Abstract
Calciphylaxis is a rare and life-threatening condition in patients with end-stage kidney disease (ESKD). In this case report, we reported a 72-year-old female who had undergone aortic and mitral mechanical valve replacement 22 years ago due to rheumatic aortic and mitral stenosis. Following the valve replacement, she initiated warfarin treatment. Five years ago, she received a diagnosis of uremia and has since been undergoing regular hemodialysis. Ten months prior to her current admission, she experienced excruciating pain and was diagnosed with calciphylaxis. Additionally, an electrocardiogram revealed atrial fibrillation, while echocardiography indicated that the aortic and mitral mechanical valves were appropriately positioned, with normal perivalvular surroundings and good valve leaflet activity. No noticeable thrombosis was observed in the left atrium or left atrial appendage. Color Doppler imaging showed moderate stenosis in the lower extremity arteries, with no venous thromboembolism present. Extensive eggshell-like calcification within the arterial media was detected. The patient was managed with regular hemodialysis, symptomatic treatments (including anticoagulation and analgesia), and sodium thiosulfate. Unfortunately, symptomatic management provided limited relief, and during the one-month follow-up period, the patient passed away due to septic shock. Currently, there is insufficient conclusive evidence regarding alternative influential anticoagulants or appropriate prosthetic valve selection. For individuals with ESKD receiving maintenance hemodialysis, early identification, diagnosis, and treatment of calciphylaxis are of paramount importance.
Background
Calciphylaxis, known as calcific uremic arteriolopathy, presents a rare and life-threatening challenge in patients with end-stage kidney disease (ESKD). Its low incidence and limited clinical studies contribute to the difficulty in diagnosis and treatment [Citation1]. Here, we present a clinical case highlighting the treatment dilemma of a 72-year-old female with calciphylaxis following 22 years of mechanical cardiac valve replacement. Additionally, we conducted a comprehensive review of reported cases of calciphylaxis in ESKD patients with mechanical valve replacement to identify practical solutions for this challenging treatment dilemma.
Case presentation
A 72-year-old female underwent aortic and mitral mechanical valve replacement in 2000 due to rheumatic aortic and mitral stenosis. To manage the mechanical valves and atrial fibrillation (AF), the patient was prescribed a daily dose of warfarin ranging from 1.25 mg to 1.875 mg. The international normalized ratio (INR) was consistently maintained between 2.5 and 3.5. However, five years ago, she was diagnosed with uremia, leading to regular hemodialysis three times a week. Around ten months prior to her current admission, she experienced excruciating pain, rated as 10 on the visual analog scale, and developed skin lesions in her right foot toes as a result of an accidental traumatic injury. Despite receiving wound care, pain medication, antibiotics, and vasodilators, there has been no significant improvement in her condition.
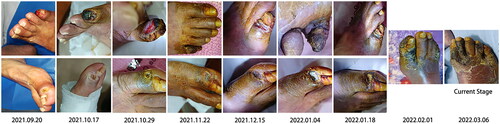
Her height was recorded as 152.0 cm, her weight was 48.0 kg, and her body mass index was calculated as 20.8 kg/㎡. During the physical examination, significantly reduced dorsalis pedis pulses were noted in both feet, along with a lower temperature in the bilateral lower extremities. Skin lesions were observed on the dorsum of the right foot, while the skin around the first and fifth toes exhibited signs of dryness, shrinking, and cutaneous darkening (, current stage). The wound appeared dry with no exudation. However, the patient’s parathyroid hormone (PTH) levels were elevated at 9.20 pmol/L (reference range, 1.60–6.90 pmol/L). Throughout the course of the disease, hemoglobin levels fluctuated between 108–121 g/L (reference range, 115–150 g/L), corrected serum calcium ranged between 2.61–2.84 mmol/L (reference range, 2.25–2.66 mmol/L), and inorganic phosphorus fluctuated between 0.8–1.7 mmol/L (reference range, 0.85–1.51 mmol/L). Despite the patient’s consistent use of warfarin and modifications to the dialysis protocol, the INR showed variations between 1.81 and 7.05 (reference range, 0.88–1.50). Additionally, the glycated hemoglobin (HbA1c) level was measured at 6.7% (reference range, 4.5–6.1%). The details of the results were shown in Supplementary Table 1.
