Abstract
Calciphylaxis, a rapidly progressive and potentially life-threatening vascular calcification syndrome that clinically presents with persistently painful, ulcerative, or necrotizing skin lesions in multiple parts of the body, is predominantly observed in patients treated with dialysis. Early diagnosis of calciphylaxis is a key measure for reducing high disability and mortality. At present, there is no unified diagnostic standard for calciphylaxis, and there is a lack of effective early screening strategies. This paper summarized and discussed the diagnostic accuracy of calciphylaxis based on the latest research worldwide. We propose a modified strategy for the early diagnosis of calciphylaxis, which is suitable for dialysis patients to help clinicians better identify such disease and improve prognosis.
Introduction
Calciphylaxis, also known as calcific uremic arteriopathy (CUA), is a rare but devastating vascular calcification syndrome. The main features are calcification of the subcutaneous adipose tissue and dermal small blood vessels, accompanied by intimal fibrosis and microthrombosis, resulting in tissue ischemic necrosis and skin ulcers [Citation1,Citation2]. The pathogenic factors and pathogenesis of calciphylaxis are unknown, and the prevalence in dialysis patients in different regions is approximately 1–4% [Citation3,Citation4]. A regional epidemiological survey in Jiangsu Province of China found that the prevalence rate in the hemodialysis population was 1.24% [Citation5]. The prognosis of this disease is poor, and several schemes have been proposed for diagnosis; however, there is currently no unified standard. Based on the latest research progress, this paper reviews research on calciphylaxis diagnosis and proposes a modified early diagnosis strategy.
Diagnostic methods
Early diagnosis and accurate evaluation are crucial to the treatment and prognosis of calciphylaxis, which requires close cooperation of multiple disciplines, including nephrology, imaging, dermatology, surgery, and pathology. The current diagnosis of the disease is mainly based on high-risk factors, characteristic skin lesions, and features of skin histopathology [Citation6], however, this approach has limitations in diagnosing calciphylaxis at an early stage. The comprehensive use of noninvasive radiological technology has been reported to be a good method for early warning of calciphylaxis [Citation7], while skin biopsy and special calcium staining tailored to high-risk patients could help confirm the diagnosis in early or atypical skin lesion cases [Citation8,Citation9].
Risk factors
Calciphylaxis is a multifactorial disease, and the study of its risk factors can provide important clues for disease diagnosis. This disease is mostly seen in patients with end-stage kidney disease (ESKD) [Citation10], and patients with non-uremic calciphylaxis account for only 15% to 20% of all cases [Citation11]. Contrary to a study in the United States showing that calciphylaxis tended to occur in women [Citation4], Liu et al. found that most cases occurred in Chinese male dialysis patients [Citation12]. Different risk factors are divided into major and minor according to the difference in their impact on the disease [Citation4,Citation12–14]. The classification of risk factors for calciphylaxis is mainly based on research results and clinical experience. The factors are considered the major risk factors that previous studies have clearly shown a correlation with the occurrence of calciphylaxis, and the OR values are significantly increased. Moreover, these factors are not common characteristics among dialysis patients, such as warfarin therapy, high-dose use of calcium-phosphate binders and active vitamin D, hyperparathyroidism, etc. Other risk factors with OR values greater than 1 are commonly shared features of uremia, or factors related to the disease only shown in case reports are considered as minor factors, such as calcium and phosphorus metabolism disorders, hypoalbuminemia, obesity, diabetes, and iron overload. In consequence, the major factors include dialysis for over 5 years, long-term use of warfarin, long-term use of high-dose calcium-phosphate binders, active vitamin D dose greater than 0.5 μg/d, plasma protein C or protein S deficiency, vitamin K deficiency, and intact parathyroid hormone (iPTH) level above 1000 pg/mL. Meanwhile, dialysis for < 5 years, obesity, diabetes, hypoalbuminemia, long-term use of immunosuppressants and glucocorticoids, hypercalcemia, hyperphosphatemia, hyperalkaline phosphatasemia, iPTH level < 300 pg/mL, subcutaneous injection of insulin or heparin, and iron overload were minor factors. However, the results of risk factor studies in various regions differ due to multifactorial differences in race, region, medication habits, and dialysis prescriptions.
Furthermore, warfarin, as an anticoagulant commonly used in clinics, is a recognized independent risk factor for calciphylaxis. It not only promotes vascular calcification by inhibiting the vitamin K-dependent matrix Gla protein (MGP) but also directly increases thrombosis by activating the coagulation pathway [Citation15]. Switching to non-warfarin anticoagulation therapy after warfarin use is associated with a reduced risk of annual mortality in patients with calciphylaxis [Citation16]. Recent studies have also observed that patients with ESKD undergoing hemodialysis are generally in a state of vitamin K deficiency [Citation17].
