Abstract
Serum magnesium levels exceeding 0.9 mmol/L are associated with increased survival rates in patients with CKD. This retrospective study aimed to identify risk factors for cardio-cerebrovascular events among patients receiving continuous ambulatory peritoneal dialysis (CAPD) and to examine their correlations with serum magnesium levels. Sociodemographic data, clinical physiological and biochemical indexes, and cardio-cerebrovascular event data were collected from 189 patients undergoing CAPD. Risk factors associated with cardio-cerebrovascular events were identified by univariate binary logistic regression analysis. Correlations between the risk factors and serum magnesium levels were determined by correlation analysis. Univariate regression analysis identified age, C-reactive protein (CRP), red cell volume distribution width standard deviation, red cell volume distribution width corpuscular volume, serum albumin, serum potassium, serum sodium, serum chlorine, serum magnesium, and serum uric acid as risk factors for cardio-cerebrovascular events. Among them, serum magnesium ≤0.8 mmol/L had the highest odds ratio (3.996). Multivariate regression analysis revealed that serum magnesium was an independent risk factor, while serum UA (<440 μmol/L) was an independent protective factor for cardio-cerebrovascular events. The incidence of cardio-cerebrovascular events differed significantly among patients with different grades of serum magnesium (χ2 = 12.023, p = 0.002), with the highest incidence observed in patients with a serum magnesium concentration <0.8 mmol/L. High serum magnesium levels were correlated with high levels of serum albumin (r = 0.399, p < 0.001), serum potassium (r = 0.423, p < 0.001), and serum uric acid (r = 0.411, p < 0.001), and low levels of CRP (r = −0.279, p < 0.001). In conclusion, low serum magnesium may predict cardio-cerebrovascular events in patients receiving CAPD.
Introduction
Chronic kidney disease (CKD) is a global health problem with a prevalence of 13.4% [Citation1]. In China, the prevalence rate of CKD was reported to be 4.86% in 2016, affecting approximately a population of 100 million [Citation2]. Peritoneal dialysis (PD) is an important home-based treatment for kidney failure that is used by approximately 11% of all dialysis patients and 9% of all patients receiving kidney replacement therapy globally [Citation3]. For patients with stage 5 CKD, chronic ambulatory peritoneal dialysis (CAPD) is the preferred clinical treatment, especially during the first 4 years, due to its ease of operation, minimal effect on hemodynamics, lack of a size limitation, strong autonomy, protection of residual renal function, low risk of the spread of blood-borne pathogens, and ability to prolong the survival of patients [Citation4]. Increased oxidative stress, production of inflammatory cytokines, overactivity of sympathetic nerves, increased molecular adhesion, inflammation, and susceptibility to cardio-cerebrovascular diseases have been reported to occur during periods of magnesium deficiency in patients with CKD [Citation5]. Magnesium deficiency has also been associated with an elevated risk of both non-fatal and fatal cardio-cerebrovascular events [Citation6,Citation7]. A previous study revealed that hypomagnesemia was significantly associated with an increased risk of cardiovascular disease mortality, which persisted even after adjustment for multiple covariates [Citation8]. Another study identified serum magnesium as a potential independent factor for predicting the cardio-cerebrovascular prognosis of patients with different stages of CKD [Citation9].
In clinical practice, restrictions on magnesium-containing foods may promote the development of hypomagnesemia, especially in patients with stage 5 CKD or end-stage renal disease (ESRD) who are asked to limit their intake of fresh green vegetables and seafood [Citation5]. Hypomagnesemia is associated with a variety of pathological conditions, including arrhythmia, hypertension, vascular calcification, cardio-cerebrovascular disease, insulin resistance, asthma, migraine, hypokalemia, hypocalcemia, osteoporosis, and other forms of terminal organ damage [Citation10]. Specifically, arrhythmias caused by hypomagnesemia can lead to fatal ventricular arrhythmias [Citation11]. Moreover, hypomagnesemia has been identified as a risk factor and accelerator for vascular aging in diabetes and CKD [Citation12]. Hypermagnesemia is related to a variety of adverse conditions, including hypotension, bradycardia, disturbance of consciousness, hypocalcemia, hyperkalemia, loss of deep tendon reflex, and respiratory distress [Citation6]. In contrast to low serum magnesium levels, relatively high serum magnesium levels may be beneficial in reducing cardiovascular risk, preventing vascular calcification, and treating hypertension in patients with CKD [Citation13]. Within a certain range of serum magnesium concentrations, a serum magnesium level exceeding 0.9 mmol/L was found to be associated with increased survival rates in the general population and patients with CKD [Citation14]. High magnesium intake may help to improve possible risk factors for the occurrence and development of cardio-cerebrovascular events, such as metabolic syndrome, diabetes, and hypertension, and in addition, higher serum magnesium levels are associated with a lower incidence of stroke and cardio-cerebrovascular diseases [Citation15].
Few studies have examined the effects of magnesium metabolism disorders in Asian patients receiving PD, especially in patients undergoing CAPD, compared with hemodialysis. Accordingly, the present study aimed to identify risk factors for cardio-cerebrovascular events among patients receiving continuous ambulatory peritoneal dialysis (CAPD) and to examine their correlations with serum magnesium. These findings may contribute to a deeper understanding of abnormal magnesium metabolism in patients with CKD, and provide guidance for a strategy to properly monitor serum magnesium to avoid magnesium metabolic disorders in these patients.