Figure 1. Progression of the cutaneous lesion (the metatarsal bone, the third toe, and the little toe suffered the most and presented with ulcers within one week, accompanied by pain and progressive cutaneous darkening and sclerosis. The skin temperature was low at the initial stage. In addition, the patient had weakened bilateral dorsalis pedis pulses, especially on the right side).
The electrocardiogram demonstrated AF. The transthoracic echocardiography showed that the aortic and mitral mechanical valves were well placed. In addition, the valve leaflets had a good activity with normal perivalvular surroundings. There was no apparent thrombosis in the left atrium or the left atrial appendage (). Transesophageal echocardiography was not performed due to intolerability. The color Doppler ultrasound showed moderate stenosis in the lower extremity arteries, and there was no venous thromboembolism. Furthermore, extensive eggshell calcification of the arterial media was detected (). Biopsies were not obtained due to concerns about potential difficulties in wound healing. In addition, X-rays of the lower limbs could not be obtained as the patient had difficulty standing.
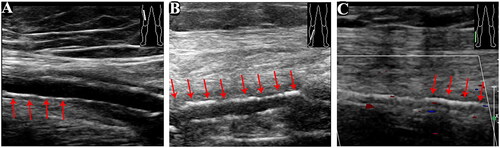
Figure 2. Echocardiography manifestations [A-B: the aortic and mitral mechanical valves (red arrows) were well placed with good activity of the valve leaflets and normal perivalvular surroundings with iso-echogenicity attached. C: There was no significant thickening of the tricuspid valve leaflet. Further, the valve showed good opening but poor closing].
![Figure 2. Echocardiography manifestations [A-B: the aortic and mitral mechanical valves (red arrows) were well placed with good activity of the valve leaflets and normal perivalvular surroundings with iso-echogenicity attached. C: There was no significant thickening of the tricuspid valve leaflet. Further, the valve showed good opening but poor closing].](/cms/asset/f2527c28-cee6-44e9-94fd-0ede2cd9fc10/irnf_a_2264401_f0002_c.jpg)
Figure 3. Vascular ultrasound of the lower extremities (there was no evident abnormality in the diameter of the bilateral lower limb artery. However, the walls were rough with multiple hyperechoic and hypoechoic plaques. Extensive calcification of the arterial media was detected. A: right femoral artery; B: right posterior tibial artery; C: right dorsalis pedis artery; red arrows show calcification areas of the arterial media).
After a multidisciplinary consultation, the patient was clinically diagnosed with calciphylaxis due to several factors including female gender, long-term use of warfarin and iron supplements, extended duration of dialysis, recurrent skin trauma, and elevated HbA1c levels. In addition, she underwent regular hemodialysis and received anticoagulants and analgesic agents (Supplementary Table 2). During the disease course, warfarin was discontinued due to the elevated INR (Supplementary Table 1). She received sodium thiosulfate (STS) for the management of calciphylaxis. An initial dose of 4 g of STS (in 100 mL of saline) is administered intravenously during the final hour of hemodialysis. The dose is then increased to 6 g and 8 g during the subsequent two hemodialysis sessions, and is maintained at 8 g (in 100 mL of saline). Unfortunately, symptomatic management offered little relief, and she forwent further treatment due to high out-of-pocket medical costs. The patient died due to septic shock during the one-month follow-up period.