Skin lesions
Skin lesions of calciphylaxis are characterized by progressive deterioration, difficulty in healing, and severe pain [Citation18]. In the early stage of this disease, localized lesions manifest as skin erythema, purpura, or livedo reticularis [Citation19]. As the disease progresses, ischemic changes cause violet plaques or indurations accompanied by intractable pain, which gradually progresses to skin ulcers (). Ulcerative lesions usually appear as black eschars, which are prone to secondary infections. The onset of pain probably precedes the progression of skin lesions, and the degree of pain may be greater than the severity of the lesions [Citation20,Citation21]. Therefore, calciphylaxis should be suspected in high-risk patients who present with extensive, painful ulcer necrosis covered with black eschar after excluding other etiologies [Citation22,Citation23]. However, many patients have atypical skin lesions at an early stage that are likely neglected, such as subcutaneous nodules, papules, purpura, and cellulitis-like erythema [Citation11,Citation22]. In a cross-sectional survey of a hemodialysis population in Jiangsu Province, China, Zhang et al. found that 394 (10.3%) of 3819 patients who did not meet the current diagnostic criteria for calciphylaxis had different types of skin lesions [Citation5]. Among them, 28.7% of the patients noticed progressive deterioration of skin lesions, such as enlargement or deepening of the damaged area, and 44.7% had moderate or severe pain under the risk of potential calciphylaxis. This finding suggested that some calciphylaxis patients with atypical skin lesions might have been overlooked, and its actual prevalence might be higher than our estimates.
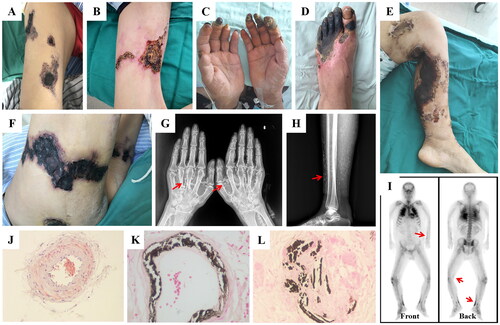
Figure 1. Clinical manifestations, imaging features and histopathology of calciphylaxis.
(A-F) Calciphylaxis patients have multiple skin lesions, that the characteristic lesions manifest as livedo reticularis, necrotic ulcers, and black eschar. (G-H) X-ray shows diffuse calcium deposition in subcutaneous tissue of the hands and lower limbs as white mass deposits (red arrows). (I) Bone scintigraphy reveals abnormal radioactivity concentrations in multiple soft tissues throughout the body, with a black linear distribution along the subcutaneous surface (red arrows). (J-L) Skin biopsy specimens of calciphylaxis show calcification of the media membrane of subcutaneous arterioles with extensive calcium deposition in extravascular interstitial tissue. Alizarin red S staining in J, von Kossa staining in K and L, magnification ×400.
According to the involved parts of skin lesions, calciphylaxis is divided into central type (involving central areas that are rich in subcutaneous fat tissue, such as the abdomen, buttocks, thighs, or breasts) and peripheral type (limited to peripheral areas with little adipose tissue, such as the hands, feet, and penis) [Citation1]. Obese patients are more likely to have central calciphylaxis [Citation24,Citation25], whereas severe pain is an important clinical feature of the peripheral type [Citation12,Citation26]. It has been reported that most calciphylaxis patients are not diagnosed until at least six months after the onset of skin lesions, by which time the disease has progressed to an advanced stage due to insufficient understanding among clinicians [Citation12]. As a result, not only the wound is protracted and difficult to heal, but also the patient suffers from long-term pain and a poor prognosis. Sepsis caused by lesions and cardiocerebrovascular accidents are common causes of death in these patients [Citation27]. According to a survey in Japan, less than 10% of nephrologists have mastered the standard diagnosis and treatment of calciphylaxis [Citation13].
Laboratory evaluation
Laboratory tests should be performed when calciphylaxis is suspected. Although many factors affect the results of blood tests, its specificity in diagnosing calciphylaxis is not high, and it can be used to further evaluate potential risk factors or rule out other diseases with similar clinical manifestations [Citation28,Citation29] (). For example, patients with calcium and phosphorus metabolism disorders, especially hypercalcemia and hyperphosphatemia, have an increased risk of calciphylaxis [Citation12]. Secondary hyperparathyroidism (SHPT) has always been considered an important feature of Chronic Kidney Disease-Mineral and Bone Disorder, which can lead to ectopic calcification of vascular and tissues in uremic patients [Citation30,Citation31]. Abnormally elevated iPTH will increase the risk of disease [Citation4]. ESKD patients receiving hemodialysis treatment are in a state of vitamin K deficiency [Citation17], leading to insufficient carboxylation and functional damage of MGP, which would also aggravate vascular calcification [Citation32].
