Abstract
Secondary hyperparathyroidism (SHPT) can progress to severe SHPT (sSHPT), which affects the survival rate and quality of life of patients. This retrospective cohort study investigated risk factors for sSHPT and the association between SHPT and mortality (all-cause and infection-related) among 771 clinically stable patients (421 male patients; mean age, 51.2 years; median dialysis vintage, 28.3 months) who underwent >3 months of regular peritoneal dialysis (PD) between January 2013 and March 2021. The sSHPT and non-sSHPT groups comprised 75 (9.7%) (median progression, 35 months) and 696 patients, respectively. sSHPT was defined as a serum intact parathyroid hormone (PTH) level >800 pg/mL observed three times after active vitamin D pulse therapy. The influence of sSHPT on the prognosis of and risk factors for sSHPT progression were evaluated using logistic and Cox regression analyses. After adjusting for confounding factors, higher (each 100-pg/mL increase) baseline PTH levels (95% confidence interval (CI) 1.206–1.649, p < .001), longer (each 1-year increase) dialysis vintages (95% CI 1.013–1.060, p = .002), higher concomitant diabetes rates (95% CI 1.375–10.374, p = .010), and lower (each 1-absolute unit decrease) Kt/V values (95% CI 0.859–0.984, p = .015) were independent risk factors for progression to sSHPT in patients on PD. During follow-up, 211 deaths occurred (sSHPT group, n = 35; non-sSHPT group, n = 176). The sSHPT group had significantly higher infection-related mortality rates than the non-sSHPT group (12.0% vs. 4.3%; p < .05), and sSHPT was associated with increased infection-related mortality. In conclusion, patients with sSHPT are at higher risk for death and infection-related mortality than patients without sSHPT.
1. Introduction
Secondary hyperparathyroidism (SHPT) is an important component of chronic kidney disease – mineral and bone disorder (CKD-MBD) that is characterized by elevated serum intact parathyroid hormone (iPTH) levels, parathyroid gland hyperplasia, and adenoma development. Elevated serum parathyroid hormone (PTH) levels can be detected by the time of stage IIIb CKD onset [Citation1–3]. Thus, early monitoring and control of calcium, phosphorus, and PTH levels are important for preventing SHPT progression. However, SHPT control is often unsatisfactory in China and other countries. According to a cross-sectional survey based on Chinese national data in 2019 [Citation4], 76% of patients on dialysis did not achieve the target serum phosphorus level range, and 20.4% did not achieve the target iPTH level range. Furthermore, 10–38% of patients with a dialysis vintage >10 years may develop severe SHPT (sSHPT) [Citation5,Citation6], which is characterized by significant parathyroid enlargement and hyperactivity and resistance to active vitamin D therapy, as well as intractable bone pain, pruritus, and fractures. This condition affects the patient’s quality of life and is associated with an increased risk of mortality [Citation4,Citation7]. Notably, most patients with sSHPT require treatment comprising parathyroidectomy (PTX) [Citation8,Citation9]. To our knowledge, studies on the relationship between sSHPT and the prognosis of patients on peritoneal dialysis (PD) have been rarely reported. In this long-term study with a relatively large sample size, we aimed to explore the association between sSHPT and the prognosis of patients on long-term PD.
2. Materials and methods
2.1. Patients
Adult patients on continuous ambulatory PD between January 2013 and March 2021 were screened for enrollment in this study. The inclusion criteria were as follows: age 18 years or older; on PD treatment for >3 months and clinically stable; participated in outpatient follow-up every 1–3 months; and signed an informed consent form to participate in this study. The exclusion criteria were as follows: cardiovascular and cerebrovascular events (angina attack, acute myocardial infarction, chronic heart failure, and stroke) diagnosed within 3 months before enrollment; acute infectious disease, or severe trauma diagnosed within 1 month before enrollment; acute renal failure; rheumatic disease, acute hepatitis, or active chronic hepatitis; acute tuberculosis; physical or mental disability that severely affected the quality of life; and previous diagnosis of primary hyperparathyroidism.
The patients were separated into two groups based on the development of sSHPT (i.e., the sSHPT group and non-sSHPT group), and their data were retrospectively evaluated. According to the 2009 Kidney Disease Outcomes Quality Initiative guidelines for the management of CKD-MBD, sSHPT was diagnosed when the patient had persistent serum iPTH levels >800 pg/mL [Citation10]. In this study, sSHPT was defined as continuously increased iPTH levels >800 pg/mL observed three times during follow-up after standard active vitamin D pulse therapy.