Discussion
The definitive diagnosis of calciphylaxis requires both clinical and histopathological criteria [Citation1]. It is worth noting that while skin biopsy remains the preferred method for confirming suspected calciphylaxis, the clinical utility of skin biopsy in calciphylaxis is still a matter of ongoing debate, and there is currently no consensus on its true effectiveness. Skin biopsy does carry a higher risk of complications, such as the development of new ulcers and infection [Citation2]. Some researchers have suggested using punch biopsy as an alternative to excisional biopsy in order to minimize further tissue damage during the diagnosis of calciphylaxis, and a telescoping punch biopsy technique may be utilized to reach deeper tissue layers [Citation3]. However, in certain cases where wound healing is challenging, and therefore this approach may not be performed [Citation4]. In our case, the eggshell calcification in Doppler indicated arterial media calcification, but it is challenging to detect calcification in micro-arteries with a diameter less than 500 μm using Doppler imaging. For maintenance hemodialysis patients with dry gangrene, especially those with an intractable ulcer, black eschar, and severe pain, calciphylaxis should be diagnosed clinically despite near-normal levels of PTH or the negative result of skin biopsy [Citation3].
The treatment of calciphylaxis involves wound care, pain management, control of risk factors, and pharmacological treatments [Citation5]. In refractory cases, advanced therapeutic approaches, such as the use of human amnion-derived mesenchymal stem cells, might be considered [Citation6]. Wound care includes surgical cleaning, analgesics, antibiotics and hyperbaric oxygen therapy (HBOT). In this case, surgical treatment is not recommended by orthopedic surgeons, as the deep tissue was not completely necrotic, which means an unclear amputation plane. Parathyroidectomy might be of help in patients with secondary hyperparathyroidism [Citation7], which was also not applicable in our patient. HBOT optimizes the fungicidal ability of neutrophils, which is of help to wound healing [Citation8]. However, rheumatic heart disease combined with AF lower the tolerability of HBOT. Moreover, HBOT is limited by the present paucity of evidence-based proof.
Common pharmacological treatments include STS and vitamin K. STS is a promising approach in the treatment of calciphylaxis [Citation9], which is able to prevent calcium phosphate precipitation by sequestering calcium ions to form highly soluble calcium thiosulfate complexes and alleviate pain [Citation10]. However, the therapeutic effect is not obvious, as the use of off-label STS requires long-term treatment. Matrix Gla protein (MGP) is a vitamin K-dependent protein synthesized by vascular smooth muscle cells and endothelial cells. Carboxylated MGP is a potent inhibitor of calcification, and carboxylation is dependent on vitamin K. However, vitamin K, an antagonist to warfarin, was not taken either. A dilemma existed due to the paradoxical use of warfarin. On the one hand, warfarin should be taken after mechanical valve replacement. On the other hand, despite the diagnosis of vascular calcifications and calciphylaxis, warfarin is a risk factor for both [Citation1].
The identification of effective non-vitamin K antagonists (VKAs) for patients with ESKD and a mechanical cardiac valve remains challenging and uncertain during these years [Citation11,Citation12]. The clinical scenarios and prognosis of anticoagulation therapy in ESKD patients with calciphylaxis also vary from person to person (). Some reported a significant clinical improvement after changing warfarin into unfractionated heparin [Citation20] or enoxaparin [Citation19], however, in some cases, the lesion still progressed even after the anticoagulation regimen was altered or halted [Citation18–20]. Warfarin was proved to have a higher risk of all‐cause hospitalization in ESKD patients with AF [Citation21], there is need to look for alternatives. Heparin is often used, and might be a therapeutic option [Citation22], but the need for subcutaneous injections made it inconvenient in patients’ daily life. Direct oral anticoagulants (DOAC) are now the first alternatives to VKAs in ESKD patients with AF [Citation23], in these days, considering rare complications such as calciphylaxis, more DOACs were prescribed to ESKD patients with AF [Citation24]. Based on the clinical experience of King et al. [Citation25], it has been found that the use of DOACs in patients with calciphylaxis is safe and well tolerated. Additionally, Russ et al. [Citation26] indicated that calciphylaxis progresses and develops as a chronic process rather than an acute one. Therefore, the early use of DOACs may be beneficial in reducing the incidence of calciphylaxis. This suggests that DOACs have potential clinical significance in the prevention and management of calciphylaxis.