Table 1. Blood test items for reference in the diagnosis of calciphylaxis.
Imaging examination
As a noninvasive method, imaging can provide earlier and more comprehensive clinical clues, helping identify patients at risk of calciphylaxis before the appearance of typical skin lesions. Low-cost X-ray imaging techniques can be used to identify extraosseous calcifications initially [Citation33]. Shmidt et al. [Citation34] found that reticular calcification of the subcutaneous soft tissue was closely related to calciphylaxis, with a specificity of 90%. Radiographic findings of reticular calcification may be observed on plain radiographs of any part of the body, implying that this disease is systemic, but skin lesions are usually localized. Yu et al. [Citation35] investigated the diagnostic value of radiomics-based CT for calciphylaxis. This study extracted CT radiomics features hidden in skin lesions and preliminarily proved the feasibility and diagnostic efficacy of the radiomics model as a noninvasive technology to diagnose calciphylaxis in patients with chronic kidney disease. This also suggests that it is possible to distinguish calciphylaxis from common types of vascular calcification by developing radiological technology to quantitatively analyze the distribution characteristics of multilevel vascular calcification in patients with calciphylaxis [Citation36].
Recent studies have revealed that the abnormal uptake of tracers by soft tissue in bone scintigraphy is associated with calciphylaxis, which has high sensitivity and specificity in diagnosing the disease and can assess the exact location and scope of the lesions [Citation37–39]. Bone scintigraphy identifies subtle calcifications by taking advantage of the high affinity between 99mTc-methylene diphosphonate (99mTc-MDP) and calcium salt. Radiotracers are attached to hydroxyapatite crystals in calcified areas of soft tissues, such as subcutaneous fat, and then the optical signal is picked up and visualized by SPECT/CT [Citation40]. Although other diseases also have soft tissue calcification, such as tumoral calcinosis, myositis ossificans, osteosarcoma, and dermatomyositis, they tend to appear as focal clumpy shadows on bone scintigraphy rather than as significant subcutaneous linear soft tissue uptake [Citation41,Citation42].
Advanced radiological technologies have provided new insights and valuable clues for calciphylaxis diagnosis. In-depth studies of various imaging techniques, such as molybdenum target [Citation43,Citation44], ultrasonography [Citation7,Citation45], and magnetic resonance imaging, are vital for future research. The application of multiple imaging techniques in clinical practice to screen high-risk patients can also provide a basis for determining whether to conduct further invasive pathological examination of skin biopsy.
Skin biopsy and dermatopathology
Histopathological examination of skin biopsy specimens remains the gold standard for confirming calciphylaxis, particularly in atypical skin lesions [Citation20]. Typical histopathological features include calcification of the medial membrane of subcutaneous arterioles, intimal fibrosis, and microthrombosis [Citation46]. The diameter of the involved microvessels was approximately 40–600 μm [Citation1]. Intravascular calcium deposition alone is not sufficient to diagnose calciphylaxis, particularly in patients with ESKD. Extravascular calcium deposition and ischemic necrosis of the epidermis, dermis, and subcutaneous tissue should also be considered during diagnosis [Citation2]. Ischemic skin injury results from the narrowing of the diameter of small arterioles in the dermis and subcutaneous tissue, caused by calcium and phosphorus deposits and microthrombosis [Citation47]. Local inflammatory reactions and increased blood viscosity also exacerbate ischemia [Citation48].
The choice of appropriate sampling sites and biopsy methods should be based on the different types of skin lesions. Thus, it is a feasible option to use materials from active lesions. Sampling at the edges of lesions rather than eschars should be given priority to avoid taking necrotic tissue without diagnostic value [Citation1]. The samples were supposed to include the entire layer of the skin and part of the subcutaneous tissue. There are generally three biopsy methods to choose from. The biopsy of a deep wedge-shaped incision obtains sufficient tissue specimens, but a deep incision probably leads to poor healing in the later stage [Citation19]. A punch biopsy (3-5 mm) is performed on the edge of the ulcer lesions or small skin lesions, such as macules, papules, and scleromas, which provide complete and continuous subcutaneous tissue samples of sufficient depth [Citation10]. This method causes less trauma to the sampling area than incision biopsy, which is safer and easier to control. For patients with deep or severe skin lesions that cannot tolerate the above procedures, ultrasound-guided needle biopsy is considered [Citation49]. In summary, the selection of the skin biopsy site, sampling method, and biopsy depth will affect the accuracy of pathological diagnosis [Citation50,Citation51]. It is worth mentioning that skin biopsy carries a risk of aggravation or the induction of new lesions. Before conducting a biopsy, it is crucial to weigh the pros and cons.