Materials and methods
Study design
In this retrospective study, the clinical data of 189 patients undergoing CAPD at the Peritoneal Dialysis Center of Inner Mongolia Medical University between January 2021 and December 2022 were collected. The inclusion criteria were: (1) age >18 years, (2) stable vital signs, and (3) treatment with PD for ≥1 year. The exclusion criteria were: (1) missing data for serum magnesium levels or other relevant clinical data; (2) acute renal injury; (3) prior renal transplantation; (4) any type of tumor or clinical manifestations and laboratory tumor markers indicating the possibility of a tumor; (5) severe infection; and (6) refusal to participate. This study was approved by the ethics committee of the Affiliated Hospital of Inner Mongolia Medical University and conducted in accordance with the Declaration of Helsinki (S.2021035). Following the national legislation and institutional requirements, an opt-out procedure replaced the need for patients’ provision of written consent.
Collection of clinical data
The demographic information, clinical diagnosis, and laboratory data of all selected patients were collected from the electronic medical records system of the Affiliated Hospital of Inner Mongolia Medical University. The collected data included: age, sex, cardio-cerebrovascular events, blood test indexes such as C-reactive protein (CRP), red cell volume distribution width standard deviation (RDW-SD), red cell volume distribution width corpuscular volume (RDW-CV), hemoglobin, white blood cell count, platelet count, erythrocyte sedimentation rate, alanine aminotransferase (ALT), aspartate aminotransferase (AST), ALT/AST ratio, alkaline phosphatase, total protein, albumin, globulin, albumin/globulin (A/G) ratio, total bilirubin, direct bilirubin, indirect bilirubin, potassium, sodium, chloride, calcium, phosphorus, magnesium, urea, serum creatinine, uric acid (UA), serum cystatin C, retinol-binding protein quantification, lactate dehydrogenase, creatine kinase, creatine kinase isoenzyme, α-hydroxybutyrate dehydrogenase, homocysteine, lipoprotein a, apolipoprotein AI, apolipoprotein B100, apolipoprotein A/B, glucose, glycosylated hemoglobin, parathyroid hormone determination, procalcitonin, high-density lipoprotein cholesterol, low density lipoprotein cholesterol, small dense low-density lipoprotein cholesterol, total cholesterol, triglyceride, and complement c1q. Hypoalbuminemia can lead to a decrease in total serum calcium, resulting in a decrease in bound calcium even though the level of ionic calcium remains normal. Therefore, the effect of serum albumin content on serum total calcium was corrected according to Penn’s formula [Citation16]: Corrected serum calcium (mmol/L) = serum calcium (mmol/L) + (40 − serum albumin) × 0.025 (mmol/L). The product of calcium and phosphorus was calculated according to the correlation formula Ca-P= (4 × serum calcium) × (3.1 × serum phosphorus) [Citation17]. The relevant clinical tests were carried out by the Clinical Laboratory Department of the Affiliated Hospital of Inner Mongolia Medical University.
Cardio-cerebrovascular events
Total cardio-cerebrovascular events include myocardial infarction, cerebral infarction, and cerebral hemorrhage [Citation18]. If an event occurred two or more times, it was recorded only once, taking the time of the first occurrence and the time the event concluded. Cases with transient ischemic attack were not included.
Kt/V was calculated using the formula Kt/V = –ln(R − 0.008 × t) + (4 − 3.5 × R) × UF/W, where ln represents the natural logarithm, R denotes the ratio of blood urea after dialysis to before dialysis, K signifies the urea clearance rate of the dialyzer, t indicates the dialysis time, V represents the distribution volume of urea in the body, UF represents the ultrafiltration rate in liters, and W represents the patient’s weight after dialysis.
Measurement of serum magnesium concentration
The normal range for serum magnesium concentration was 1.70–2.4 mg/dL (0.8–1.1 mmol/L). Thus, hypomagnesemia was defined as a serum magnesium concentration <0.8 mmol/L, and hypermagnesemia was defined as a serum magnesium >1.1 mmol/L [Citation19].
Statistical analysis
All data were analyzed using SPSS version 25.0. All grouped data were tested for normality before further analysis. Count data that conformed to the normal distribution were compared between two groups using an independent t-test. A non-parametric rank-sum test was used if the data for any group in the comparison did not conform to a normal distribution. Normally distributed data were expressed as means (x) ± standard deviation (SD). The Shapiro–Wilk test, median, and interquartile range were used to check the degree of dispersion of the data. Risk factors associated with cardio-cerebrovascular events were identified by univariate binary logistic regression analysis. Correlations between two types of data were analyzed by Pearson’s rank correlation if both groups followed a normal distribution and by Spearman’s rank correlation if any group did not follow a normal distribution. Cross-tabulation χ2 test was used for comparisons between genders. p values <0.05 indicated statistical significance.
Results
Patients’ clinical characteristics
Among the 189 patients receiving CAPD who were included in this study, 41 patients had cardio-cerebrovascular events and 148 patients did not experience cardio-cerebrovascular events. shows the statistical comparisons of data for patients who did and did not experience cardio-cerebrovascular events. Age, CRP, RDW-SD, RDW-CV, AST, and serum UA were significantly higher in the group with cardio-cerebrovascular events than in the group without cardio-cerebrovascular events, whereas serum albumin, A/G, serum potassium, serum sodium, serum chloride, serum magnesium, and retinol-binding protein quantification were significantly lower in the group with cardio-cerebrovascular events than in the group without cardio-cerebrovascular events (all p < 0.05). All other examined indexes showed no significant difference between the groups with and without cardio-cerebrovascular events ().
Table 1. Clinical characteristics of the patients receiving CAPD (N = 189).
