Abstract
Aims
This study aimed to investigate the efficacy and safety of sacubitril/valsartan in abnormal renal function (eGFR < 60 ml/min/1.73m2) patients combined with heart failure based on randomized controlled trials (RCTs) and observational studies.
Methods
The Embase, PubMed and the Cochrane Library were searched for relevant studies from inception to December 2023. Dichotomous variables were described as event counts with the odds ratio (OR) and 95% confidence interval (CI) values. Continuous variables were expressed as mean standard deviation (SD) with 95% CIs.
Results
A total of 6 RCTs and 8 observational studies were included, involving 17335 eGFR below 60 ml/min/1.73m2 patients combined with heart failure. In terms of efficacy, we analyzed the incidence of cardiovascular events and found that sacubitril/valsartan significantly reduced the risk of cardiovascular death or heart failure hospitalization in chronic kidney disease (CKD) stages 3–5 patients with heart failure (OR: 0.65, 95%CI: 0.54–0.78). Moreover, sacubitril/valsartan prevented the serum creatinine elevation (OR: 0.81, 95%CI: 0.68–0.95), the eGFR decline (OR: 0.83, 95% CI: 0.73–0.95) and the development of end-stage renal disease in this population (OR:0.73, 95%CI:0.60–0.89). As for safety outcomes, we did not find that the rate of hyperkalemia (OR:1.31, 95%CI:0.79–2.17) and hypotension (OR:1.57, 95%CI:0.94–2.62) were increased in sacubitril/valsartan group among CKD stages 3–5 patients with heart failure.
Conclusions
Our meta-analysis proves that sacubitril/valsartan has a favorable effect on cardiac function without obvious risk of adverse events in abnormal renal function patients combined with heart failure, indicating that sacubitril/valsartan has the potential to become perspective treatment for these patients.
Introduction
Chronic kidney disease (CKD) is a global health problem with an increased risk of cardiovascular disease [Citation1,Citation2], often manifested as heart failure [Citation2,Citation3]. Moreover, studies have documented that concomitant heart failure is associated with elevated morbidity and mortality of patients with CKD stages 3–5 [Citation3–5]. However, in these special patients, the management of heart failure remains a huge challenge, potentially due to adverse drug reactions and their limited response to conventional therapies [Citation6]. So, it is imperative to explore new therapeutic strategies for abnormal renal function patients combined with heart failure.
In recent years, sacubitril/valsartan has been confirmed to ameliorate the prognosis of heart failure through vasodilatation, diuresis, natriuresis and anti-remodeling [Citation2] by simultaneously restraining natriuretic peptides degradation and renin-angiotensin-aldosterone system (RAAS) activation [Citation7]. Current clinical guidelines also have recommended sacubitril/valsartan for patients with heart failure [Citation8,Citation9] to mitigate the risk of cardiovascular death [Citation10]. However, these guidelines primarily pertain to patients with normal renal function. Whether sacubitril/valsartan is safe and effective in patients with impaired renal function, especially in advanced kidney disease, is still unclear.
Some randomized controlled trials (RCTs) once suggested that CKD stage 3 patients with heart failure might have received renal and cardiovascular benefits from sacubitril/valsartan [Citation11–13]. But these results are unsystematic and incomplete because they have to be extrapolated from subgroup analyses, as well as lack of safety outcomes. Additionally, patients with CKD at stage 4 or higher were excluded from RCTs investigating the effects of sacubitril/valsartan; however, they were included in several observational studies. One of the observational studies involving 49 patients with eGFR < 15 mL/min/1.73m2 concomitant with heart failure demonstrated that sacubitril/valsartan effectively enhanced cardiac function without eliciting significant adverse effects [Citation14]. But the findings of another observational study including 1039 patients with CKD stages 3–5 and heart failure indicated that, after a duration of 12 months, the sacubitril/valsartan group exhibited a higher incidence of adverse drug reactions than control group [Citation15]. It follows that these observational studies have inconsistent conclusions, small sample sizes and short follow-up periods, leading to a bias when assessing the effect of sacubitril/valsartan in CKD stages 4–5 patients with heart failure.
Previously, our research team has completed a study on the effect of sacubitril/valsartan in patients with CKD, while mainly emphasizing patients with CKD stage 3 and only including RCTs [Citation16]. Consequently, to evaluate the efficacy and safety of sacubitril/valsartan in a broader population and the real-world clinical practice, we conducted this meta-analysis on patients with CKD stages 3–5 combined with heart failure, based on both RCTs and observational studies.