2.2. Ethics statement and approval
Data were obtained from patients on regular PD. All patients signed an informed consent form for the use of their clinical and laboratory data available from their medical records and follow-up evaluations for scientific research without compensation. The study protocol ethical approval number was 2018-315.
2.3. Demographic and biochemistry data
Demographic data, serological measurements, and PD details (modality, dosage, and dialysate glucose concentration) were collected retrospectively from the medical records and routine follow-up evaluations. Both the 24-h urine volume and PD effluent were collected at the same time to calculate the weekly urea clearance (Kt/V), creatinine clearance, and normalized protein clearance and to measure the glomerular filtration rate, which was calculated as the average 24-h kidney creatinine and urea clearance and normalized according to the 1.73 m2 body surface area using Adequest 2.0 software (Baxter Healthcare, Deerfield, IL). The average daily dialysate glucose concentration was calculated using the following formula: average daily dialysate glucose concentration = (1.5% abdominal dialysate (L) + 2.5% abdominal dialysate (L))/total abdominal dialysate volume (L).
iPTH levels were measured using a radioimmunoassay and regularly reassessed during follow-up according to the Kidney Disease: Improving Global Outcomes clinical practice guidelines for the diagnosis, evaluation, prevention, and treatment of CKD-MBD [Citation9,Citation10].
2.4. Treatments
A trained dietician adjusted the dose of phosphate binders to the estimated phosphate content of a meal and educated the patients about how to avoid phosphate (e.g., animal, vegetable, and additive sources of phosphate) when making dietary decisions. Oral treatment of CKD-MBD was aimed at lowering high serum phosphate levels and maintaining serum calcium levels using calcium-based phosphate binders, short-term aluminum-containing phosphate binders, and calcium-free phosphate binders. Calcitriol and calcimimetics were used to control abnormal PTH levels in CKD-MBD. Dose adjustments were performed to maintain predialysis serum phosphate levels at <2.0 mmol/L, serum calcium levels between 2.1 and 2.5 mmol/L, and iPTH levels in the range of approximately two- to nine-times the upper normal limit for the assay. However, vitamin D analogues were not used during this study because they were not locally available.
Continuous ambulatory PD comprising four to five daily exchanges with a volume of 2000 mL was performed. A weekly Kt/V value >1.7 (including the residual renal function) was the goal. Standard PD solution contained 1.75 mmol/L calcium and was used during the entire study period; however, a solution with 1.25 mmol/L calcium was used since 2010.
2.5. Study endpoint
The primary study endpoint was death. Renal transplantation, transfer to another center, loss to follow-up, and conversion to hemodialysis were recorded as censors. All patients were followed up until 31 March 2021. The dialysis vintage was the time between the initiation of PD and either death or the end of the follow-up period (31 December 2021).
2.6. Statistical analysis
Data are presented as the mean ± standard deviation unless otherwise stated. For comparisons between groups, a two-tailed Student’s t-test was used for continuous variables. For non-normally distributed variables, the Wilcoxon rank sum test was used to analyze differences between groups. A Chi-squared analysis or Fisher’s exact test was performed for discrete variables.
To evaluate the association between sSHPT and outcomes, a time-dependent Cox regression analysis was performed [Citation11,Citation12]. In the Cox regression models, to mitigate immortal time bias, sSHPT was considered a time-varying exposure. In time-varying analysis, data were reconstructed according to the time of sSHPT onset. Therefore, the time from dialysis to the time of sSHPT was categorized as unexposed. The multivariable analysis was adjusted for age, sex, diabetes mellitus, hemoglobin levels, serum albumin levels, blood phosphorus levels, and high-sensitivity C-reactive protein levels. The hazards ratios (HRs) and 95% confidence intervals (CIs) of the Cox regression models were reported. Kaplan–Meier’s survival curves were used to compare the survival rates of the two groups, and a log-rank test was performed. Propensity score (PS)-driven analyses (overlap weighting (OW) and propensity score matching (PSM)) were performed to account for confounding by indication bias in the time-dependent Cox regression analysis [Citation11,Citation12]. Multivariable logistic regression with sSHPT as a binary outcome was performed to obtain the predicted probability of sSHPT, which was used as the PS. All baseline characteristics were used to construct the PS for each group of patients. OW is a method of obtaining the PS. Specifically, treated patients were weighted by the probability of not receiving treatment (1 − PS), and untreated patients were weighted by the probability of receiving treatment (PS). These weights were smaller for extreme PS values so that outliers who were nearly always treated (PSnear 1) or never treated (PSnear 0) did not dominate the results and worsen the precision, which can occur with inverse probability of treatment weighting (IPTW) [Citation13]. In PSM, the caliper value was set as 20% of the standard deviation of the PS. During the PSM study, a 2:1 ratio (one patient with sSHPT for every two patients without sSHPT) was used. We reported the statistical results (crude HR and adjusted HR) of the Cox regression analysis, Cox regression with time-varying exposure analysis, IPTW-based analysis, and PSM-based analysis. The difference was considered statistically significant when p < .05. All analyses were performed using SPSS 19 (SPSS Inc., Chicago, IL) and R 4.3.1 software (R Foundation for Statistical Computing, Vienna, Austria).