Prevalence of cardio-cerebrovascular events
The prevalence of cardio-cerebrovascular events among the included CAPD patients was 25.4%. For the most common cardio-cerebrovascular events, the prevalence rates were 6.9% for cerebral infarction, 2.6% for myocardial infarction, and 2.1% for cerebral hemorrhage.
Risk factors of cardio-cerebrovascular events
After classifying clinical indicators with differences according to the normal value range of clinical indicators and performing univariate binary logistic regression analysis, a total of 10 clinical indicators were found to be statistically significant risk factors for cardio-cerebrovascular events: age (odds ratio [OR] = 2.319, 95% confidence interval [CI] 1.147–4.692), CRP (OR = 2.531, 95%CI 1.249–5.128), RDW-SD (OR = 3.225, 95%CI 1.581–6.578), RDW-CV (OR = 2.370, 95%CI 1.053–5.333), serum albumin (OR = 2.269, 95%CI 1.055–4.882), serum potassium (OR = 2.523, 95%CI 1.141–5.582), serum sodium (OR = 3.870, 95%CI 1.776–8.435), serum chlorine (OR = 2.417, 95%CI 1.146–5.095), serum magnesium (OR = 3.996, 95%CI 1.760–9.070), and serum UA (OR = 3.289, 95%CI 1.299–8.329) (). Diabetes and Kt/v were also significant risk factors for cardio-cerebrovascular events (Supplementary Table 1). Further multivariate regression analysis revealed that serum magnesium (OR = 2.834, 95%CI 1.069–7.510) was an independent risk factor, while serum UA (OR = 0.430, 95%CI 0.217–0.853) was an independent protective factor for cardio-cerebrovascular events ().
Table 2. Univariate analysis of risk factors affecting cardio-cerebrovascular events (N = 189).
Table 3. Multivariate analysis of risk factors affecting cardio-cerebrovascular events (N = 189).
Cardio-cerebrovascular events in patients with different serum magnesium levels
From the analysis of risk factors for cardio-cerebrovascular events, serum magnesium had the highest OR value. We further categorized serum magnesium levels into three different ranges of <0.8 mmol/L, 0.8–1.1 mmol/L, and >1.1 mmol/L. We found that the cardio-cerebrovascular event rate among patients with serum magnesium <0.8 mmol/L was 45.2%, while this rate was 17.1% for patients with serum magnesium of 0.8–1.1 mmol/L and patients with serum magnesium >1.1 mmol/L. Further analysis confirmed that the incidence of cardio-cerebrovascular events differed significantly among patients with different grades of serum magnesium (χ2=12.023, p = 0.002; ). These data demonstrated that the incidence of cardio-cerebrovascular events differed significantly among patients with different grades of serum magnesium, with the highest incidence observed in those with a serum magnesium concentration <0.8 mmol/L.
Table 4. Incidence of cardio-cerebrovascular events in patient groups with different serum magnesium levels (N = 189).
Clinical factors associated with serum magnesium
Among all patients receiving CAPD, Spearman correlation analysis showed significant correlations between the serum magnesium concentration and the serum concentrations of albumin, potassium, UA, and CRP. Within a specific range of serum magnesium concentrations, positive correlations were observed between serum magnesium and serum albumin (r = 0.399, p < 0.001), serum potassium (r = 0.423, p < 0.001), and UA (r = 0.411, p < 0.001). Conversely, serum magnesium was negatively correlated with CRP (r= −0.279, p < 0.001) (, ).
Figure 1. Serum magnesium concentration was correlated with risk factors for cardio-cerebrovascular events, including serum albumin, serum potassium, uric acid (UA), and C-reactive protein (CRP) among patients undergoing continuous ambulatory peritoneal dialysis (CAPD).
A: Positive correlation between serum magnesium and serum albumin (r = 0.399, p < 0.001).
B: Positive correlation between serum magnesium and serum potassium (r = 0.423, p < 0.001).
C: Positive correlation between serum magnesium and UA (r = 0.411, p < 0.001).
D: Negative correlation between serum magnesium and CRP (r= −0.279, p < 0.001).
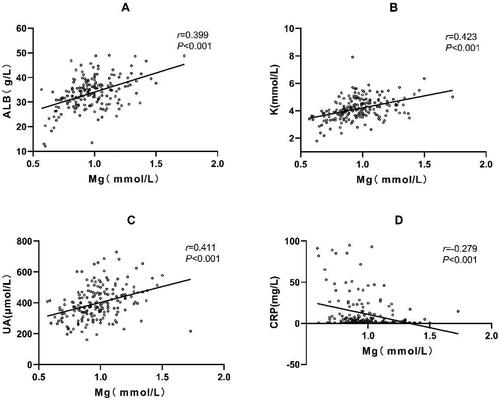
Table 5. Clinical factors associated with serum magnesium (N = 189).
Clinical factors associated with serum magnesium in different groups
In patients who experienced cardiovascular events, a positive correlation was observed between A/G and serum magnesium (r2 = 0.475). Conversely, the erythrocyte sedimentation rate (r2=-0.395) and alkaline phosphatase (r2= −0.443) were negatively correlated with serum magnesium. However, these correlations were not found in patients without cardiovascular events. Among those without cardiovascular events, retinol-binding protein (r1 = 0.278), creatine kinase (r1 = 0.019), homocysteine (r1 = 0.313), glucose (r1= −0.192), and complement c1q (r1= −0.262) were negatively correlated with serum magnesium. However, these associations were not observed in those with cardiovascular events. Furthermore, irrespective of the occurrence of cardiovascular and cerebrovascular events, many clinical factors such as total protein, serum albumin, serum potassium, serum phosphorus, calcium-phosphorus product, serum urea, serum creatinine, and serum UA were positively correlated with serum magnesium. In addition, CRP was negatively correlated with serum magnesium in both groups ().