Materials and methods
This meta-analysis was conducted in compliance with the Preferred Reporting Items for Reporting Systematic Reviews and Meta-analyses (PRISMA) statement [Citation17].
Search strategy
Online databases including the Cochrane Library, Embase and PubMed were searched by Yang and Jin for relevant studies published as of December 9th, 2023, using the following terms: ‘sacubitril valsartan’, ‘sacubitril and valsartan’, ‘sacubitril/valsartan’, ‘sakubitril valsartan’, ‘entresto’, ‘neprilysin inhibitor’, ‘LCZ696’, ‘LCZ-696’, ‘AHU377’, ‘ARNI’, ‘angiotensin receptor neprilysin inhibitor’, ‘angiotensin receptor neprilysin blocker’. Details were provided in the Supplementary Materials. Disagreements were solved through consulting with the third reviewer (Xu).
Study selection
Two reviewers (Yang and Cheng) screened all titles, abstracts and full texts, as well as ultimately determined the inclusion of studies by the consensus. Any disagreements were referred to the third researcher (Bai) for advice. The following inclusion criteria were required for eligible literature:
Participants: the population of trial contained abnormal renal function (eGFR < 60 ml/min/1.73m2) patients combined with heart failure;
Intervention: the experimental group used sacubitril/valsartan;
Comparisons: the control group without sacubitril/valsartan;
Outcomes: cardiovascular events or safety outcomes were available;
Study design: the number of patients with CKD and heart failure was definite or can be calculated; the type of study was RCT or observational study.
We identified and excluded studies which met at least one of the following criteria: (a) repeated literature; (b) title or content not relevant to our meta-analysis; (c) animal or cell trial; (d) article not published in English; (e) did not meet the inclusion criteria; (f) review, case, report, new, meta-analysis, conference abstract, letter, erratum or comment.
Data extraction and quality assessment
We extracted the following information from the eligible trials: the trial name, number of patients, baseline eGFR, drug names and doses, duration of treatment, efficacy outcomes (the incidence of cardiovascular death or hospitalization for heart failure) and safety outcomes (changes in renal function; the incidence of hyperkalemia and hypotension). Discrepancies were addressed by consensus with a third author (Xu).
The bias of RCTs was assessed using the Cochrane risk of bias (ROB) tool according to 5 items: according to random sequence generation (selection bias), allocation concealment (selection bias), blinding of participants and personnel (performance bias), incomplete outcome data (attribution bias), and selective reporting (reporting bias) and other bias [Citation17].
In addition, the Risk of bias in Studies of Non-randomized Interventions Scale (ROBINS-I) was used to evaluate the quality of observational studies in 6 aspects: bias due to confounding, bias in selection of participants into the study, bias in classification of interventions, bias due to deviations from intended interventions, bias due to missing data, bias in measurement of outcomes, bias in selection of the reported results [Citation18]. These assessments were done by two reviewers (Cheng and Yang) separately. Conflicts were resolved by the third investigator (Jin).
Statistical analysis
All statistical analysis in this meta-analysis were performed on Review Manager 5.3. Quality assessment of observational studies and funnel plot of cardiovascular events was drawn by RStudio 4.1.3. Dichotomous variables were described as event counts with the odds ratio (OR) and 95% confidence interval (CI) values. Continuous variables were expressed as mean ± standard deviation (SD) with 95% CIs. The heterogeneity of studies was assessed with the Q test, I2 statistic and forest maps. Heterogeneity was low when I2 was less than 25%, moderate when I2 was between 25% and 50% and high when I2 was greater than 50%. When Ι2 was less than 50%, heterogeneity was considered acceptable. We chose a fixed-effects model when I2<50%, otherwise the random-effects model was picked. Funnel plots and Egger’s test were used to assess publication bias if at least ten studies were included. There is no missing data in the included articles, and we have received all answers from studies.
Results
Literature search results
Using our search strategy, we retrieved 10128 studies from target databases, removed 2854 duplicate records and further screened 1125 full texts for eligibility. Finally, six RCTs [Citation11–13,Citation19–21] and eight observational studies [Citation14,Citation15,Citation22–27], involving a total of 17335 CKD stages 3–5 patients with heart failure, were included (Cohen’s kappa = 0.832, p < 0.01, the consistency of literature search is strong). The flow diagram of literature searching and screening was detailed in the .