3. Results
3.1. Baseline data
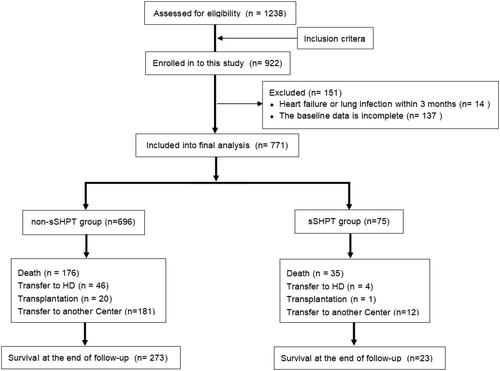
A total of 771 patients (421 male patients; 54.6%) were enrolled in this study. The patients had a mean age of 51.2 years (standard deviation, 14.3 years) and a median dialysis vintage of 38 (range, 3–187.6) months. The primary diseases included chronic glomerulonephritis, diabetic nephropathy, and hypertensive nephropathy. Overall, 75 (9.7%) patients experienced progression to sSHPT. The flowchart of patient enrollment and follow-up is shown in . Compared with patients in the non-sSHPT group, patients in the sSHPT group were older and had longer dialysis vintages; fewer diabetes-related complications; higher baseline PTH levels, serum phosphorus levels, serum creatinine levels, serum calcium levels, and uric acid levels; and lower hemoglobin levels. The sSHPT group was administered a higher dialysis dose to achieve a larger 24-h ultrafiltration volume and lower Kt/V value (p < .05) ().
Table 1. Baseline clinical characteristics and serum parameters of patients with sSHPT on PD.
3.2. Analysis of risk factors for progression to sSHPT in patients on PD
Seventy-five (9.7%) patients experienced progression to sSHPT. After adjusting for confounding factors, higher baseline PTH levels (odds ratio (OR), 1.410; 95% CI, 1.206–1.649; p < .001), longer dialysis vintages (OR, 1.036; 95% CI, 1.013–1.060; p = .002), higher rates of concomitant diabetes (OR, 3.776; 95% CI, 1.375–10.374; p = .010), and lower Kt/V values (OR, 0.919; 95% CI, 0.859–0.984; p = .015) were independent risk factors for progression to sSHPT in patients on PD ().
Table 2. Risk factors for progression to sSHPT in patients on PD in multivariable logistic regression analysis.
3.3. Prognostic analysis
During a median follow-up time of 38 months (range, 3–187.6 months), 211 all-cause deaths occurred (sSHPT group, n = 35 [46.67%]; non-sSHPT group, n = 176 [25.29%]). The main causes of death were cardiovascular and cerebrovascular events (n = 90 [42.65%]), infection (n = 39 [18.48%]), and wasting/cachexia (n = 23 [10.90%]).
3.4. Association between sSHPT and all-cause mortality for patients on PD
A Kaplan–Meier survival analysis revealed that the cumulative mortality rate of the sSHPT group was significantly higher than that of the non-sSHPT group (46.67% vs. 25.29%; log-rank test, p < .001). The results of the univariate regression model are shown in Supplemental Table 1. In the multivariable Cox model with time-varying exposures, sSHPT was independently associated with an increased cumulative mortality rate (HR, 1.57; 95% CI, 1.08–2.30; p = .02) (). After OW and PSM (baseline shown in Supplemental Table 1), an association between sSHPT and increased cumulative mortality was observed (IPTW: HR, 2.50; 95% CI, 1.66–3.76; p < .001; PSM: HR, 2.75; 95% CI, 1.56–4.87; p < .001) (). Kaplan–Meier’s survival curves created after OW and PSM are shown in and .
Figure 2. Kaplan–Meier’s survival curves of the sSHPT group and non-rSHPT group for OW cumulative mortality.
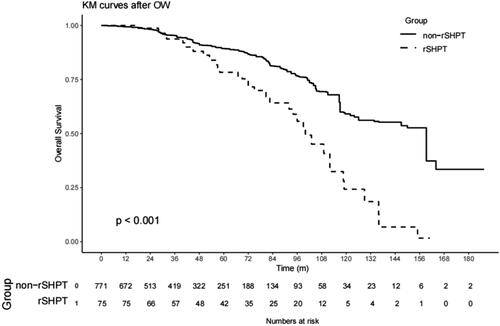
Figure 3. Kaplan–Meier’s survival curves of the sSHPT group and non-rSHPT group for PSM cumulative mortality.