In terms of pathological detection methods, HE staining shows calcification of subcutaneous small vessels with uniform blue-purple granular calcium deposition but is less sensitive to the diagnosis of microcalcifications. The combined use of special calcium staining methods, such as von Kossa and alizarin red S staining, significantly improved the detection rate of small punctate calcium deposition and increased the sensitivity and specificity to 85% and 88% respectively [Citation9,Citation52]. Zhu et al. recently discovered that Fluo-3 AM is a rapid, sensitive, and reliable fluorescent probe for the detection of calcium deposits, and is presumably a promising diagnostic tool for calciphylaxis [Citation8]. Although skin biopsy is an invasive procedure, it is indispensable to confirm the diagnosis in early and atypical patients with suspected calciphylaxis. Early diagnosis contributes to early treatment and the chance of significantly improving the prognosis of calciphylaxis.
Differential diagnosis
The treatment of calciphylaxis is often delayed owing to missed diagnosis and misdiagnosis, which is mainly caused by its confusing skin lesions. Diseases that need to be identified include diabetic ulcer, atherosclerotic vascular disease, thromboangiitis obliterans, cellulitis, traumatic ulcer, dystrophic calcification, cholesterol embolism syndrome, vasculitis, pyoderma gangrenosum, stasis ulcers, neuropathic ulcers, warfarin-induced skin necrosis, nephrogenic systemic fibrosis, and cryofibrinogenemia [Citation18,Citation20,Citation53,Citation54] (). In general, most lesions that are similar to calciphylaxis do not show evidence of vascular calcification on skin biopsy [Citation54].
Table 2. Differential diagnosis of calciphylaxis.
Diagnostic process
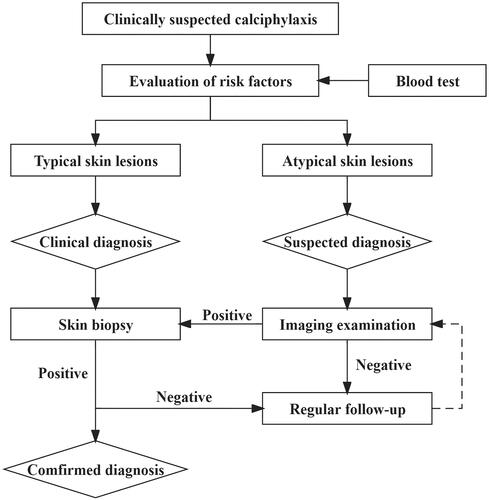
Early diagnosis of calciphylaxis is difficult. On the basis of relevant research, the Institute of Nephrology of Southeast University has developed a modified diagnosis program for calciphylaxis, which is called the “Zhong Da Diagnostic Approach” [Citation55]. In this protocol, dialysis patients with clinical suspicion of calciphylaxis are stratified into “suspected diagnosis, clinical diagnosis, and confirmed diagnosis” to establish a scheme for early diagnosis and early warning for calciphylaxis. When dialysis patients present with chronically progressive and unhealed skin lesions, calciphylaxis can be clinically suspected, after excluding other diseases. (i) When there are early suspicious skin atypical changes, such as unexplained skin induration, plaques, erythema, and purpura, combined with multiple potential risk factors for calciphylaxis, the “suspected diagnosis” should be considered (). (ii) Patients with recommended risk factors for calciphylaxis, which have typical skin lesions such as livedo reticularis, ulcers, and eschar, are given a “clinically diagnosed”. (iii) Combined with clinical symptoms, the diagnosis could be “confirmed” when skin biopsy revealed pathological features of calciphylaxis, such as calcification of subcutaneous adipose tissue and dermal small blood vessels, intimal hyperplasia, and thrombosis. A comprehensive radiographic assessment, including X-ray, molybdenum target, CT, and bone scintigraphy, will be performed in a timely manner in patients with a suspected diagnosis. Pathological examination of the skin biopsy of the involved area should be carried out when meaningful abnormalities on imageological examination are found as soon as possible to confirm the early diagnosis of calciphylaxis ().