Table 6. Clinical factors associated with serum magnesium in different groups (N = 189).
Discussion
This study showed that serum magnesium was the clinical index with the highest OR value as a protective factor for cardio-cerebrovascular events in patients undergoing CAPD. In addition, the incidence of cardio-cerebrovascular events varied among patients with different grades of serum magnesium concentration and was higher in patients with a serum magnesium concentration <0.8 mmol/L. Univariate analysis showed that CRP was negatively correlated with serum magnesium, while serum albumin, serum potassium, serum chlorine, and serum UA were positively correlated with serum magnesium. Multivariate regression analysis revealed that serum magnesium was an independent risk factor, while serum UA was an independent protective factor for cardio-cerebrovascular events.
Diabetes is a multifactorial disease affected by heredity, social factors, lifestyle, and the environment [Citation20]. The incidence of diabetes is associated with age, family history diabetes, obesity, and insulin resistance. Approximately 75% of diabetic patients die from cardio-cerebrovascular disease annually [Citation21,Citation22]. Biological dysfunction of insulin regulation often results in lipid metabolism disorder and concurrent hyperlipidemia in diabetic patients [Citation23]. The combination of diabetes and hyperlipidemia accelerates atherosclerosis progression and increases the risk of cardiovascular disease [Citation24]. Moreover, the coexistence of hyperlipidemia and elevated high sensitivity CRP levels may be a key factor in promoting vascular endothelial injury in hypertensive patients and increasing the incidence of cardiovascular disease.
Our correlation analysis revealed that serum magnesium and CRP were negatively correlated among patients undergoing CAPD and that a high CRP level was a risk factor for cardio-cerebrovascular events. Previous research has shown that magnesium ions have anti-inflammatory and antioxidant effects [Citation15]. Another study reported that low serum magnesium accelerates vascular calcification and atherosclerosis, both of which contribute to cardiovascular events and may increase the risk of sudden cardiac death [Citation25]. Both CKD and cardiovascular diseases are characterized by a high inflammatory burden, chronic systemic inflammation, and oxidative stress and are associated with altered magnesium metabolism, more specifically hypomagnesemia [Citation26]. However, in patients with chronic diseases, the CRP level may be chronically elevated, making it difficult to determine whether a high CRP level represents an independent risk factor for cardiovascular events in patients with altered magnesium metabolism. Magnesium has also been administered during seizure events to induce dilatation of cerebral vessels and thereby prevent cerebral vasospasm. However, a previous study on women with severe preeclampsia showed that the vasodilatory property of magnesium had no short-term therapeutic effect on the glomerular filtration rate [Citation27]. Given this, it is worth examining whether the vasodilatory property of magnesium has an effect in patients undergoing CAPD.
Kt/V is an indicator reflecting the clearance of small molecule solutes, water, and sodium during peritoneal dialysis, providing insights into the adequacy of dialysis procedure. Clinical practice guidelines recommend a weekly total Kt/V of 8-membrane dialysis to be at least 1.7 for optimal peritoneal dialysis adequacy [Citation28]. A decrease in Kt/V indicates that insufficient removal of excess water and urea nitrogen, leading to the formation of urea nitrogen decomposition products, which can contribute to protein carbamylation modification. A previous study [Citation29] has shown that protein carbamylation inhibits ectonucleoside pyrophosphate/phosphodiesterase 1 expression, exacerbating vascular smooth muscle cell calcification. Lower Kt/V values indicate inadequate dialysis, correlating with a more pronounced disruption in calcium and phosphorus metabolism, a recognized risk factor for vascular calcification [Citation30,Citation31]. Vascular calcification on the peritoneal surface directly impacts blood vessel elasticity and blood flow supply, affecting the ultrafiltration function in peritoneal dialysis patients. Furthermore, lower Kt/V values correlate with a decreased ability to remove inflammatory mediators, resulting in an increase in inflammatory factors in peritoneal dialysis patients, as evidenced by a study [Citation31] showing that inflammation is related to vascular calcification. Inflammatory conditions also lead to a reduction in ultrafiltration volume compared to normal conditions during peritoneal dialysis. Therefore, these factors collectively contribute to the more severe vascular calcification observed with reduced Kv/V. A previous study showed that, anuric patients, even with low Kt/V, can achieve nitrogen balance and maintain good nutritional status with appropriate dietary protein intake [Citation32].
We also identified a low serum albumin level as a risk factor for cardiovascular events in patients receiving CAPD and showed a significant positive correlation between serum albumin and serum magnesium. In general, albumin is considered a biomarker for the assessment of nutritional status. Hypoalbuminemia in dialysis patients is primarily a consequence of a reduced albumin synthesis rate [Citation33]. Previous studies have shown that reduced hepatic albumin synthesis due to malnutrition as well as protein loss through the kidneys [Citation34] or gastrointestinal tract leads to hypoproteinemia [Citation35]. However, in patients receiving CAPD, low serum albumin is to a greater extent attributable to systemic inflammation than to malnutrition. Adequate amounts of albumin mobilize polyunsaturated fatty acids from the liver and allow the synthesis of various anti-inflammatory lipids such as lipoxins, lysins, and protections [Citation36]. Therefore, it can be hypothesized that hypoalbuminemia may contribute to increased morbidity and mortality from cardio-cerebrovascular events through pro-inflammatory effects. A previous study reported that A/G is associated with all-cause patient mortality and the occurrence of cardiovascular events, independently of various clinical confounders, with patients with higher A/G levels having lower overall and cardiovascular mortality compared with those with lower A/G levels [Citation37]. Therefore, A/G may serve as an important predictor of cardio-cerebrovascular events in patients with CKD.