Table 3. Association between the sSHPT and all-cause mortality in patients on PD in multivariable Cox regression analysis.
3.5. Association between sSHPT and infection-associated mortality
Thirty-nine deaths were attributable to infections (sSHPT group, n = 9; non-sSHPT group, n = 30). The main causes of infection-related death were peritonitis (48.72%) and pulmonary infection (25.64%).
The sSHPT group had a significantly higher infection-related mortality rate than the non-sSHPT group (12.0% vs. 4.3%; log-rank test, p < .05). The results of the univariate regression model are shown in Supplemental Table 2. After OW and PSM (baseline shown in Supplemental Table 2), an association between sSHPT and increased infection-related mortality was observed (IPTW: HR, 4.78; 95% CI, 2.06–11.12; p < .001; PSM: HR, 7.47; 95% CI, 1.42–39.29; p < .001) (). Kaplan–Meier’s survival curves created after OW and PSM are shown in and .
Figure 4. Kaplan–Meier’s survival curves of the sSHPT group and non-rSHPT group for OW infection mortality.
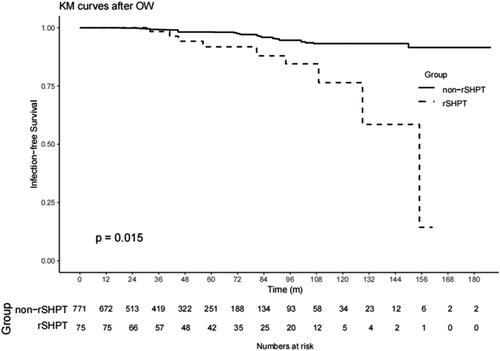
Figure 5. Kaplan–Meier’s survival curves of the sSHPT group and non-sSHPT group for PSM infection mortality.
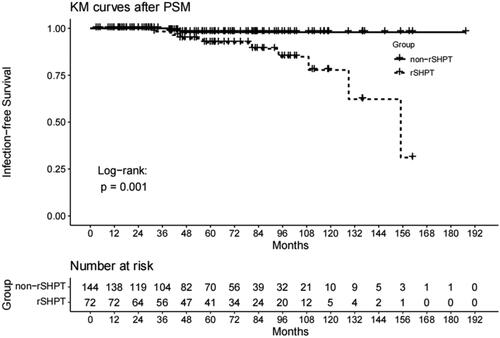
Table 4. Association between the sSHPT and mortality due to infection in patients on PD in multivariable Cox regression analysis.
3.6. Association between sSHPT and peritonitis in patients on PD
Peritonitis occurred in 302 patients on PD (sSHPT group, n = 39; non-sSHPT group, n = 263). The results of the univariate regression model are shown in Supplemental Table 3.
In the multivariable Cox model with time-varying exposures, sSHPT was associated with the development of peritonitis in patients on PD (HR, 1.57; 95% CI, 1.07–2.30; p = .022) (). However, after OW and PSM (baseline shown in Supplemental Table 3), sSHPT was not associated with the development of peritonitis (IPTW: HR, 1.23; 95% CI, 0.86–1.75; p = .255; PSM: HR, 1.24; 95% CI, 0.78–1.96; p = .368) (). Kaplan–Meier’s survival curves created after OW and PSM are shown in and .
Figure 6. Kaplan–Meier’s survival curves of the sSHPT group and non-sSHPT group for OW peritonitis incidence.
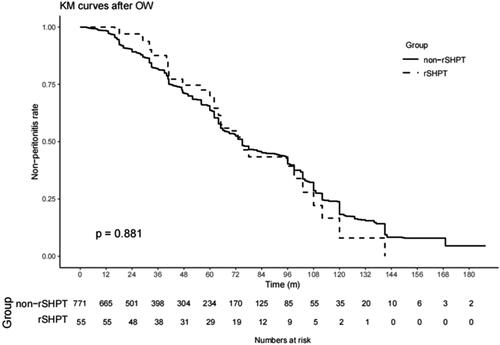
Figure 7. Kaplan–Meier’s survival curves of the sSHPT group and non-sSHPT group for PSM peritonitis incidence.