Table 1. A Summary of reported cases of calciphylaxis and prosthetic cardiac valve replacement treated with warfarin.
Nevertheless, DOACs seem to be contraindicated in patients with mechanical valves [Citation27]. Compared with warfarin, patients with mechanical cardiac valves, who were treated with dabigatran, had increased rates of thromboembolic and bleeding complications, showing no benefit and an excess risk of dabigatran [Citation28]. In a small investigator-initiated trial, rivaroxaban was found to have comparable rates of thromboembolic events and bleeding events when compared to warfarin [Citation29]. However, it is important to note that the evidence from this trial is not conclusive due to the limitations of a small sample size, as only 44 participants were randomized, and there was minimal follow-up conducted, with a duration of only 90 days. It should be noted that all the above studies excluded patients with chronic kidney disease or ESKD, whether dabigatran and rivaroxaban are applicable in ESKD patients with mechanical cardiac valve replacement still lacks sufficient high-quality clinical trial evidence [Citation30]. Also, evidence regarding the use of edoxaban in patients with mechanical aortic valve remains limited, as the ENGAGE AF-TIMI 48 Trial specifically excluded patients with mechanical aortic valve replacement [Citation31].
It seems that warfarin is commonly prescribed as an oral anticoagulant for patients with ESKD. However, in cases where patients are experiencing calciphylaxis, apixaban has been recommended as an alternative option [Citation32]. However, the early cessation of the PROACT Xa trial indicated that the rates of thromboembolism and valve thrombosis causing stroke were higher in the apixaban group compared to the warfarin group. This finding suggests that apixaban may not be as effective as warfarin in preventing these complications in patients with a mechanical cardiac valve [Citation33]. The optimal anticoagulation therapy needs further exploration.
Some researchers believe that the biological valves might be better in prosthetic valve selection in ESKD patients [Citation13,Citation18]. They deem that the hemodialysis-dependent patients experiencing the valve replacement might have a poorer prognosis than normal patients, and biological valves might be better to avoid further comorbidities. Therefore, the selection of the prosthetic valves in ESKD patients might be essential as well, as a mechanical valve requires a life-long administration of warfarin, whilst a biological valve only needs 2 to 3 months of anticoagulation, which might be better to avoid further comorbidities. In current guidelines [Citation34,Citation35], age is an important factor in prosthetic valve selection. A mechanical valve is recommended in patients aged <50 years in America and <60 years in Europe, respectively. Large retrospective studies found that biological valves have comparable rates of survival and valve-related reoperation with mechanical valves in ESKD patients who need dialysis, with lower bleeding and thromboembolic events [Citation36,Citation37]. However, calciphylaxis can occur rapidly in a few cases [Citation17,Citation20], and even in patients with biological valves [Citation16]. In addition, although no significant difference of the morbidity was found between the mechanical and biological mitral valve replacement, the mechanical aortic valve replacement was associated with a lower hazard of death [Citation38]. Currently, there is no definitive consensus on whether bioprosthetic valves are more suitable for ESKD patients, as not all patients who undergo mechanical valve replacement will develop calciphylaxis. However, we believe it is important to consider the potential risk of calciphylaxis when selecting valves for ESKD patients.
