Abstract
Aims
Recent accumulating evidence has recently documented a significant prevalence of right ventricular dysfunction (RVD) in end-stage renal disease (ESRD) patients. Tricuspid annular plane systolic excursion (TAPSE)/pulmonary-artery systolic pressure (PASP) ratio assessed with echocardiography might be a useful clinical index of right ventricular (RV) -pulmonary arterial (PA) coupling. The current study aimed to investigate the value of the TAPSE/PASP ratios in patients on maintenance hemodialysis (MHD).
Methods
We studied 83 times echocardiographic tests from 68 patients with MHD. The associations of TAPSE/PASP ratios with echocardiography variables, clinical characteristics, and biochemical parameters were analyzed, as well as the associations of TAPSE/PASP ratios with odds of all-cause mortality, cardiovascular disease (CVD) events and frequent intermittent dialysis hypotension (IDH).
Results
Correlation analysis showed TAPSE/PASP ratios positively correlated with LVEF and negatively correlated with E/A and E/e’ values. For clinical and biochemical parameters, TAPSE/PASP ratios negatively correlated with BNP, NT-proBNP, age, CRP, and average interdialysis weight gain (ΔBW) and positively correlated with albumin. Logistic regression analysis, which induced the TAPSE/PASP ratio as a continuous variable (per 0.1 mm/mmHg increase), identified that the TAPSE/PASP ratio was associated with decreased CVD events (OR 0.386 [95% CI 0.231–0.645], p < 0.001) and frequent IDH odds (OR 0.571 [95% CI 0.397–0.820], p = 0.002). Moreover, the TAPSE/PASP ratio independently predicted CVD events (adjusted HR 0.539 [95% CI 0.391–0.743], p < 0.001) during a follow-up period of 12 months.
Conclusions
RVD, assessed by echocardiography TAPSE/PASP ratio, was found to be associated with increased risks of CVD events and frequent IDH in patients with MHD.
1. Introduction
Hemodialysis is the most common modality of renal replacement therapy worldwide in patients with end-stage renal disease (ESRD). Recent studies have shown that the number of patients undergoing maintenance hemodialysis (MHD) is increasing annually worldwide [Citation1]. Cardiovascular diseases (CVD) such as atherosclerosis, hypertension, heart failure (HF), and coronary artery disease are the leading cause of death in patients with MHD, which is significantly higher than that among their counterparts in the general population [Citation2,Citation3].
While left ventricular dysfunction (LVD) was widely investigated in the field of dialysis [Citation4–7], increasing studies have recently documented a significant prevalence of right ventricular dysfunction (RVD) in ESRD patients, including patients with dialysis [Citation8–10]. In addition, right ventricular (RV) function was poorer in patients with hemodialysis compared to that in patients with peritoneal dialysis, regardless of left ventricular (LV) function and pulmonary hypertension [Citation11]. Moreover, greater risks of death, arrhythmias, and cardiogenic shock have been identified in the presence of RVD [Citation12–14].
Tricuspid annular plane systolic excursion (TAPSE), measured employing M-mode echocardiography, is decreased with impaired RV function [Citation14] In the absence of right ventricular outflow tract obstruction or pulmonic stenosis, RV systolic pressure equals pulmonary-artery systolic pressure (PASP). Thus, the ratio of TAPSE to PASP estimates right ventricular function and its afterload coupling. Guazzi et al. first reported that the TAPSE/PASP ratio assessed with echocardiography might be a useful clinical index of RV function [Citation15]. Moreover, the ratio is thought to provide more comprehensive information about right heart performance than TAPSE or PASP alone [Citation16].
Previous studies showed the prognostic significance of the TAPSE/PASP ratio in patients with HF even with preserved ejection fraction [Citation16–21]. Currently, limited evidence about its predictive value in patients with MHD is available. The aim of this analysis was to investigate the value of noninvasive RV–pulmonary arterial (PA) coupling in predicting mortality and CV events, evaluated using the TAPSE/PASP ratio, in patients with MHD, as well as the association between TAPSE/PASP ratio and metabolic, nutritional and inflammatory parameters.
2. Methods
2.1. Study population
This study was a single-center, retrospective clinical study approved by the Ethics Committee of Peking University First Hospital and conducted according to the Declaration of Helsinki. 68 patients with dialysis vintage greater than one year at Peking University First Hospital and 83 echocardiographic tests were involved from December 2021 to March 2023. The participants were clinically stable and with a proper acoustic window for echocardiography and strain analysis. All the tests were conducted on the non-dialysis day after the first or second dialysis session of the week, and the interval between the period of each echocardiographic test in the same patient was longer than one year. The exclusion criteria were unwillingness to participate in the study, dialysis for acute kidney disease, severe pulmonary hypertension due to pulmonary diseases or pulmonary vascular diseases, other severe valvular diseases, arrhythmias (including atrial fibrillation), disabling stroke and active malignancy.