A low serum potassium level was also identified as a risk factor for the development of cardio-cerebrovascular events in patients receiving CAPD, and a positive correlation was observed between serum magnesium and serum potassium. In patients with ESRD, hypokalemia and hypomagnesemia can occur together for a variety of reasons, including as adverse reactions to diuretics, chronic diarrhea, use of drugs that induce nephrotoxic tauopathies such as aminoglycosides and cisplatin, alcohol abuse, and reduced intake due to dietary restrictions for patients with CKD [Citation5]. Intracellular magnesium binds to ROMK channels and inhibits the outward transfer of potassium, and thus, lower serum magnesium promotes potassium excretion [Citation38]. Hyperkalemia and hypomagnesemia can occur together in all patients with CKD [Citation39]. Previous research demonstrated that persistent hypokalemia is associated with higher mortality and peritonitis in CAPD patients [Citation40]. These findings suggest that clinicians should promptly measure serum magnesium and potassium concentrations when patients receiving CAPD have unexplained test abnormalities.
In the present study, serum UA was positively correlated with serum magnesium. However, multivariate analysis suggested that serum magnesium was an independent risk factor, while serum UA (<440 μmol/L) was an independent protective factor for cardio-cerebrovascular events in patients undergoing CAPD. Most UA is derived from dietary intake and endogenous purine catabolism, and about two-thirds of UA is excreted through the urine [Citation41]. Previous studies have shown that the incidence of cardiovascular events, such as hypertension, coronary heart disease, heart failure, or stroke, increases with increasing UA level [Citation42,Citation43]. UA may also act as an endogenous risk signal to trigger arrhythmias [Citation44]. Moreover, a recent meta-analysis of data from more than 172,000 individuals confirmed that UA is an independent predictor of cardiovascular mortality [Citation45]. However, interventions that reduce urate production or increase urate excretion in patients with hyperuricemia consistently improve cardiorenal prognosis [Citation46]. The controversial role of UA as either a promoter or protector in these pathological conditions warrants further investigation.
Increased RDW has been reported to be an emerging biomarker of abnormal red blood cell metabolism and survival, potentially representing oxidative stress, inflammation, and various diseases; thus, an increased RDW may be considered an indicator of inflammation [Citation47]. Additionally, abnormally increased RDW has been identified as a negative prognostic factor in patients with advanced heart failure who have recently experienced decompensation, independent of the presence of anemia or malnutrition, that offers superior performance compared with more traditionally used indicators [Citation48]. In addition, increased RDW is a predictor of early anemia associated with iron, vitamin B12, or folic acid deficiency, as it reflects the presence of peripheral immature erythrocytes due to increased erythrocyte destruction, pathological iron homeostasis, and ineffective erythropoiesis [Citation49]. Due to their ability to deform and flow in the microvascular network, erythrocytes have various essential functions in the body, such as blood and tissue-to-tissue gas exchange [Citation50]. In patients with CKD, erythrocyte deformability is significantly reduced due to changes in osmotic pressure, and the consequent expansion or contraction of erythrocytes within the vasculature can lead to microvascular perfusion as well as tissue hypoxia, which contributes to the development of heart failure symptoms. This may be one of the mechanisms through which elevated RDW is associated with congestive heart failure [Citation51]. However, a correlation between RDW and serum magnesium was not found in the present study.
Hyponatremia is the most common electrolyte abnormality among hospitalized patients and is associated with increased morbidity and mortality due to cardio-cerebrovascular events, including heart failure, in a variety of clinical settings [Citation52]. Gunal et al. demonstrated the importance of these strategies specifically for dialysis patients based on their study showing that strict restriction of salt intake and aggressive dialysis could prevent or minimize left ventricular hypertrophy and dilation with only a reduced dose or entirely without antihypertensive medications [Citation53]. Chloride and sodium ions are the major ions in extracellular fluid, and a previous study found that abnormal serum sodium metabolism is a predictor of poor prognosis in patients with acute or chronic heart failure [Citation54]. However, few studies have considered the serum chloride level in their analyses. In patients with heart failure, serum chloride is a stronger predictor of outcome than serum sodium, and changes in plasma volume, pressor secretion, and renin-angiotensin-aldosterone system (RAAS) expression at the onset of heart failure are primarily mediated by serum chloride rather than serum sodium [Citation55]. Activation of chloride channels disrupts the membrane potential and action potential duration in the cardiac sinus node, leading to the development of arrhythmias. Such arrhythmias are mediated in part by dysregulated intracellular pH and serum potassium levels in myocytes, and abnormal chloride levels cause pathological changes that can lead to sudden cardiac death [Citation56,Citation57]. Hypochlorhydria lowers chloride delivery to the dense spots of the renal unit via tubular bulb feedback in the kidney, which in turn leads to increased renin secretion from the juxtaglomerular cells, and this physiological feedback depends on chloride rather than sodium [Citation58]. When elevated serum potassium levels lead to increased secretion of aldosterone, serine-threonine kinase activity is then inhibited [Citation59]. A recent study reported that chloride ions can bind directly to a catalytic site of the serine-threonine kinase, phosphorylating the sodium regulatory pathway and thereby regulating blood pressure and electrolyte balance [Citation60]. This represents a possible mechanistic link between the pathology of heart failure and chloride imbalance. In the present study, no correlation between serum magnesium levels, serum sodium levels, and serum chlorine levels has been observed.