Table 3. Hierarchical diagnosis of calciphylaxis in dialysis patients.
Although there is still a lack of guidelines and expert consensus on the diagnosis and treatment of calciphylaxis, as well as a lack of large-scale RCT research, some scholars have clearly proposed recommended calciphylaxis diagnostic strategies. Hayashi M’s diagnostic criteria emphasize the presence of multiple painful and intractable skin ulcers with painful purpura and focus on a subset of patients who already have typical skin lesions in the advanced stages of the disease [Citation56]. The diagnostic criteria proposed by the Mayo Clinic in 2016 are set as major and minor factors based on patient’s clinical symptoms and skin biopsy results, and their diagnosis is divided into “Definite, Probable, Possible or No” according to the number of factors satisfied by the patients [Citation6]. On this basis, our proposed “Zhong Da Diagnostic Approach” is characterized by the concept of “suspected diagnosis”. We have found significant abnormalities in the imaging examination of patients with calciphylaxis, which could early indicate this disease. Patients who fail to meet the clinical diagnostic criteria are capable of early diagnosis with the aid of supportive clues provided by imaging technology and timely pathological examination of skin biopsy, while traditional diagnostic programs are prone to misdiagnosis of such patients. When a patient with the suspected diagnosis is definitively diagnosed with calciphylaxis, it is called early diagnosis.
We tried and preliminarily validated this protocol in a Chinese dialysis population. From 2018 to 2020, a total of 51 patients with calciphylaxis were treated in our center, and hierarchical diagnosis resulted in an early diagnosis rate of 29.4% (15 cases) [Citation57], preliminarily suggesting the effectiveness of this approach. Meanwhile, the survival rate of early cases was higher, indicating the necessity of the application of this scheme. All patients were treated with sodium thiosulfate-based comprehensive treatment, and 87.1% of the patients survived after 9 months of follow-up, and the prognosis was notably improved [Citation58]. Previous reports have shown that the one-year mortality rate of calciphylaxis patients is as high as 45–80%, which varies depending on comorbidities and lesion location [Citation1]. This suggests that the “Zhong Da Diagnostic Approach” has made preliminary achievements in the early diagnosis of calciphylaxis and improvement of the therapeutic effect. Due to the limited sample size at present, we are unable to conduct modeling and validation, and the strength of the evidence is insufficient. We have now established a long-term observation queue, and in future prospective cohort studies, we will provide data on the effectiveness of this scheme for early diagnosis.
Conclusion
Normally, the prevalence of vascular calcification is extremely high in the dialysis population [Citation59], but its progression is usually relatively slow. In some cases, there was also explosive onset such as mitral annular calcification among them [Citation60]. Effective control of calcium and phosphorus metabolism can slow calcification progression to a certain extent. In contrast, calciphylaxis is a rare, progressive, and lethal disease of microvascular calcification with an increasing incidence. The disease progresses aggressively, with high disability and mortality rates, and there are currently no specific therapeutic drugs for the indications of calciphylaxis. Some drugs, such as sodium thiosulfate, are only used as off-label drugs [Citation61]. Thus, early diagnosis is a key strategy for increasing the cure rate and improving the prognosis. Attention to atypical skin lesions, taking full advantage of new radiological technology, and conducting a standardized pathological examination of skin biopsy in a timely manner are vital strategies for the early diagnosis of calciphylaxis, which deserve further attention and more in-depth study.
Authors’ contributions
Review conception and design: Yuqiu Liu, Xiaoliang Zhang; Drafting and editing the manuscript: Yuqiu Liu; Providing intellectual content of critical importance to the work described: all authors; Review supervision and mentorship: Xiaoliang Zhang. Each author read and approved the final manuscript and agreed to ensure that questions about the accuracy or integrity of any portion of the work were appropriately investigated and resolved.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Nigwekar SU, Thadhani R, Brandenburg VM. Calciphylaxis. N Engl J Med. 2018;378(18):1–9. doi: 10.1056/NEJMra1505292.
- Colboc H, Moguelet P, Bazin D, et al. Localization, morphologic features, and chemical composition of calciphylaxis-related skin deposits in patients with calcific uremic arteriolopathy. JAMA Dermatol. 2019;155(7):789–796. doi: 10.1001/jamadermatol.2019.0381.
- Angelis M, Wong LL, Myers SA, et al. Calciphylaxis in patients on hemodialysis: a prevalence study. Surgery. 1997;122(6):1083–1090. doi: 10.1016/s0039-6060(97)90212-9.