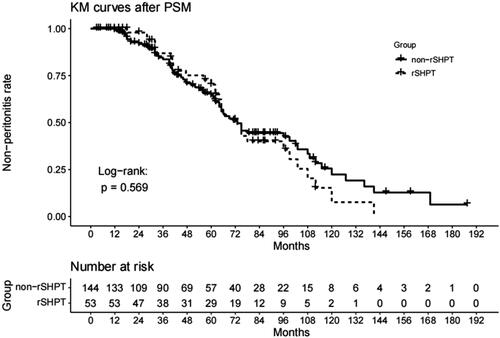
Table 5. Association between the rSHPT and peritonitis incidence in patients on PD in multivariable Cox regression analysis.
4. Discussion
CKD-MBD is a common complication among patients with CKD that can progress to sSHPT and affect their survival rate and quality of life [Citation4,Citation7]. This study analyzed a cohort of patients on regular PD and included a long-term follow-up period of 8 years. During follow-up, 75 of 771 patients experienced progression to sSHPT. After adjusting for confounding factors, higher baseline PTH levels, longer dialysis times, higher diabetes rates, and lower Kt/V values were independent risk factors for progression to sSHPT in patients on PD. The sSHPT group had a ninefold higher risk of death than the non-sSHPT group, and sSHPT was an independent risk factor for mortality among patients on PD. The sSHPT group also had a significantly higher infection-related mortality rate than the non-sSHPT group.
In this study, patients on PD with higher baseline iPTH levels, longer dialysis times, higher concomitant diabetes rates, and lower Kt/V values were at higher risk for progression to sSHPT. An international, multicenter, prospective study that included 5683 patients who were on hemodialysis from 21 countries reported that the overall prognosis was affected by the PTH levels at the time of hemodialysis initiation [Citation14]. Furthermore, early iPTH control could reduce the amount of medications required to treat CKD-MBD during dialysis, thus improving patient outcomes. Additionally, in patients with chronic renal failure, persistent iPTH elevation and time on dialysis before renal transplantation were important risk factors associated with persistent hyperparathyroidism after renal transplantation [Citation15]. A single-center study of 173 patients on PD (SHPT incidence of 29.48%) in Thailand reported that dialysis time was positively correlated with baseline iPTH level [Citation16], and it concluded that increased PTH levels before PD predicted sustained increased PTH levels at 1 year. A correlation between increased PTH levels before PD initiation and the use of more medications during the first year of dialysis was also observed. SHPT first appears during the early stages of renal failure, and the main factors that contribute to its pathogenesis are phosphorus retention, hypocalcemia, insufficient levels of 1,25(OH)2D and its receptor, elevated fibroblast growth factor-23 levels, and skeletal resistance to PTH [Citation1,Citation17]. During dialysis, the residual renal function gradually decreases until it is completely lost. Additionally, disorders of the calcium and phosphorus metabolic axis and significant reductions in fibroblast growth factor receptor-1 and Klotho protein expression in parathyroid tissues, which are also mechanisms underlying enhanced parathyroid transcription, occur [Citation18,Citation19]. Progressively proliferating parathyroid tissues form autonomous nodules that render drug therapy ineffective. In this study, elevated iPTH levels of patients with sSHPT could not be effectively controlled with the available drug treatment (i.e., calcitriol and vitamin D analogs). However, during this study, calcimimetics (e.g., cinacalcet), which exhibit a unique mode of action at the calcium-sensing receptor and comprise an important step toward controlling CKD-MBD [Citation20], were not commercially available. This may have contributed to the ineffective control of CKD-MBD in these patients. Mak reported that patients on dialysis with 1,25-(OH)2D3 deficiency and SHPT exhibited glucose intolerance and insulin resistance [Citation21]. After intravenous administration of 1,25-(OH)2D3, increased insulin secretion and improved glucose tolerance were observed. The Kt/V value was correlated with the adequacy of dialysis. Low Kt/V values indicated inadequate dialysis, which can lead to the accumulation of phosphorus and other related toxins in the body and eventually cause SHPT [Citation22].
sSHPT is an integral part of CKD-MBD progression that often presents with clinical symptoms such as pruritus, bone pain, fractures, skeletal deformities, and sarcopenia, as well as various degrees of cardiovascular calcification, resulting in greatly increased cardiovascular mortality [Citation23,Citation24]. Several studies have confirmed that severe mineral metabolism abnormalities in these patients are closely related to clinical events, such as fractures, cardiovascular disease, and death [Citation25]. Based on the baseline comparisons, the overall clinical status of patients with sSHPT was markedly poorer and associated with older age, longer dialysis times, lower hemoglobin levels, higher serum creatinine levels, and higher uric acid levels. However, there were no differences in the adequacy of dialysis and residual renal function, which are critical to SHPT control. After further adjusting for confounding factors (age, sex, hemoglobin levels, serum albumin levels, serum phosphorus levels, and high-sensitivity C-reactive protein levels, and concomitant diabetes), sSHPT remained an independent risk factor for death among patients on PD.