The present study has several limitations that should be considered when interpreting the results. First, this study was a retrospective study. Additionally, many patients had to be excluded due to missing data for serum magnesium concentrations and other relevant clinical data. However, the clinical characteristics of the patients included in the study did not differ markedly from those who were excluded. In addition, the sample size for this study was relatively small, increasing the probability of bias and error. Third, based on the observational nature of the study, we cannot determine whether the observed associations are causal. Finally, the current study only provides evidence for relationships between clinical indicators and the baseline serum magnesium concentration and a link between the baseline serum magnesium concentration and cardio-cerebrovascular events, but it remains unclear whether the total amount of magnesium in the body can be evaluated only by measuring the serum magnesium concentration. Moreover, due to dynamic changes in the magnesium concentration over time, our findings are limited by the collection of serum magnesium data at a single point. Further research including data from repeated measurements is needed to verify the conclusions of the present study. Therefore, future large-scale interventional studies are needed to elucidate the importance of serum magnesium concentration and the effect of magnesium supplementation in patients receiving CAPD.
Conclusions
Low serum magnesium is a risk factor for cardio-cerebrovascular events among CKD patients undergoing CAPD. Thus, careful monitoring of serum magnesium in these patients may help to improve outcomes. Serum magnesium also was found to be positively correlated with serum albumin, serum potassium, and serum UA, and negatively correlated with CRP. The mechanisms responsible for these correlations are not yet known, and research to elucidate these mechanisms will likely provide important insight for new strategies to improve the prognosis of patients with CKD.
Authors’ contributions
Peng-Lei Li and Wen-Lu Yu collected data, analyzed data, and drafted the manuscript. Yuan-Yuan Lu and Yuan-Yuan Li conducted experiments. Tie-Gang Lv participated in revising the manuscript. Li-Ping Xu participated and supervised the experiments. Jian Hao conceived the study, participated in revising the manuscript and provided final approval to submit the version of the document. All authors read and approved the manuscript.
Supplemental Material
Download MS Word (15.2 KB)Acknowledgments
The authors acknowledge the patients and staff of the Affiliated Hospital of Inner Mongolia Medical University.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Additional information
Funding
References
- Hill NR, Fatoba ST, Oke JL, et al. Global prevalence of chronic kidney disease - a systematic review and meta-analysis. Plos One. 2016;11(7):1. doi: 10.1371/journal.pone.0158765.
- Zhang L, Zhao MH, Zuo L, et al. China kidney disease network (CK-NET) 2016 annual data report. Kidney Int Suppl (2011). 2020;10(2):e97–12. doi: 10.1016/j.kisu.2020.09.001.
- Bello AK, Okpechi IG, Osman MA, et al. Epidemiology of peritoneal dialysis outcomes. Nat Rev Nephrol. 2022;18(12):779–793. doi: 10.1038/s41581-022-00623-7.
- Andreoli MCC, Totoli C. Peritoneal dialysis. Rev Assoc Med Bras (1992). 2020;66(Suppl 1):s37–s44. doi: 10.1590/1806-9282.66.S1.37.
- Sakaguchi Y. The emerging role of magnesium in CKD. Clin Exp Nephrol. 2022;26(5):379–384. doi: 10.1007/s10157-022-02182-4.
- Van Laecke S. Hypomagnesemia and hypermagnesemia. Acta Clin Belg. 2019;74(1):41–47. doi: 10.1080/17843286.2018.1516173.
- Vormann J. Magnesium: nutrition and homoeostasis. AIMS Public Health. 2016;3(2):329–340. doi: 10.3934/publichealth.2016.2.329.
- Sakaguchi Y, Fujii N, Shoji T, et al. Hypomagnesemia is a significant predictor of cardiovascular and non-cardiovascular mortality in patients undergoing hemodialysis. Kidney Int. 2014;85(1):174–181. doi: 10.1038/ki.2013.327.
- M de Francisco AL, Rodríguez M. Magnesium - its role in CKD. Nefrologia. 2013;33(3):389–399. doi: 10.3265/Nefrologia.pre2013.Feb.11840.
- Pham PC, Pham PA, Pham SV, et al. Hypomagnesemia: a clinical perspective. Int J Nephrol Renovasc Dis. 2014;7:219–230. doi: 10.2147/ijnrd.S42054.
- Negru AG, Pastorcici A, Crisan S, et al. The role of hypomagnesemia in cardiac arrhythmias: a clinical perspective. Biomedicines. 2022;10(10):2356. doi: 10.3390/biomedicines10102356.
- Pethő ÁG, Tapolyai M, Browne M, et al. Hypomagnesemia as a risk factor and accelerator for vascular aging in diabetes mellitus and chronic kidney disease. Metabolites. 2023;13(2):306. doi: 10.3390/metabo13020306.
- Floege J. Magnesium in CKD: more than a calcification inhibitor? J Nephrol. 2015;28(3):269–277. doi: 10.1007/s40620-014-0140-6.
- Ye H, Cao P, Zhang X, et al. Serum magnesium and cardiovascular mortality in peritoneal dialysis patients: a 5-year prospective cohort study. Br J Nutr. 2018;120(4):415–423. doi: 10.1017/s0007114518001599.