Baseline characteristics and quality assessment of included studies
As specified in , the characteristics of the included studies were summarized. A total of 17335 CKD stages 3–5 patients with heart failure were involved in our meta-analysis.
Table 1. Baseline characteristic of included studies.
In terms of RCTs, three of the them compared sacubitril/valsartan with angiotensin converting enzyme inhibitor (ACEI) [Citation12,Citation19,Citation21], two of them compared sacubitril/valsartan with angiotensin receptor blocker (ARB) [Citation11,Citation13], and the last one compared the sacubitril/valsartan with routine treatment of heart failure which contained ACEI or ARB [Citation20]. The duration of treatment ranged from 8 weeks to 35 months. Regarding observational studies, patients in the experimental group received sacubitril/valsartan, while the control group did not receive sacubitril/valsartan. The period of therapy ranged from 6.9 to 15 months.
The average dosage of sacubitril/valsartan in most studies was 100 mg/bid or 200 mg/bid. And the mean dose of sacubitril/valsartan in two studies conducted by Chang et al. was 112.5 ± 58.7 mg/qd [Citation22] and 143 ± 84mg/qd [Citation23], respectively.
Based on the ROB tool, all included RCTs were of high quality (Supplementary figure 1). According to the ROBINS-I tool, eight observational studies were assessed as having a relatively low risk of bias in six areas (Supplementary figure 2).
Cardiovascular events
A total of five RCTs and five observational studies described cardiovascular events (cardiovascular death or heart failure hospitalization). Compared with control group, sacubitril/valsartan treatment reduced the incidence of cardiovascular events in CKD stages 3–5 patients with heart failure (12236 patients, OR: 0.65, 95%CI: 0.54–0.78, p < 0.00001, I2=73%, ). Additionally, subgroup analysis based on CKD stage showed that even patients with eGFR below 30 mL/min/1.73m2 were able to derive cardiovascular benefits from sacubitril/valsartan (OR: 0.60, 95%CI: 0.42–0.87, p = 0.007, I2=67%, ). Both funnel plot () and Egger’s test (p = 0.602) did not show significant publication bias. Overall, sacubitril/valsartan exhibited cardioprotective effects in abnormal renal function patients combined with heart failure.
Figure 2. Results of cardiovascular events. (A) Forest plot showing the difference in cardiovascular events between sacubitril/valsartan and control group. (B) Funnel plot of cardiovascular events. Note: Berg et al. defined cardiovascular events as a composite of cardiovascular death or rehospitalization for heart failure [Citation12]; Chen et al. described cardiovascular events as rehospitalization for heart failure and all-cause death [Citation24]; Damman et al. Chang et al. Hsiao et al. Lee et al. and Tsutsui et al. described cardiovascular events as cardiovascular death or heart failure hospitalization [Citation15,Citation19,Citation21–23,Citation26]; Mc Causland et al. defined cardiovascular events as a composite of total (first and recurrent) hospitalizations for heart failure and death from cardiovascular causes; In Sheng et al.’s research, cardiovascular events were regarded as the rehospitalization of patients due to acute myocardial ischemia, HF, thromboembolic or hemorrhagic stroke, arrhythmia, and peripheral vascular disease [Citation20]; Niu et al. regarded cardiovascular events as hospitalization because of cardiovascular diseases [Citation14].
![Figure 2. Results of cardiovascular events. (A) Forest plot showing the difference in cardiovascular events between sacubitril/valsartan and control group. (B) Funnel plot of cardiovascular events. Note: Berg et al. defined cardiovascular events as a composite of cardiovascular death or rehospitalization for heart failure [Citation12]; Chen et al. described cardiovascular events as rehospitalization for heart failure and all-cause death [Citation24]; Damman et al. Chang et al. Hsiao et al. Lee et al. and Tsutsui et al. described cardiovascular events as cardiovascular death or heart failure hospitalization [Citation15,Citation19,Citation21–23,Citation26]; Mc Causland et al. defined cardiovascular events as a composite of total (first and recurrent) hospitalizations for heart failure and death from cardiovascular causes; In Sheng et al.’s research, cardiovascular events were regarded as the rehospitalization of patients due to acute myocardial ischemia, HF, thromboembolic or hemorrhagic stroke, arrhythmia, and peripheral vascular disease [Citation20]; Niu et al. regarded cardiovascular events as hospitalization because of cardiovascular diseases [Citation14].](/cms/asset/6a299761-1ba3-462c-a8ba-90a076c057ee/irnf_a_2349135_f0002_c.jpg)
Renal events
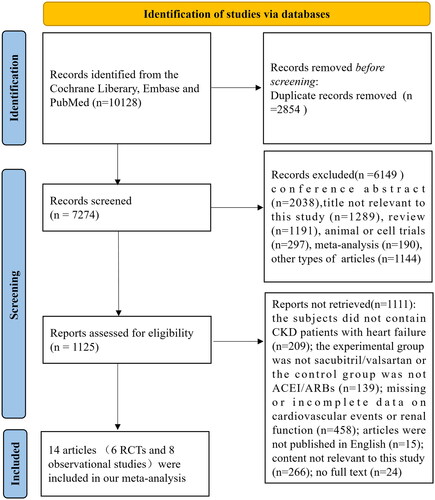
Changes in serum creatinine (scr)
Three RCTs and one observational study, involving 7030 CKD stages 3–5 patients with heart failure, analyzed the changes in serum creatinine. The pooled outcomes indicated that the risk of Scr elevation was obviously decreased in the sacubitril/valsartan group (OR: 0.81, 95% CI: 0.68–0.95, p = 0.01, I2=0%, ).