Among the observed deaths, 42.65% were attributable to cardiovascular causes. Therefore, early control of CKD-MBD is important to improve the survival rate and quality of life of patients and warrants attention. Current treatment methods for sSHPT include drug therapy, surgery, and renal replacement therapy. The 2017 Kidney Disease Improving Global Outcomes guidelines suggested that patients with stage 5D CKD should receive standardized drug treatment, including active vitamin D and analogs and calcimimetic agents, based on the dialysis adequacy. However, patients with sSHPT and spontaneous proliferative adenomas generally respond poorly to these drugs; therefore, PTX is often required [Citation5]. Jorna et al. performed a univariate analysis and reported that the initial PTH level was significantly associated with the need for PTX, although this association became statistically insignificant in the multivariable analysis because of the greater effect of the PTH level at 1 year [Citation26]. Although PTX can effectively reduce iPTH levels of most patients, it is also associated with the risk of complications and recurrence; during long-term follow-up, the recurrence rate was approximately 10% [Citation27]. During the Dialysis Outcomes and Practice Patterns Study, only 52% of Chinese patients achieved the target range for iPTH [Citation28]. Hence, successful prevention of CKD-MBD and early-stage treatment of CKD-MBD are necessary. This would involve selecting reasonable, standardized, and individualized treatment and strengthening cooperation across fields, such as nephrology, endocrinology, thyroid surgery, nuclear medicine, ultrasonography, pathology, and evidence-based medicine, which would help improve the diagnosis and treatment of CKD-MBD and enhance the survival rate and quality of life of patients during its advanced stage.
Our study demonstrated that infection-related mortality (death associated with peritonitis [48.72%] and pulmonary infection [25.64%]) was closely related to sSHPT. This has been rarely reported in the literature. Chuang et al. found that patients with serum calcium, phosphorous, and PTH levels within the target of the Kidney Disease: Improving Global Outcomes guidelines had significantly fewer episodes of peritonitis and exit site infections at 1 year [Citation29], which indicated an association between CKD-MBD and infection. This is possibly because poorly controlled SHPT can play a pivotal role in the development of extraosseous calcifications in patients with peritonitis [Citation30,Citation31]. Both vascular and peritoneal calcifications are associated with high rates of peritonitis; however, the achievement of serum calcium targets may be associated with a lower risk of such calcification [Citation32]. Previous studies reported that patients with CKD-MBD can develop SHPT, which is associated with extraosseous calcification; furthermore, extraosseous calcification leads to vascular and peritoneal calcifications, resulting in an increased incidence of peritonitis [Citation29–31]. Grzegorzewska et al. reported that variant homozygosity of calcium-sensing receptor gene rs7652589 was associated with PTH levels >500 pg/mL, and that the allelic purity of the calcium-sensing receptor gene rs1801725 variant resulted in increased infection-related mortality among patients on hemodialysis [Citation33–35].
This study had some limitations. First, during this retrospective study, the strong ability of sSHPT to predict death did not necessarily imply causality because of the complicated interactions among hyperphosphatemia, hypocalcemia, inhibited active 1,25(OH)2 vitamin D levels, elevated fibroblast growth factor-23 levels, and elevated iPTH levels. Therefore, the results of the regression analysis were insufficient and could not be used to identify risk factors that lead to these outcomes. Second, economic limitations, limitations imposed by medical insurance, and other factors such as the location of this study resulted in inadequate CKD-MBD treatment during follow-up, which may have resulted in progression to sSHPT. However, these factors were not included as risk factors in the analysis. Third, because calcimimetics were not commercially available in our region during this study, the patients had to purchase calcimimetics in other regions; therefore, very few patients were treated with calcimimetics. Finally, the present study included a Chinese population; thus, extrapolation of these results to other populations is limited.
In conclusion, sSHPT was found to be an independent risk factor for mortality among patients on PD. Patients with sSHPT on long-term PD had poorer outcomes and had a nine- and fourfold higher risks for death and infection-related mortality, respectively, than patients without sSHPT. Therefore, more attention should be focused on patients with higher iPTH levels and lower Kt/V values at the time of PD initiation and those with longer dialysis vintages.
5. Conclusions
Concomitant sSHPT is an independent risk factor for all-cause and infectious mortality in PD patients. Notably, sSHPT patients had a ninefold higher risk of death and more than fourfold infection mortality than non-sSHPT patients.