One lesson we learned from these cases is that prevention is more significant than treatment. For primary care physicians, especially those not specialized in nephrology, education on calciphylaxis prevention knowledge should be strengthened [Citation24]. High risk factor identification, adjustments to the dialysis prescription, avoidance of a high calcium bath and calcium-based phosphate binders, and maintenance of calcium-phosphate metabolism are of vital importance to prevent the occurrence of calciphylaxis [Citation39]. In the case of patients with ESKD who have been diagnosed with anemia, mineral and bone disorders, it is crucial to exercise caution while prescribing medications like calcium-based binders and iron supplements, which have the potential to escalate the risk of developing calciphylaxis [Citation40]. Additionally, necessary tests such as serum Vitamin D levels can provide valuable insights [Citation41]. However, in this particular case, the optimal treatment timing was missed due to multiple clinical department visits in the early stages, and some important tests, such as serum Vitamin D and Vitamin K levels, were not completed.
Conclusion
Calciphylaxis is a rare but devastating disease in ESKD patients with mechanical cardiac valve replacement treated with warfarin. The currently available evidence of the effective alternative anticoagulants or the suitable selection of the prosthetic valves is inconclusive so far. For ESKD patients with maintenance hemodialysis, early identification, diagnosis and treatment of calciphylaxis are essential to arrest the progression of the disease and prevent serious complications such as amputation, sepsis, and death.
Ethical approval
The study was approved by the ethics committee of West China Hospital, Sichuan University.
Supplemental Material
Download PDF (161.7 KB)Acknowledgments
The authors thank our patient for allowing for her case to be presented.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Rick J, Strowd L, Pasieka HB, et al. Calciphylaxis: part I. Diagnosis and pathology. J Am Acad Dermatol. 2022;86(5):1–7. doi:10.1016/j.jaad.2021.10.064.
- Nigwekar SU, Thadhani R, Brandenburg VM. Calciphylaxis. N Engl J Med. 2018;378(18):1704–1714. doi:10.1056/NEJMra1505292.
- Gallo Marin B, Aghagoli G, Hu SL, et al. Calciphylaxis and kidney disease: a review. Am J Kidney Dis. 2023;81(2):232–239. doi:10.1053/j.ajkd.2022.06.011.
- Dobry AS, Nguyen ED, Shah R, et al. The role of skin biopsy in diagnosis and management of calciphylaxis: a retrospective analysis. J Am Acad Dermatol. 2021;85(3):765–767. doi:10.1016/j.jaad.2020.05.101.
- Oliveira TM, Frazão JM. Calciphylaxis: from the disease to the diseased. J Nephrol. 2015;28(5):531–540. doi:10.1007/s40620-015-0192-2.
- Bian A, Ye X, Wang J, et al. Therapeutic effects and mechanism of human amnion-derived mesenchymal stem cells on hypercoagulability in a uremic calciphylaxis patient. Ren Fail. 2023;45(1):2218483. doi:10.1080/0886022X.2023.2218483.
- Roy S, Reddy SN, Garcha AS, et al. Successful treatment of calciphylaxis in a young female with End-Stage renal disease on peritoneal dialysis with parathyroidectomy, intensification of dialysis, and sodium Thiosulphate-A case report and literature review. J Investig Med High Impact Case Rep. 2021;9:23247096211060580. doi:10.1177/23247096211060580.
- McCarthy JT, El-Azhary RA, Patzelt MT, et al. Survival, risk factors, and effect of treatment in 101 patients with calciphylaxis. Mayo Clin Proc. 2016;91(10):1384–1394. doi:10.1016/j.mayocp.2016.06.025.
- Yang X, Liu Y, Xie X, et al. Use of the optimized sodium thiosulfate regimen for the treatment of calciphylaxis in chinese patients. Ren Fail. 2022;44(1):914–922. doi:10.1080/0886022X.2022.2081179.
- Schlieper G, Brandenburg V, Ketteler M, et al. Sodium thiosulfate in the treatment of calcific uremic arteriolopathy. Nat Rev Nephrol. 2009;5(9):539–543. doi:10.1038/nrneph.2009.99.
- Krüger T, Brandenburg V, Schlieper G, et al. Sailing between scylla and charybdis: oral long-term anticoagulation in dialysis patients. Nephrol Dial Transplant. 2013;28(3):534–541. doi:10.1093/ndt/gfs485.