Figure 3. Results about cardiovascular events. (A) Forest plot about changes in Scr. (B) Forest plot regarding changes in eGFR. (C) Meta-analysis of the incidence of ESRD in CKD stages 3–5 patients with heart failure. *Berg et al. defined the change of Scr as an increase in serum creatinine of at least 0.5 mg/dL; **Mc Causland et al. were described the change of Scr as elevated serum creatinine ≥ 2.0 mg/dL; *** Tan et al. described the change of Scr as doubling of serum creatinine; ****Voors et al. defined the change of Scr as >0.3 mg/dL increase in creatinine in combination with an increase of more than 25% in serum creatinine between two time points. †In the Chen’s study, the PARADIGM-HF trial and the PARAGON-HF trial, eGFR level declined >50% from baseline; ††In the Berg et al.’s trial, eGFR level declined >25% from baseline; †††In the Tan’s study, eGFR level declined >30% or more from baseline.
Estimated glomerular filtration rate (eGFR)
Three RCTs and two observational studies reported the data on the incidence of eGFR reduction. As shown in , CKD stages 3–5 patients with heart failure in the sacubitril/valsartan group had a slower eGFR decline compared with the control group (OR: 0.83, 95% CI: 0.73–0.95, p = 0.007, I2=31%, ).
The incidence of end-stage renal disease (ESRD)
The incidence of ESRD was provided in two RCTs and four observational studies which contained 14008 CKD stages 3–5 patients with heart failure. Compared with control group, patients receiving sacubitril/valsartan had a lower incidence of ESRD (OR:0.73, 95%CI:0.60–0.89, p = 0.002, I2=46%, ).
To sum up, these findings indicated that sacubitril/valsartan might attenuate the progression of kidney function in abnormal renal function patients combined with heart failure.
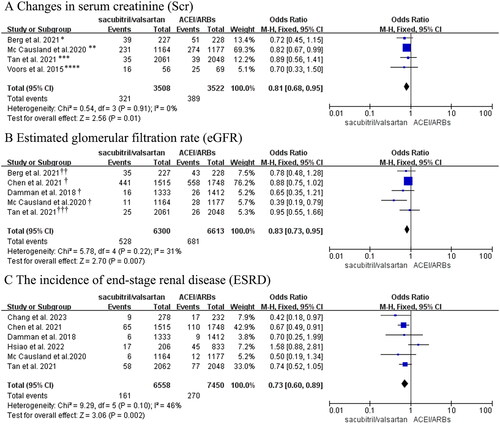
Hyperkalemia
Meta-analysis of two RCTs and three observational studies (with a total of 4131 CKD 3–5 stages patients with heart failure) showed that the risk of hyperkalemia in the sacubitril/valsartan group was comparable to that in the control group (OR: 1.31, 95% CI: 0.79–2.17, p = 0.29, ), but with high heterogeneity (I2=69%). To explore the source of heterogeneity, subgroup analysis was performed according to eGFR level, which illustrated a decrease in heterogeneity with no change in the conclusions (OR: 0.90, 95% CI: 0.70–1.17, p = 0.45, I2=16%; OR: 1.86, 95% CI: 0.87–3.98, p = 0.11, I2=45%, ). In summary, sacubitril/valsartan might not increase the risk of hyperkalemia among abnormal renal function patients combined with heart failure.