- Rosique-Esteban N, Guasch-Ferré M, Hernández-Alonso P, et al. Dietary magnesium and cardiovascular disease: a review with emphasis in epidemiological studies. Nutrients. 2018;10(2):168. doi: 10.3390/nu10020168.
- Parent X, Spielmann C, Hanser AM. ["corrected" calcium: calcium status underestimation in non-hypoalbuminemic patients and in hypercalcemic patients]. Ann Biol Clin. 2009;67(4):411–418. doi: 10.1684/abc.2009.0348.
- Ying P, Gu M, Jiang X, et al. Serum calcium-phosphorus product for predicting the risk of osteoporotic vertebral compression fractures in elderly patients: a retrospective observational study. J Orthop Surg Res. 2022;17(1):57. doi: 10.1186/s13018-022-02953-5.
- Luepker RV, Lakshminarayan K. 971 Cardiovascular and cerebrovascular diseases. In: Detels R, Beaglehole R, Lansang MA, Gulliford M, editors. Oxford textbook of public health. 5th ed. Oxford: Oxford University Press, 2009. doi: 10.1093/med/9780199218707.003.0059.
- van de Wal-Visscher ER, Kooman JP, van der Sande FM. Magnesium in chronic kidney disease: should we care? Blood Purif. 2018;45(1–3):173–178. doi: 10.1159/000485212.
- Lin CC, Li CI, Hsiao CY, et al. Time trend analysis of the prevalence and incidence of diagnosed type 2 diabetes among adults in Taiwan from 2000 to 2007: a population-based study. BMC Public Health. 2013;13(1):318. doi: 10.1186/1471-2458-13-318.
- Go AS, Chertow GM, Fan D, et al. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351(13):1296–1305. doi: 10.1056/NEJMoa041031.
- Chen XT. Study on the effect of hypertension and diabetes for elderly with cardiovascular disease. Mod Prev Med. 2011;38:3253–3254.
- Xin W. The effect of arterial stiffness index for Xuzhikang capsule in patients with type 2 diabetes. Chinese J Mod Drug Appl. 2015;9:127–128.
- Barzilay JI, Spiekerman CF, Kuller LH, et al. Prevalence of clinical and isolated subclinical cardiovascular disease in older adults with glucose disorders: the cardiovascular health study. Diabetes Care. 2001;24(7):1233–1239. doi: 10.2337/diacare.24.7.1233.
- Massy ZA, Drüeke TB. Magnesium and outcomes in patients with chronic kidney disease: focus on vascular calcification, atherosclerosis and survival. Clin Kidney J. 2012;5(Suppl 1):i52–i61. doi: 10.1093/ndtplus/sfr167.
- Rodelo-Haad C, Pendón-Ruiz de Mier MV, Díaz-Tocados JM, et al. The role of disturbed Mg homeostasis in chronic kidney disease comorbidities. Front Cell Dev Biol. 2020;8:543099. doi: 10.3389/fcell.2020.543099.
- Kreepala C, Luangphiphat W, Villarroel A, et al. Effect of magnesium on glomerular filtration rate and recovery of hypertension in women with severe preeclampsia. Nephron. 2018;138(1):35–41. doi: 10.1159/000481463.
- Peritoneal Dialysis Adequacy Work Group. Clinical practice guidelines for peritoneal dialysis adequacy. Am J Kidney Dis. 2006;48(Suppl 1):S98–S129. doi: 10.1053/j.ajkd.2006.04.006.
- Mori D, Matsui I, Shimomura A, et al. Protein carbamylation exacerbates vascular calcification. Kidney Int. 2018;94(1):72–90. doi: 10.1016/j.kint.2018.01.033.
- Ray M, Jovanovich A. Mineral bone abnormalities and vascular calcifications. Adv Chronic Kidney Dis. 2019;26(6):409–416. doi: 10.1053/j.ackd.2019.09.004.
- Viegas C, Araújo N, Marreiros C, et al. The interplay between mineral metabolism, vascular calcification and inflammation in chronic kidney disease (CKD): challenging old concepts with new facts. Aging. 2019;11(12):4274–4299. doi: 10.18632/aging.102046.
- Su CY, Lu XH, Tang W, et al. Peritoneal dialysis can maintain nitrogen balance with low Kt/V in anuric patients. Clin Nephrol. 2022;97(4):206–214. doi: 10.5414/cn110482.
- Kaysen GA. Biological basis of hypoalbuminemia in ESRD. J Am Soc Nephrol. 1998;9(12):2368–2376. doi: 10.1681/asn.v9122368.
- Lacson E, Jr., Wang W, Ma L, et al. Serum magnesium and mortality in hemodialysis patients in the United States: a cohort study. Am J Kidney Dis. 2015;66(6):1056–1066. doi: 10.1053/j.ajkd.2015.06.014.
- Gupta D, Lis CG. Pretreatment serum albumin as a predictor of cancer survival: a systematic review of the epidemiological literature. Nutr J. 2010;9(1):69. doi: 10.1186/1475-2891-9-69.
- Das UN. Albumin to globulin ratio and/or plasma albumin in predicting long-term mortality. Am J Surg. 2014;208(1):157–158. doi: 10.1016/j.amjsurg.2013.08.055.
- Wu PP, Hsieh YP, Kor CT, et al. Association between albumin-Globulin ratio and mortality in patients with chronic kidney disease. J Clin Med. 2019;8(11):1991. doi: 10.3390/jcm8111991.