- Thomson BKA, Pilkey NG, Monteith B, et al. A scoping review of alternative anticoagulation strategies for hemodialysis patients with a mechanical heart valve. Am J Nephrol. 2021;52(10-11):861–870. doi:10.1159/000519921.
- Cadavid JC, DiVietro ML, Torres EA, et al. Warfarin-induced pulmonary metastatic calcification and calciphylaxis in a patient with end-stage renal disease. Chest. 2011;139(6):1503–1506. doi:10.1378/chest.10-1322.
- Disbrow MB, Qaqish I, Kransdorf M, et al. Calcific uraemic arteriolopathy. BMJ Case Rep. 2015;2015:bcr2014207935. doi:10.1136/bcr-2014-207935.
- Berry A, Degheim G, Saba S. Arteriolar vs. valvular thrombosis: pick your evil!. Thromb J. 2018;16:23. doi:10.1186/s12959-018-0175-3.
- Sohal R, George T. Sodium-Thiosulfate induced Life-Threatening metabolic acidosis limiting treatment of calciphylaxis. Am J Case Rep. 2020;21:e919926. doi:10.12659/AJCR.919926.
- Diasty ME, Cuenca J. New-onset acute rapidly deteriorating case of calciphylaxis after open heart surgery: a case report. Eur Heart J Case Rep. 2021;5(3):ytab060. doi:10.1093/ehjcr/ytab060.
- Hirooka K, Anju K, Moriyama Y, et al. Calciphylaxis after aortic valve replacement in a patient with End-Stage renal disease. Ann Vasc Dis. 2021;14(4):376–379. doi:10.3400/avd.cr.21-00040.
- Smith MC, Gleaves E, Singh A, et al. Anticoagulation in a patient with mechanical prosthetic valves and calcific uremic arteriolopathy on warfarin. Cureus. 2021;13(3):e14196. doi:10.7759/cureus.14196.
- Nyembo PF, Eidman KE, Simegn M, et al. Rapidly progressive and catastrophic calciphylaxis after mechanical valve replacement: an anticoagulation dilemma. JACC Case Rep. 2022;4(19):1319–1323. doi:10.1016/j.jaccas.2022.07.034.
- Steiner D, Schmaldienst S, Lorenz M, et al. Atrial fibrillation and anticoagulation are associated with hospitalisations in patients with end-stage kidney disease on haemodialysis: a prospective population-based cohort study. Thromb J. 2022;20(1):71. doi:10.1186/s12959-022-00434-7.
- Carter A, Ortega-Loayza AG, Barrett J, et al. Calciphylaxis with evidence of hypercoagulability successfully treated with unfractionated heparin: a multidisciplinary approach. Clin Exp Dermatol. 2016;41(3):275–278. doi:10.1111/ced.12729.
- Laugesen EK, Staerk L, Carlson N, et al. Non-vitamin K antagonist oral anticoagulants vs. vitamin-K antagonists in patients with atrial fibrillation and chronic kidney disease: a nationwide cohort study. Thromb J. 2019;17:21. doi:10.1186/s12959-019-0211-y.
- Halperin LF, Lee MK, Liew J, et al. Anticoagulation for patients with atrial fibrillation and End-Stage renal disease on dialysis: a national survey. Can J Cardiol. 2021;37(6):924–928. doi:10.1016/j.cjca.2020.12.005.
- King BJ, El-Azhary RA, McEvoy MT, et al. Direct oral anticoagulant medications in calciphylaxis. Int J Dermatol. 2017;56(10):1065–1070. doi:10.1111/ijd.13685.
- Russ P, Russwurm M, Kortus-Goetze B, et al. Phenprocoumon based anticoagulation is an underestimated factor in the pathogenesis of calciphylaxis. BMC Nephrol. 2019;20(1):114. doi:10.1186/s12882-019-1301-6.