- Nigwekar SU, Zhao S, Wenger J, et al. A nationally representative study of calcific uremic arteriolopathy risk factors. J Am Soc Nephrol. 2016;27(11):3421–3429. doi: 10.1681/ASN.2015091065.
- Liu Y, Yang C, Yang X, et al. Prevalence and clinical characteristics of calciphylaxis in Chinese hemodialysis patients. Front Med. 2022;9:902171. doi: 10.3389/fmed.2022.902171.
- McCarthy JT, El-Azhary RA, Patzelt MT, et al. Survival, risk factors, and effect of treatment in 101 patients with calciphylaxis. Mayo Clin Proc. 2016;91(10):1384–1394. doi: 10.1016/j.mayocp.2016.06.025.
- Bonchak JG, Park KK, Vethanayagamony T, et al. Calciphylaxis: a case series and the role of radiology in diagnosis. Int J Dermatol. 2016;55(5):e275-279–e279. doi: 10.1111/ijd.13043.
- Zhu X, Liu Y, Yang X, et al. Identifying subcutaneous tissue microcalcification by fluo-3 AM imaging in cutaneous calciphylaxis. Exp Dermatol. 2022;31(10):1632–1634. doi: 10.1111/exd.14579.
- Mochel MC, Arakaki RY, Wang G, et al. Cutaneous calciphylaxis: a retrospective histopathologic evaluation. Am J Dermatopathol. 2013;35(5):582–586. doi: 10.1097/DAD.0b013e31827c7f5d.
- García-Lozano JA, Ocampo-Candiani J, Martínez-Cabriales SA, et al. An update on calciphylaxis. Am J Clin Dermatol. 2018;19(4):599–608. doi: 10.1007/s40257-018-0361-x.
- Ghosh T, Winchester DS, Davis MDP, et al. Early clinical presentations and progression of calciphylaxis. Int J Dermatol. 2017;56(8):856–861. doi: 10.1111/ijd.13622.
- Liu Y, Zhang X, Xie X, et al. Risk factors for calciphylaxis in chinese hemodialysis patients: a matched case-control study. Ren Fail. 2021;43(1):406–416. doi: 10.1080/0886022X.2021.1884094.
- Hayashi M, Takamatsu I, Kanno Y, et al. A case-control study of calciphylaxis in Japanese end-stage renal disease patients. Nephrol Dial Transplant. 2012;27(4):1580–1584. doi: 10.1093/ndt/gfr658.
- Gaisne R, Péré M, Menoyo V, et al. Calciphylaxis epidemiology, risk factors, treatment and survival among French chronic kidney disease patients: a case-control study. BMC Nephrol. 2020;21(1):63. doi: 10.1186/s12882-020-01722-y.
- Lehman JS, Chen TY, Lohse CM, et al. Evaluating the validity of subclassifying warfarin-associated nonuremic calciphylaxis: a retrospective cohort study. Int J Dermatol. 2018;57(5):572–574. doi: 10.1111/ijd.13884.
- Gabel CK, Nguyen ED, Chakrala T, et al. Assessment of outcomes of calciphylaxis. J Am Acad Dermatol. 2021;85(4):1057–1064. doi: 10.1016/j.jaad.2020.10.067.
- Mizuiri S, Nishizawa Y, Yamashita K, et al. Relationship of matrix Gla protein and vitamin K with vascular calcification in hemodialysis patients. Ren Fail. 2019;41(1):770–777. doi: 10.1080/0886022X.2019.1650065.
- Jeong HS, Dominguez AR. Calciphylaxis: controversies in pathogenesis, diagnosis and treatment. Am J Med Sci. 2016;351(2):217–227. doi: 10.1016/j.amjms.2015.11.015.
- Sreedhar A, Sheikh HA, Scagliotti CJ, et al. Advanced-stage calciphylaxis: think before you punch. Cleve Clin J Med. 2016;83(8):562–564. doi: 10.3949/ccjm.83a.15103.
- Nigwekar SU, Kroshinsky D, Nazarian RM, et al. Calciphylaxis: risk factors, diagnosis, and treatment. Am J Kidney Dis. 2015;66(1):133–146. doi: 10.1053/j.ajkd.2015.01.034.
- El-Azhary RA, Patzelt MT, McBane RD, et al. Calciphylaxis: a disease of pannicular thrombosis. Mayo Clin Proc. 2016;91(10):1395–1402. doi: 10.1016/j.mayocp.2016.06.026.
- Chiriac A, Grosu OM, Terinte C, et al. Calcific uremic arteriolopathy (calciphylaxis) calls into question the validity of guidelines of diagnosis and treatment. J Dermatolog Treat. 2020;31(5):545–548. doi: 10.1080/09546634.2019.1618435.