- Kaneko S, Ookawara S, Morishita Y. Clinical factors associated with serum magnesium concentration in patients undergoing peritoneal dialysis: a Single-Center observational study. Int J Nephrol Renovasc Dis. 2022;15:185–195. doi: 10.2147/ijnrd.S357130.
- Gröber U, Schmidt J, Kisters K. Magnesium in prevention and therapy. Nutrients. 2015;7(9):8199–8226. doi: 10.3390/nu7095388.
- Davies SJ, Zhao J, Morgenstern H, et al. Low serum potassium levels and clinical outcomes in peritoneal dialysis-international results from PDOPPS [published correction appears in kidney int rep. 2021 Mar 11;6(5):1485]Kidney Int Rep. 2021;6(2):313–324. doi: 10.1016/j.ekir.2020.11.021.
- Saito Y, Tanaka A, Node K, et al. Uric acid and cardiovascular disease: a clinical review. J Cardiol. 2021;78(1):51–57. doi: 10.1016/j.jjcc.2020.12.013.
- Johnson RJ, Titte S, Cade JR, et al. Uric acid, evolution and primitive cultures. Semin Nephrol. 2005;25(1):3–8. doi: 10.1016/j.semnephrol.2004.09.002.
- Bos MJ, Koudstaal PJ, Hofman A, et al. Uric acid is a risk factor for myocardial infarction and stroke: the Rotterdam study. Stroke. 2006;37(6):1503–1507. doi: 10.1161/01.STR.0000221716.55088.d4.
- Rock KL, Kataoka H, Lai JJ. Uric acid as a danger signal in gout and its comorbidities. Nat Rev Rheumatol. 2013;9(1):13–23. doi: 10.1038/nrrheum.2012.143.
- Zhao G, Huang L, Song M, et al. Baseline serum uric acid level as a predictor of cardiovascular disease related mortality and all-cause mortality: a meta-analysis of prospective studies. Atherosclerosis. 2013;231(1):61–68. doi: 10.1016/j.atherosclerosis.2013.08.023.
- Kleber ME, Delgado G, Grammer TB, et al. Uric acid and cardiovascular events: a mendelian randomization study. J Am Soc Nephrol. 2015;26(11):2831–2838. doi: 10.1681/asn.2014070660.
- Deng X, Gao B, Wang F, et al. Red blood cell distribution width is associated with adverse kidney outcomes in patients with chronic kidney disease. Front Med. 2022;9:877220. doi: 10.3389/fmed.2022.877220.
- Chen J, Li Y, Liu P, et al. A nomogram to predict the in-hospital mortality of patients with congestive heart failure and chronic kidney disease. ESC Heart Fail. 2022;9(5):3167–3176. doi: 10.1002/ehf2.14042.
- Allen LA, Felker GM, Mehra MR, et al. Validation and potential mechanisms of red cell distribution width as a prognostic marker in heart failure. J Card Fail. 2010;16(3):230–238. doi: 10.1016/j.cardfail.2009.11.003.
- Tomaiuolo G, Lanotte L, D’Apolito R, et al. Microconfined flow behavior of red blood cells. Med Eng Phys. 2016;38(1):11–16. doi: 10.1016/j.medengphy.2015.05.007.
- Reinhart WH, Piety NZ, Goede JS, et al. Effect of osmolality on erythrocyte rheology and perfusion of an artificial microvascular network. Microvasc Res. 2015;98:102–107. doi: 10.1016/j.mvr.2015.01.010.
- Ritz E, Dikow R, Adamzcak M, et al. Congestive heart failure due to systolic dysfunction: the Cinderella of cardiovascular management in dialysis patients. Semin Dial. 2002;15(3):135–140. doi: 10.1046/j.1525-139x.2002.00044.x.
- Gunal AI, Karaca I, Aygen B, et al. Strict fluid volume control and left ventricular hypertrophy in hypertensive patients on chronic haemodialysis: a cross-sectional study. J Int Med Res. 2004;32(1):70–77. doi: 10.1177/147323000403200112.
- Romanovsky A, Bagshaw S, Rosner MH. Hyponatremia and congestive heart failure: a marker of increased mortality and a target for therapy. Int J Nephrol. 2011;2011:732746–732747. doi: 10.4061/2011/732746.
- Kataoka H. Proposal for heart failure progression based on the ‘chloride theory’: worsening heart failure with increased vs. non-increased serum chloride concentration. ESC Heart Fail. 2017;4(4):623–631. doi: 10.1002/ehf2.12191.
- Adkins GB, Curtis MJ. Potential role of cardiac chloride channels and transporters as novel therapeutic targets. Pharmacol Ther. 2015;145:67–75. doi: 10.1016/j.pharmthera.2014.08.002.
- Cuthbert JJ, Pellicori P, Rigby A, et al. Low serum chloride in patients with chronic heart failure: clinical associations and prognostic significance. Eur J Heart Fail. 2018;20(10):1426–1435. doi: 10.1002/ejhf.1247.
- Kazory A, Ronco C. Emergence of chloride as an overlooked cardiorenal connector in heart failure. Blood Purif. 2020;49(1-2):219–221. doi: 10.1159/000503774.
- Maynard N, Bihari D, Beale R, et al. Assessment of splanchnic oxygenation by gastric tonometry in patients with acute circulatory failure. JAMA. 1993;270(10):1203–1210. doi: 10.1001/jama.1993.03510100053032.
- Testani JM, Hanberg JS, Arroyo JP, et al. Hypochloraemia is strongly and independently associated with mortality in patients with chronic heart failure. Eur J Heart Fail. 2016;18(6):660–668. doi: 10.1002/ejhf.477.