Figure 4. (A) Forest plot of the incidence of hyperkalemia. (B) Meta-analysis of the incidence of hypotension in CKD stages 3-5 patients with heart failure. (C) Forest plots of changes in blood pressure. *Hyperkalemia was defined as potassium > 5.5 mmol/L in these studies; ** Hyperkalemia was defined as potassium > 6mmol/L in Hsiao’s study. †Berg et al. regarded hypotension as symptomatic hypotension; ††Hypotension in the Mc Causland et al.’s trial was regarded as SBP < 100 mmHg.
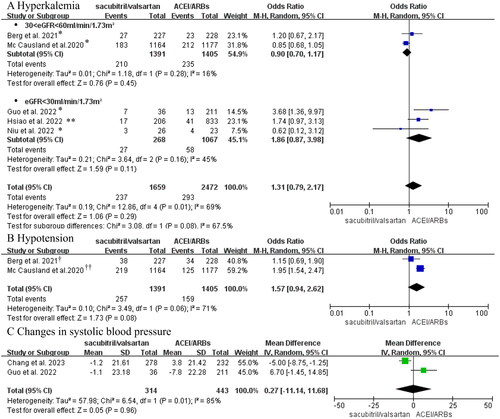
Changes in blood pressure
Regarding the changes in blood pressure, we applied dichotomous and continuous variables to perform the meta-analysis, respectively. Two RCTs consisting of 2796 CKD 3 stage patients with heart failure provided information about the incidence of hypotension (dichotomous variable). The results showed that sacubitril/valsartan did not increase the risk of hypotension (OR:1.57, 95%CI:0.94–2.62, p = 0.08, I2=71%, ).
Moreover, the pooled analysis of two observational studies involving 757 CKD 4–5 stages patients with heart failure indicated no statistically significant difference in systolic blood pressure between the two groups after follow-up (continuous variable, MD = 0.27 mmHg, 95% CI: −11.14–11.68, p = 0.96, ).
Above all, we believed that the treatment with sacubitril/valsartan might not influence blood pressure in abnormal renal function patients combined with heart failure.
Discussion
In the current study, we conducted a meta-analysis for the first time by combining RCTs and observational studies to comprehensively evaluate the efficacy and safety of sacubitril/valsartan in abnormal renal function patients with heart failure. With respect to efficacy, we found that sacubitril/valsartan significantly reduced the rate of cardiovascular death or heart failure hospitalization and the risk of kidney function worsening among eGFR below 60 mL/min/1.73m2 patients with heart failure. As for safety outcomes, the risk of hyperkalemia and hypotension was not increased in patients receiving sacubitril/valsartan. To sum up, data from our study showed an apparent cardioprotective effect and good tolerability of sacubitril/valsartan, which could provide favorable evidence for the clinical management of abnormal renal function patients combined with heart failure.
It is clear that sacubitril/valsartan concurrently inhibits neprilysin and RAAS, thereby exerting effects on sympathetic tone alleviating, vasodilation, natriuresis, and diuresis [Citation28,Citation29]. Thus, sacubitril/valsartan has an absolute cardiovascular protective effect on heart failure patients with normal renal function [Citation30,Citation31]. For heart failure patients with abnormal renal function, a meta-analysis by Kang et al. revealed that the sacubitril/valsartan group reduced NT-proBNP levels, indicating the positive effect on cardiovascular system [Citation32]. But this article focused on patients with CKD stage 3 and involved only three original studies. Based on this, our meta-analysis further included patients with CKD stages 4–5 and more relevant researches. Similar to the above study, we also found cardiovascular benefits of sacubitril/valsartan in CKD stages 3–5 patients with heart failure. To be specific, sacubitril/valsartan decreased risk of cardiovascular death or heart failure hospitalization. In summary, sacubitril/valsartan could improve cardiovascular prognosis in abnormal renal function patients combined with heart failure.
Theoretically, because of RAAS inhibition, sacubitril/valsartan can cause dilation of the efferent arterioles, subsequently leading to a decrease in glomerular pressure and eGFR [Citation33]. However, our previous study [Citation16] on patients with CKD stage 3 did not observe a significant reduction in eGFR among patients treated with sacubitril/valsartan. In the present meta-analysis, we further assessed the renal impact of sacubitril/valsartan on patients with lower eGFR based on RCTs and real-world observational studies. Consistently, the findings demonstrated that sacubitril/valsartan could effectively prevent the deterioration of renal function. This may be related to the increase in renal perfusion after improvement of heart failure by sacubitril/valsartan [Citation14,Citation25]. Furthermore, with the extension of follow-up (ranged from 8 weeks to 35 months), sacubitril/valsartan also revealed a favorable long-term prognosis for kidney function.