Author contributions
NT: designed the research study. YL performed the research. XF, NC, SS, MY, YW, HZ, LW, and MC provided help and advice. YL: analyzed the data. YL and NT: wrote the manuscript. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript.
Ethical approval
Data were obtained from our regular follow-up dialysis patients, and all patients signed informed consent to use the clinical and laboratory data from their medical records and follow-ups for scientific research without compensation. Study protocol ethical approval number was 2018-315.
Supplemental Material
Download MS Word (22.7 KB)Acknowledgements
The authors would like to thank Prof. Qian Zhou for supporting with statistical analysis.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Additional information
Funding
References
- Lau WL, Obi Y, Kalantar-Zadeh K. Parathyroidectomy in the management of secondary hyperparathyroidism. Clin J Am Soc Nephrol. 2018;13(6):1–13.
- Bouma-de Krijger A, Bots ML, Vervloet MG, et al. Time-averaged level of fibroblast growth factor-23 and clinical events in chronic kidney disease. Nephrol Dial Transplant. 2014;29(1):88–97. doi: 10.1093/ndt/gft456.
- Steinl GK, Kuo JH. Surgical management of secondary hyperparathyroidism. Kidney Int Rep. 2021;6(2):254–264. doi: 10.1016/j.ekir.2020.11.023.
- Liu ZH, Yu XQ, Yang JW, et al. Prevalence and risk factors for vascular calcification in Chinese patients receiving dialysis: baseline results from a prospective cohort study. Curr Med Res Opin. 2018;34(8):1491–1500. doi: 10.1080/03007995.2018.1467886.
- Block GA, Cannataandia JB, Goldsmith D. Morbidity and mortality related to disturbances in mineral metabolism in CKD. 2nd ed. The Spectrum of Mineral and Bone Disorders in Chronic Kidney Disease; 2010. p. 261–578.
- Pasnicu C, Radu PA, Garofil D, et al. Clinical aspects of parathyroid hyperplasia in secondary renal hyperparathyroidism. Chirurgia. 2019;114(5):594–601. doi: 10.21614/chirurgia.114.5.594.
- Leifheit-Nestler M, Große Siemer R, Flasbart K, et al. Induction of cardiac FGF23/FGFR4 expression is associated with left ventricular hypertrophy in patients with chronic kidney disease. Nephrol Dial Transplant. 2016;31(7):1088–1099. doi: 10.1093/ndt/gfv421.
- Ketteler M, Block GA, Evenepoel P, et al. Diagnosis, evaluation, prevention, and treatment of chronic kidney disease-mineral and bone disorder: synopsis of the Kidney Disease: Improving Global Outcomes 2017 clinical practice guideline update. Ann Intern Med. 2018;168(6):422–430. doi: 10.7326/M17-2640.
- Saoposky D, Rown A, Dusso AJKI. Pathogenesis of secondary hyperparathyroidism. Kidney Int Suppl. 1999;73:S14–S19.
- Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Work Group. KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease-mineral and bone disorder (CKD-MBD). Kidney Int Suppl. 2009;S1–130. doi: 10.1038/ki.2009.188.
- Ali MS, Groenwold RH, Belitser SV, et al. Reporting of covariate selection and balance assessment in propensity score analysis is suboptimal: a systematic review. J Clin Epidemiol. 2015;68(2):112–121.
- Mansournia MA, Altman DG. Inverse probability weighting. BMJ. 2016;352:i189. doi: 10.1136/bmj.i189.
- Thomas LE, Li F, Pencina MJ. Overlap weighting: a propensity score method that mimics attributes of a randomized clinical trial. JAMA. 2020;323(23):2417–2418. doi: 10.1001/jama.2020.7819.
- Tabibzadeh N, Karaboyas A, Robinson BM, et al. The risk of medically uncontrolled secondary hyperparathyroidism depends on parathyroid hormone levels at haemodialysis initiation. Nephrol Dial Transplant. 2021;36(1):160–169. doi: 10.1093/ndt/gfaa195.
- Kirnap NG, Kirnap M, Sayin B, et al. Risk factors and treatment options for persistent hyperparathyroidism after kidney transplantation. Transplant Proc. 2020;52(1):157–161. doi: 10.1016/j.transproceed.2019.11.020.
- Suwan N. Secondary hyperparathyroidism and risk factors in patients undergoing peritoneal dialysis in a tertiary hospital. J Med Assoc Thailand. 2011;94(Suppl. 4):S101–S105.
- Akimbekov NS, Digel I, Sherelkhan DK, et al. Vitamin D and phosphate interactions in health and disease. Adv Exp Med Biol. 2022;1362:37–46. doi: 10.1007/978-3-030-91623-7_5.