- Kodumudi V, Jeha GM, Mydlo N, et al. Management of cutaneous calciphylaxis. Adv Ther. 2020;37(12):4797–4807. doi: 10.1007/s12325-020-01504-w.
- Weenig RH, Sewell LD, Davis MD, et al. Calciphylaxis: natural history, risk factor analysis, and outcome. J Am Acad Dermatol. 2007;56(4):569–579. doi: 10.1016/j.jaad.2006.08.065.
- Davis JM. The relationship between obesity and calciphylaxis: a review of the literature. Ostomy/Wound Manage. 2016;62(1):12–18.
- Yang CL, Liu YQ, Ni HF, et al. Potential effect of sodium thiosulfate in calciphylaxis: remission of intractable pain. J Pak Med Assoc. 2021;71(2):340–342.
- Patecki M, Lehmann G, Brasen JH, et al. A case report of severe calciphylaxis - suggested approach for diagnosis and treatment. BMC Nephrol. 2017;18(1):137. doi: 10.1186/s12882-017-0556-z.
- Mormile I, Mosella F, Turco P, et al. Calcinosis cutis and calciphylaxis in autoimmune connective tissue diseases. Vaccines. 2023;11(5):898. doi: 10.3390/vaccines11050898.
- Byers A, Herrera N, Owoyemi I. Chronic inflammation and calciphylaxis. BMJ Case Rep. 2022;15(4):e248668. doi: 10.1136/bcr-2021-248668.
- Cunningham J, Locatelli F, Rodriguez M. Secondary hyperparathyroidism: pathogenesis, disease progression, and therapeutic options. Clin J Am Soc Nephrol. 2011;6(4):913–921. doi: 10.2215/CJN.06040710.
- Chandran M, Wong J. Secondary and tertiary hyperparathyroidism in chronic kidney disease: an endocrine and renal perspective. Indian J Endocrinol Metab. 2019;23(4):391–399. doi: 10.4103/ijem.IJEM_292_19.
- Nigwekar SU, Bloch DB, Nazarian RM, et al. Vitamin K-dependent carboxylation of matrix gla protein influences the risk of calciphylaxis. J Am Soc Nephrol. 2017;28(6):1717–1722. doi: 10.1681/ASN.2016060651.
- Idris KM, Laing ME. Xeroradiography as a noninvasive tool in diagnosing calciphylaxis. JAMA Dermatol. 2022;158(9):1080–1082. doi: 10.1001/jamadermatol.2022.2158.
- Shmidt E, Murthy NS, Knudsen JM, et al. Net-like pattern of calcification on plain soft-tissue radiographs in patients with calciphylaxis. J Am Acad Dermatol. 2012;67(6):1296–1301. doi: 10.1016/j.jaad.2012.05.037.
- Yu Q, Liu Y, Xie X, et al. Radiomics-based method for diagnosis of calciphylaxis in patients with chronic kidney disease using computed tomography. Quant Imaging Med Surg. 2021;11(11):4617–4626. doi: 10.21037/qims-20-1211.
- Zhang L, Li L, Feng G, et al. Advances in CT techniques in vascular calcification. Front Cardiovasc Med. 2021;8:716822. doi: 10.3389/fcvm.2021.716822.
- Paul S, Rabito CA, Vedak P, et al. The role of bone scintigraphy in the diagnosis of calciphylaxis. JAMA Dermatol. 2017;153(1):101–103. doi: 10.1001/jamadermatol.2015.4591.
- Martineau P, Pelletier-Galarneau M, Bazarjani S. The role of bone scintigraphy with single-photon emission computed tomography-computed tomography in the diagnosis and evaluation of calciphylaxis. World J Nucl Med. 2017;16(2):172–174. doi: 10.4103/1450-1147.203076.
- Shi W, Xie X, Liu Y, et al. The mystery of black lungs in a patient with calciphylaxis. J Nephrol. 2021;34(5):1553–1555. doi: 10.1007/s40620-021-01121-y.
- Itani M, Matesan M, Behnia S, et al. Calciphylaxis on bone scan: correlation between molecular and cross-sectional findings. Radiol Case Rep. 2017;12(1):175–178. doi: 10.1016/j.radcr.2016.11.027.
- Raduka J, Aggarwal A, Johnson K, et al. Bone scintigraphy findings in calciphylaxis. Radiol Case Rep. 2018;13(2):315–319. doi: 10.1016/j.radcr.2017.12.005.