- Chan N, Sobieraj-Teague M, Eikelboom JW. Direct oral anticoagulants: evidence and unresolved issues. Lancet. 2020;396(10264):1767–1776. doi:10.1016/S0140-6736(20)32439-9.
- Eikelboom JW, Connolly SJ, Brueckmann M, et al. Dabigatran versus warfarin in patients with mechanical heart valves. N Engl J Med. 2013;369(13):1206–1214. doi:10.1056/NEJMoa1300615.
- Duraes AR, de Souza Lima Bitar Y, Schonhofen IS, et al. Rivaroxaban versus warfarin in patients with mechanical heart valves: open-Label, proof-of-Concept trial-The RIWA study. Am J Cardiovasc Drugs. 2021;21(3):363–371. doi:10.1007/s40256-020-00449-3.
- Ryu R, Tran R. DOACs in mechanical and bioprosthetic heart valves: a narrative review of emerging data and future directions. Clin Appl Thromb Hemost. 2022;28:10760296221103578. doi:10.1177/10760296221103578.
- Kato ET, Giugliano RP, Ruff CT, et al. Efficacy and safety of edoxaban in elderly patients with atrial fibrillation in the ENGAGE AF-TIMI 48 trial. J Am Heart Assoc. 2016;5(5):e003432. doi:10.1161/JAHA.116.003432.
- Jain N, Reilly RF. Clinical pharmacology of oral anticoagulants in patients with kidney disease. Clin J Am Soc Nephrol. 2019;14(2):278–287. doi:10.2215/CJN.02170218.
- Ryu R. DOACs in valvular heart disease: latest key updates on PROACT Xa (apixaban) and INVICTUS (rivaroxaban). Clin Appl Thromb Hemost. 2022;28:10760296221144044. doi:10.1177/10760296221144044.
- Otto CM, Nishimura RA, Bonow RO, et al. 2020 ACC/AHA guideline for the management of patients with valvular heart disease: executive summary: a report of the American college of cardiology/American heart association joint committee on clinical practice guidelines. Circulation. 2021;143(5):e35–e71. doi:10.1161/CIR.0000000000000932.
- Vahanian A, Beyersdorf F, Praz F, et al. 2021 ESC/EACTS guidelines for the management of valvular heart disease. Eur Heart J. 2022;43(7):561–632. doi:10.1093/eurheartj/ehab395.
- Ikeno Y, Mukohara N, Fukumura Y, et al. Outcomes of valve replacement with mechanical prosthesis versus bioprosthesis in dialysis patients: a 16-year multicenter experience. J Thorac Cardiovasc Surg. 2019;158(1):48–56.e4. doi:10.1016/j.jtcvs.2018.11.089.
- Li GY, Chen YY, Chung FP, et al. Long-Term outcomes of bioprosthetic or mechanical valve replacement in End-Stage renal disease: a nationwide Population-Based retrospective study. Front Cardiovasc Med. 2021;8:745370. doi:10.3389/fcvm.2021.745370.
- Ngendahimana DK, Deo SV, Sundaram V, et al. Outcomes of surgical mitral and aortic valve replacements among kidney transplant candidates: implications for valve selection. J Am Heart Assoc. 2021;10(5):e018971. doi:10.1161/JAHA.120.018971.
- Liu Y, Zhang X, Xie X, et al. Risk factors for calciphylaxis in chinese hemodialysis patients: a matched case-control study. Ren Fail. 2021;43(1):406–416. doi:10.1080/0886022X.2021.1884094.
- Farah M, Crawford RI, Levin A, et al. Calciphylaxis in the current era: emerging ‘ironic’ features? Nephrol Dial Transplant. 2011;26(1):191–195. doi:10.1093/ndt/gfq407.
- Monegal A, Peris P, Alsina M, et al. Development of multiorganic calciphylaxis during teriparatide, vitamin D, and calcium treatment. Osteoporos Int. 2016;27(8):2631–2634. doi:10.1007/s00198-016-3571-1.