Hyperkalemia is a potentially fatal side effect of sacubitril/valsartan in patients with CKD [Citation34]. But, the latest RCT involving CKD patients without heart failure found no statistical difference in hyperkalemia events between the sacubitril/valsartan and the control group [Citation35]. Our meta-analysis, focusing on CKD patients combined with heart failure, also did not discover a trend of increased risk of hyperkalemia after sacubitril/valsartan treatment. Collectively, these findings indicated that sacubitril/valsartan may be safe for abnormal renal function patients combined with heart failure.
Previous studies found that in heart failure patients with normal kidney function, hypotension (SBP < 90mmHg) was more frequent in sacubitril/valsartan group than ACEI/ARBs [Citation21]. Mechanistically, the use of sacubitril/valsartan in patients with renal impairment may increase the risk of hypotension, because the metabolism of sacubitril is dependent on renal function [Citation33]. However, our results showed that sacubitril/valsartan did not exert a marked impact on blood pressure alterations among eGFR below 60 mL/min/1.73m2 patients with heart failure. Generally, apart from RAAS overactivation and volume overload, several hormones such as endothelin [Citation36] and thromboxane [Citation37,Citation38] also play a role in the development of hypertension in CKD patients. But these hormones are not inhibited by sacubitril-valsartan, which may explain why the incidence of hypotension in CKD stages 3–5 patients with heart failure receiving sacubitril/valsartan has not increased. Noteworthily, recent studies have demonstrated a dose-dependent effect of sacubitril/valsartan on anti-hypertension [Citation39–41]. In clinical practice, the risk of hypotension may elevate with the increase in dosage of the drug. Thus, it is crucial to customize personalized therapy for individuals based on fluctuations in blood pressure.
There were several limitations in our study. First, our meta-analysis included several observational studies which lacked the experimental random allocation to assess outcomes accurately. But the risk of bias was relatively low in these observational studies, which meant that confounding factors were controlled to some extent. Second, some outcomes defined differently in these trials, but very slightly. Third, RCTs about the role of sacubitril-valsartan in patients with eGFR < 30 mL/min/1.73m2 are still rare, and further large-scale RCTs should be carried out to validate above results.
Conclusions
Our meta-analysis proves that sacubitril/valsartan has a favorable effect on cardiac function without obvious risk of adverse events in abnormal renal function patients combined with heart failure, indicating that sacubitril/valsartan has the potential to become perspective treatment for these patients.
Supplemental Material
Download PDF (443.8 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
All data generated or analyzed during this study are included in this article or Supplementary files.
Additional information
Funding
References
- Gan L, Lyu X, Yang X, et al. Application of angiotensin receptor-neprilysin inhibitor in chronic kidney disease patients: chinese expert consensus. Front Med (Lausanne). 2022;9:1. doi:10.3389/fmed.2022.877237.
- Kuang H, Huang X, Zhou Z, et al. Sacubitril/valsartan in chronic kidney disease: from pharmacological mechanism to clinical application. Eur J Pharmacol. 2021;907:174288. doi:10.1016/j.ejphar.2021.174288.
- Mehta R, Ning H, Bansal N, et al. Ten-Year risk-Prediction equations for incident heart failure hospitalizations in chronic kidney disease: findings from the chronic renal insufficiency cohort study and the Multi-Ethnic study of atherosclerosis. J Card Fail. 2022;28(4):540–11. doi:10.1016/j.cardfail.2021.10.007.
- Joseph MS, Palardy M, Bhave NM. Management of heart failure in patients with end-stage kidney disease on maintenance dialysis: a practical guide. Rev Cardiovasc Med. 2020;21(1):31–39. doi:10.31083/j.rcm.2020.01.24.
- Rangaswami J, McCullough PA. Heart failure in End-Stage kidney disease: pathophysiology, diagnosis, and therapeutic strategies. Semin Nephrol. 2018;38(6):600–617. doi:10.1016/j.semnephrol.2018.08.005.
- House AA, Wanner C, Sarnak MJ, et al. Heart failure in chronic kidney disease: conclusions from a kidney disease: improving global outcomes (KDIGO) controversies conference. Kidney Int. 2019;95(6):1304–1317. doi:10.1016/j.kint.2019.02.022.
