imaizumi T, Aizawa T, et al. Endothelial expression of fractalkine (CX3CL1) is induced by toll-like receptor 3 signaling in cultured human glomerular endothelial cells. Mod Rheumatol. 2020;30(6):1074–1081. doi: 10.1080/14397595.2019.1682768.
- Ding X, Ren Y, He X. IFN-I mediates lupus nephritis from the beginning to renal fibrosis. Front Immunol. 2021;12:676082. doi: 10.3389/fimmu.2021.676082.
- Kawai T, Akira S. TLR signaling. Cell Death Differ. 2006;13(5):816–825. doi: 10.1038/sj.cdd.4401850.
- Anders HS. Pseudoviral immunity - a novel concept for lupus. Trends Mol Med. 2009;15(12):553–561. doi: 10.1016/j.molmed.2009.10.004.
- Tanaka H, Imaizumi T. Inflammatory chemokine expression via toll-like receptor 3 signaling in normal human mesangial cells. Clin Dev Immunol. 2013;2013:984708. doi: 10.1155/2013/984708.
- Watanabe S, Imaizumi T, Tsuruga K, et al. Glomerular expression of myxovirus resistance protein 1 (Mx1) in human mesangial cells: possible activation of innate immunity in the pathogenesis of lupus nephritis. Nephrology (Carlton). 2013;18(12):833–837. doi: 10.1111/nep.12155.
- Imaizumi T, Aizawa T, Segawa C, et al. Toll-like receptor 3 signaling contributes to the expression of a neutrophil chemoattractant, CXCL1, in human mesangial cells. Clin Exp Nephrol. 2015;19(5):761–770. doi: 10.1007/s10157-014-1060-4.
- Lorenz G, Lech M, Anders HJ. Toll-like receptor activation in the pathogenesis of lupus nephritis. Clin Immunol. 2017;185:86–94. doi: 10.1016/j.clim.2016.07.015.
- Conti F, Spinelli FR, Truglia S, et al. Kidney expression of toll-like receptors in lupus nephritis: quantification and clinicopathological correlations. Mediators Inflamm. 2016;2016:7697592. doi: 10.1155/2016/7697592.
- Imaizumi T, Yano C, Numata A, et al. Interferon (IFN)-induced protein 35 (IFI35), a type I interferon-dependent transcript, upregulates inflammatory signaling pathways by activating toll-like receptor 3 in human mesangial cells. Kidney Blood Press Res. 2016;41(5):635–642. doi: 10.1159/000447932.
- Hashimoto S, Imaizumi T, Aizawa T, et al. Expression of IFN-induced transmembrane protein 1 in glomerular endothelial cells. Pediatr Int. 2021;63(9):1075–1081. doi: 10.1111/ped.14579.
- Karasawa T, Sato R, Imaizumi T, et al. Glomerular endothelial expression of type I IFN-stimulated gene, DExD/H-Box helicase 60 via toll-like receptor 3 signaling: possible involvement in the pathogenesis of lupus nephritis. Ren Fail. 2022;44(1):137–145. doi: 10.1080/0886022X.2022.2027249.
- Crosse KM, Monson EA, Beard MR, et al. Interferon-stimulated genes as enhancers of antiviral signaling. J Innate Immun. 2018;10(2):85–93. doi: 10.1159/000484258.
- Espert L, Degols G, Gongora C, et al. ISG20, a new interferon-induced RNase specific for single-stranded RNA, defines an alternative antiviral pathway against RNA. J Biol Chem. 2003;278(18):16151–16158. doi: 10.1074/jbc.M209628200.
- Imaizumi T, Shimada T, Matsumiya T, et al. Interferon-stimulated gene 15, a type I interferon-dependent transcript, is involved in a negative feedback loop in innate immune reactions in human mesangial cells. Nephron. 2016;132(2):144–152. doi: 10.1159/000443934.
- Imaizumi T, Tanaka H, Mechti N, et al. Polyinosinic-polycytidylic acid induces the expression of interferon-stimulated gene 20 in mesangial cells. Nephron Exp Nephrol. 2011;119(2):e40–e48. doi: 10.1159/000328923.
- Aizawa-Yashiro T, Imaizumi T, Tsuruga K, et al. Glomerular expression of fractalkine is induced by polyinosinic-polycytidylic acid in human mesangial cells: possible involvement of fractalkine after viral infection. Pediatr Res. 2013;73(2):180–186. doi: 10.1038/pr.2012.165.
- Anders HJ, Vielhauer V, Schlöndorff D. Chemokines and chemokine receptors are involved in the resolution or progression of renal disease. Kidney Int. 2003;63(2):401–415. doi: 10.1046/j.1523-1755.2003.00750.x.
- Weiss CM, Trobaugh DW, Sun C, et al. The interferon-induced exonuclease ISG20 exerts antiviral activity through upregulation of type I interferon response proteins. mSphere. 2018;3(5):e00209–e00218. doi: 10.1128/mSphere.00209-18.
- Gao M, Lin Y, Liu X, et al. ISG20 promotes local tumor immunity and contributes to poor survival in human glioma. Oncoimmunol. 2019;8(2):e1534038. doi: 10.1080/2162402X.2018.1534038.