- Morevati M, Mace ML, Egstrand S, et al. Extrarenal expression of α-klotho, the kidney related longevity gene, in Heterocephalus glaber, the long living naked mole rat. Sci Rep. 2021;11(1):15375. doi: 10.1038/s41598-021-94972-1.
- Sun XM, Kong WX, Lu Y, et al. Different expressions of fibroblast growth factor receptor 1 (FGFR1) and Klotho in parathyroid cells with different hyperplasia from secondary hyperparathyroidism patients. Fudan Univ J Med Sci. 2018;45:823–829.
- Bover J, Ureña P, Ruiz-García C, et al. Clinical and practical use of calcimimetics in dialysis patients with secondary hyperparathyroidism. Clin J Am Soc Nephrol. 2016;11(1):161–174. doi: 10.2215/CJN.01760215.
- Mak RH. Intravenous 1,25 dihydroxycholecalciferol corrects glucose intolerance in hemodialysis patients. Kidney Int. 1992;41(4):1049–1054. doi: 10.1038/ki.1992.159.
- Hong-Mei Z, Qing-Dong X, Zhi-Hua Z, et al. Analysis of associated factors in secondary hyperparathyroidism of continuous ambulatory peritoneal dialysis patients. Chin J Nephrol. 2012;28:933–936.
- Rodriguez M, Canalejo A, Garfia B, et al. Pathogenesis of refractory secondary hyperparathyroidism. Kidney Int Suppl. 2002;61(80):155–160. doi: 10.1046/j.1523-1755.61.s80.26.x.
- Li Y, Ling Z, Pen L, et al. Evaluation of the efficacy of parathyroidectomy for refractory hyperparathyroidism in 89 cases. Chin J Blood Purif. 2009;8(8):431–436.
- Goldsmith D, Kothawala P, Chalian A, et al. Systematic review of the evidence underlying the association between mineral metabolism disturbances and risk of fracture and need for parathyroidectomy in CKD. Am J Kidney Dis. 2009;53(6):1002–1013. doi: 10.1053/j.ajkd.2009.02.010.
- Jorna FH, Tobé TJ, Huisman RM, et al. Early identification of risk factors for refractory secondary hyperparathyroidism in patients with long-term renal replacement therapy. Nephrol Dial Transplant. 2004;19(5):1168–1173. doi: 10.1093/ndt/gfh018.
- Lorenz K, Bartsch DK, Sancho JJ, et al. Surgical management of secondary hyperparathyroidism in chronic kidney disease – a consensus report of the European Society of Endocrine Surgeons. Langenbecks Arch Surg. 2015;400(8):907–927. doi: 10.1007/s00423-015-1344-5.
- Cozzolino M, Shilov E, Li Z, et al. Pattern of laboratory parameters and management of secondary hyperparathyroidism in countries of Europe, Asia, the Middle East, and North America. Adv Ther. 2020;37(6):2748–2762. doi: 10.1007/s12325-020-01359-1.
- Chuang SH, Wong HC, Vathsala A, et al. Prevalence of chronic kidney disease-mineral and bone disorder in incident peritoneal dialysis patients and its association with short-term outcomes. Singapore Med J. 2016;57(11):603–609. doi: 10.11622/smedj.2015195.
- Francis DM, Busmanis I, Becker G. Peritoneal calcification in a peritoneal dialysis patient: a case report. Perit Dial Int. 1990;10(3):237–240. doi: 10.1177/089686089001000310.
- Inoshita H, Gohda T, Io H, et al. Improvement of peritoneal calcification after parathyroidectomy in a peritoneal dialysis patient. Clin Nephrol. 2008;69(1):58–62. doi: 10.5414/cnp69058.
- Huang JW, Lien YC, Yang CY, et al. High peritoneal Kt/V and peritonitis rates are associated with peritoneal calcification. PLOS One. 2013;8(8):e71636. doi: 10.1371/journal.pone.0071636.
- Grzegorzewska AE, Bednarski D, Świderska M, et al. The calcium-sensing receptor gene polymorphism rs1801725 and calcium-related phenotypes in hemodialysis patients. Kidney Blood Press Res. 2018;43(3):719–734. doi: 10.1159/000489747.
- Cheng SX, Lightfoot YL, Yang T, et al. Epithelial CaSR deficiency alters intestinal integrity and promotes proinflammatory immune responses. FEBS Lett. 2014;588(22):4158–4166. doi: 10.1016/j.febslet.2014.05.007.
- Cheng SX. Calcium-sensing receptor: a new target for therapy of diarrhea. World J Gastroenterol. 2016;22(9):2711–2724. doi: 10.3748/wjg.v22.i9.2711.