- Ayalasomayajula S, Langenickel T, Pal P, et al. Clinical pharmacokinetics of sacubitril/valsartan (LCZ696): a novel angiotensin receptor-neprilysin inhibitor. Clin Pharmacokinet. 2017;56(12):1461–1478. doi:10.1007/s40262-017-0543-3.
- Yancy CW, Jessup M, Bozkurt B, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American college of cardiology/American heart association task force on clinical practice guidelines and the heart failure society of america. Circulation. 2017;136(6):e137–e161. doi:10.1161/cir.0000000000000509.
- Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the european society of cardiology (ESC)developed with the special contribution of the heart failure association (HFA) of the ESC. Eur Heart J. 2016;37(27):2129–2200. doi:10.1093/eurheartj/ehw128.
- McMurray JJ, Packer M, Desai AS, et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371(11):993–1004. doi:10.1056/NEJMoa1409077.
- Mc Causland FR, Lefkowitz MP, Claggett B, et al. Angiotensin-Neprilysin inhibition and renal outcomes in heart failure with preserved ejection fraction. Circulation. 2020;142(13):1236–1245. doi:10.1161/circulationaha.120.047643.
- Berg DD, Samsky MD, Velazquez EJ, et al. Efficacy and safety of sacubitril/valsartan in high-risk patients in the PIONEER-HF trial. Circ Heart Fail. 2021;14(2):e007034. doi:10.1161/circheartfailure.120.007034.
- Voors AA, Gori M, Liu LC, et al. Renal effects of the angiotensin receptor neprilysin inhibitor LCZ696 in patients with heart failure and preserved ejection fraction. Eur J Heart Fail. 2015;17(5):510–517. doi:10.1002/ejhf.232.
- Niu CY, Yang SF, Ou SM, et al. Sacubitril/valsartan in patients with heart failure and concomitant End-Stage kidney disease. J Am Heart Assoc. 2022;11(18):e026407. doi:10.1161/jaha.122.026407.
- Hsiao FC, Lin CP, Yu CC, et al. Angiotensin receptor-neprilysin inhibitors in patients with heart failure with reduced ejection fraction and advanced chronic kidney disease: a retrospective Multi-Institutional study. Front Cardiovasc Med. 2022;9:794707. doi:10.3389/fcvm.2022.794707.
- Zhou W, Yang X, Jin J, et al. The efficacy and safety of sacubitril/valsartan in chronic kidney disease: a systematic review and meta-analysis. Int Urol Nephrol. 2023;56(1):181–190. doi:10.1007/s11255-023-03599-w.
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi:10.1371/journal.pmed.1000097.
- Sterne JA, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. doi:10.1136/bmj.i4919.
- Damman K, Gori M, Claggett B, et al. Renal effects and associated outcomes during angiotensin-neprilysin inhibition in heart failure. JACC Heart Fail. 2018;6(6):489–498. doi:10.1016/j.jchf.2018.02.004.
- Sheng Y, Ma X, Liu Y, et al. Study on the efficacy of sacubitril/valsartan in patients with heart failure with preserved ejection fraction undergoing peritoneal dialysis. Cardiology. 2023;148(5):385–394. doi:10.1159/000531217.
- Tsutsui H, Momomura SI, Saito Y, et al. Efficacy and safety of sacubitril/valsartan in japanese patients with chronic heart failure and reduced ejection Fraction – Results from the PARALLEL-HF study. Circ J. 2021;85(5):584–594. doi:10.1253/circj.CJ-20-0854.
- Chang HY, Feng AN, Fong MC, et al. Sacubitril/valsartan in heart failure with reduced ejection fraction patients: real world experience on advanced chronic kidney disease, hypotension, and dose escalation. J Cardiol. 2019;74(4):372–380. doi:10.1016/j.jjcc.2019.03.010.
- Chang HY, Lin CC, Chao CJ, et al. Real-World experience of angiotensin receptor-neprilysin inhibition in reduced ejection fraction heart failure patients with advanced kidney disease. Mayo Clin Proc. 2023;98(1):88–99. doi:10.1016/j.mayocp.2022.06.007.
- Chen DY, Chen CC, Tseng CN, et al. Clinical outcomes of sacubitril/valsartan in patients with acute heart failure: a multi-institution study. EClinicalMedicine. 2021;41:101149. doi:10.1016/j.eclinm.2021.101149.