- Peller PJ, Ho VB, Kransdorf MJ. Extraosseous Tc-99m MDP uptake: a pathophysiologic approach. Radiographics. 1993;13(4):715–734. doi: 10.1148/radiographics.13.4.8356264.
- Hall DJ, Gentile LF, Duckworth LV, et al. Calciphylaxis of the breast: a case report and literature review. Breast J. 2016;22(5):568–572. doi: 10.1111/tbj.12632.
- AlQattan AS, Ghulam WZ, Aldaoud N, et al. Breast fat necrosis secondary to warfarin-induced calciphylaxis, a rare mimicker of breast cancer: a case report and a review of literature. Breast J. 2021;27(3):258–263. doi: 10.1111/tbj.14160.
- Tobarran N, Collin M. Point-of-care ultrasound in the diagnosis of calciphylaxis. Clin Pract Cases Emerg Med. 2020;4(3):495–496. doi: 10.5811/cpcem.2020.7.47886.
- Pyle HJ, Shedd CM, Begovic J, et al. Complications and histopathological findings of image-guided core needle biopsy in diagnosis of cutaneous calciphylaxis. Am J Dermatopathol. 2023;45(6):414–417. doi: 10.1097/DAD.0000000000002426.
- Chen TY, Lehman JS, Gibson LE, et al. Histopathology of calciphylaxis: cohort study with clinical correlations. Am J Dermatopathol. 2017;39(11):795–802. doi: 10.1097/DAD.0000000000000824.
- Yu WY, Bhutani T, Kornik R, et al. Warfarin-associated nonuremic calciphylaxis. JAMA Dermatol. 2017;153(3):309–314. doi: 10.1001/jamadermatol.2016.4821.
- Mask-Bull L, Lee MP, Wang A. Image-guided core-needle biopsy for the diagnosis of cutaneous calciphylaxis. JAMA Dermatol. 2019;155(7):856–857. doi: 10.1001/jamadermatol.2019.0413.
- Dobry AS, Nguyen ED, Shah R, et al. The role of skin biopsy in diagnosis and management of calciphylaxis: a retrospective analysis. J Am Acad Dermatol. 2021;85(3):765–767. doi: 10.1016/j.jaad.2020.05.101.
- Rotondi S, De Martini N, Tartaglione L, et al. On the role of skin biopsy in the diagnosis of calcific uremic arteriolopathy: a case-based discussion. J Nephrol. 2020;33(4):859–865. doi: 10.1007/s40620-019-00678-z.
- Magro CM, Simman R, Jackson S. Calciphylaxis: a review. J Am Col Certif Wound Spec. 2010;2(4):66–72. doi: 10.1016/j.jcws.2011.03.001.
- Musso CG, Enz PA, Kowalczuk A, et al. Differential diagnosis of calciphylaxis in chronic dialysis patients. Int Urol Nephrol. 2020;52(3):595–597. doi: 10.1007/s11255-020-02388-z.
- Ng AT, Peng DH. Calciphylaxis. Dermatol Ther. 2011;24(2):256–262. doi: 10.1111/j.1529-8019.2011.01401.x.
- Liu Y, Xie X, Zhang X. Diagnosis strategy of calciphylaxis in dialysis patients. Chin J Nephrol. 2022;38(6):561–566.
- Hayashi M. Calciphylaxis: diagnosis and clinical features. Clin Exp Nephrol. 2013;17(4):498–503. doi: 10.1007/s10157-013-0782-z.
- Jiao Y, Sun L, Xie X, et al. Clinical features and outcomes of calciphylaxis in chinese patients with chronic kidney disease. Nephrology. 2023;28(6):305–314. doi: 10.1111/nep.14156.
- Yang X, Liu Y, Xie X, et al. Use of the optimized sodium thiosulfate regimen for the treatment of calciphylaxis in Chinese patients. Ren Fail. 2022;44(1):914–922. doi: 10.1080/0886022X.2022.2081179.
- Liu ZH, Yu XQ, Yang JW, et al. Prevalence and risk factors for vascular calcification in Chinese patients receiving dialysis: baseline results from a prospective cohort study. Curr Med Res Opin. 2018;34(8):1491–1500. doi: 10.1080/03007995.2018.1467886.
- Vallabhajosyula S, Said SM, Kitzmann AS, et al. Accelerated extensive mitral valve calcification in a young end-stage kidney disease patient. Eur Heart J. 2020;41(45):4361–4361. doi: 10.1093/eurheartj/ehaa715.
- Generali JA, Cada DJ. Sodium thiosulfate: calciphylaxis. Hosp Pharm. 2015;50(11):975–977. doi: 10.1310/hpj5011-975.