- Guo Y, Ren M, Wang T, et al. Effects of sacubitril/valsartan in ESRD patients undergoing hemodialysis with HFpEF. Front Cardiovasc Med. 2022;9:955780. doi:10.3389/fcvm.2022.955780.
- Lee WC, Liao TW, Chen TY, et al. Sacubitril/valsartan improves all-cause mortality in heart failure patients with reduced ejection fraction and chronic kidney disease. Cardiovasc Drugs Ther. 2023 [cited 2023 Jan 7]. doi:10.1007/s10557-022-07421-0.
- Tan NY, Deng Y, Yao X, et al. Renal outcomes in patients with systolic heart failure treated with sacubitril-valsartan or angiotensin converting enzyme inhibitor/angiotensin receptor blocker. Mayo Clin Proc Innov Qual Outcomes. 2021;5(2):286–297. doi:10.1016/j.mayocpiqo.2020.10.008.
- Pontremoli R, Borghi C, Perrone Filardi P. Renal protection in chronic heart failure: focus on sacubitril/valsartan. Eur Heart J Cardiovasc Pharmacother. 2021;7(5):445–452. doi:10.1093/ehjcvp/pvab030.
- Chen X, Jin C, Xie L, et al. LCZ696 and preservation of renal function in heart failure: a meta-analysis of 6 randomized trials. Rev Cardiovasc Med. 2020;21(1):113–118. doi:10.31083/j.rcm.2020.01.2.
- Wang Y, Zhou R, Lu C, et al. Effects of the angiotensin-receptor neprilysin inhibitor on cardiac reverse remodeling: meta-analysis. J Am Heart Assoc. 2019;8(13):e012272. doi:10.1161/jaha.119.012272.
- Hsieh HL, Chen CY, Chen CH, et al. Renal protective effect of sacubitril/valsartan in patients with heart failure. Sci Rep. 2021;11(1):4593. doi:10.1038/s41598-021-84118-8.
- Kang H, Zhang J, Zhang X, et al. Effects of sacubitril/valsartan in patients with heart failure and chronic kidney disease: a meta-analysis. Eur J Pharmacol. 2020;884:173444. doi:10.1016/j.ejphar.2020.173444.
- Cho IJ, Kang SM. Angiotensin receptor-neprilysin inhibitor in patients with heart failure and chronic kidney disease. Kidney Res Clin Pract. 2021;40(4):555–565. doi:10.23876/j.krcp.21.900.
- Valdivielso JM, Balafa O, Ekart R, et al. Hyperkalemia in chronic kidney disease in the new era of kidney protection therapies. Drugs. 2021;81(13):1467–1489. doi:10.1007/s40265-021-01555-5.
- Haynes R, Judge PK, Staplin N, et al. Effects of sacubitril/valsartan versus irbesartan in patients with chronic kidney disease. Circulation. 2018;138(15):1505–1514. doi:10.1161/circulationaha.118.034818.
- Moorhouse RC, Webb DJ, Kluth DC, et al. Endothelin antagonism and its role in the treatment of hypertension. Curr Hypertens Rep. 2013;15(5):489–496. doi:10.1007/s11906-013-0380-1.
- Thomas P, Dasgupta I. The role of the kidney and the sympathetic nervous system in hypertension. Pediatr Nephrol. 2015;30(4):549–560. doi:10.1007/s00467-014-2789-4.
- Hu J, Yang Z, Chen X, et al. Thromboxane A(2) is involved in the development of hypertension in chronic kidney disease rats. Eur J Pharmacol. 2021;909:174435. doi:10.1016/j.ejphar.2021.174435.
- Zhao Y, Yu H, Zhao X, et al. The effects of LCZ696 in patients with hypertension compared with angiotensin receptor blockers: a meta-analysis of randomized controlled trials. J Cardiovasc Pharmacol Ther. 2017;22(5):447–457. doi:10.1177/1074248417693379.
- Kario K, Sun N, Chiang FT, et al. Efficacy and safety of LCZ696, a first-in-class angiotensin receptor neprilysin inhibitor, in asian patients with hypertension: a randomized, double-blind, placebo-controlled study. Hypertension. 2014;63(4):698–705. doi:10.1161/hypertensionaha.113.02002.
- Zhang Y, Zhao X, Huang H, et al. Network meta-analysis of sacubitril/valsartan for the treatment of essential hypertension. Clin Res Cardiol. 2023;112(7):855–867. doi:10.1007/s00392-022-02120-0.