2.2. Echocardiography
Echocardiographic studies were performed according to the guidelines [Citation11,Citation14]. LV systolic function is mainly assessed by left ventricular ejection fraction (LVEF) [Citation22], and the LVEF was calculated using the biplane-modified Simpson’s method. TAPSE was measured as the total displacement of the tricuspid annulus (in mm) from end diastole to end systole by two-dimensional echocardiography-guided M-mode recording from the apical 4-chamber view. The maximal peak velocity of tricuspid regurgitation was measured. PASP was calculated using the transtricuspid pressure gradient plus the estimate of the right atrial pressure by the diameter and collapsibility of the inferior vena cava.
Measurements of septal ratios of transmitral flow velocity to annular velocity (E/e’) and overall mitral inflow peak E to peak A velocity ratios (E/A), which are used as an assessment of diastolic function, and other echocardiographic parameters were obtained using the software program installed on the ultrasound machine [Citation23,Citation24].
2.3. Biochemical parameters
Routinely determined biochemical parameters were measured in the same month as echocardiography before the first dialysis session of the week. The following parameters were evaluated: serum potassium, calcium, phosphorus, serum creatinine (Scr), blood urea nitrogen (BUN), hemoglobin (Hb), intact parathormone (iPTH), albumin, C-reactive protein (CRP), B-type natriuretic peptide (BNP), and N-terminal pro-BNP (NT-proBNP).
Δ Body weight (BW) was measured using the formula, ΔBW = interdialytic weight gain/dry weight, and the average ΔBW defined as the average values of ΔBW in six dialysis sessions around echocardiography.
Dialysis adequacy was measured using the single-pool Daugirdas formula [Citation25].
2.4. Outcomes
We examined the following outcomes: CVD events (stroke, nonfatal myocardial infarction, unstable angina, and HF) and all-cause mortality within one year since echocardiography. Mortality and CVD events were identified by referring to medical records and confirmed by direct contact with the patients, relatives, and in-charge physicians.
Intradialytic hypotension (IDH) was defined as having a drop of systolic blood pressure of >20mmHg during dialysis, accompanied by any clinical events attributable to hypotension, such as dizziness, nausea, muscle cramp and other manifestations of tissue hypoperfusion, and in need of nursing interventions [Citation26,Citation27]. Frequent IDH was defined as IDH events that had occurred at least three times in all the dialysis sessions in the following month of echocardiography.
2.5. Statistical analysis
After determining the best latent profile model, statistical analyses were performed using the SPSS 26.0 software. Continuous variables with normal distribution were described by mean and standard deviation, and non-normal distribution was described by median and interquartile range. Categorical variables were described by frequency and percentage. Univariate analysis was performed by the Kruskal–Wallis test, one-way analysis of variance (ANOVA), or the chi-square test. Correlation between quantitative variables was done using Pearson’s or Spearmann’s correlations. Logistic regression tests were performed to explore the correlations among the assessed parameters. The clinical endpoint was assessed with the Kaplan–Meier method and compared with the log-rank test. Cox regression models were used to calculate hazard ratios (HR) and 95% CI for each endpoint. The predictive value of these models was assessed by the area under the receiver operating characteristic (ROC) curve (AUC). p < 0.05 was considered statistically significant.
3. Results
3.1. Characteristics of the study population
Data from show the characteristics of the analyzed patients. There were a total of six deaths and 21 CVD events during the one-year follow-up since echocardiographic tests.
Table 1. Baseline clinical characteristics divided with TAPSE/PASP.
Most studies assigned the optimal cutoff for TAPSE/PASP by Cox proportional hazards, whereas some considered the median TAPSE/PASP ratio value of the overall population as the cutoff. The current study stratified the population according to the median TAPSE/PASP value (0.65 mm/mmHg). Patients with TAPSE/APSP values 0.65 mm/mmHg or less were significantly associated with lower albumin concentrations, higher BNP or NT-proBNP, and higher CRP levels. They also had significantly higher rates of CVD events and frequent IDH.
3.2. Echocardiography characteristics and clinical parameters
Data from show the echocardiography variables of the analyzed patients.
Table 2. Echocardiography variables.
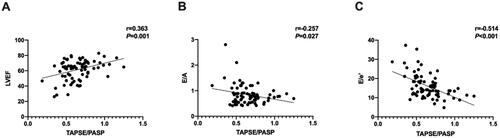
TAPSE/PASP ratios positively correlated with LVEF (r = 0.363, p = 0.001) and negatively correlated with E/A and E/e’ values (r= −0.257, p = 0.027; r=-0.514, p < 0.001, respectively) (), as LVEF negatively correlated with E/e’ values (r= −0.398, p < 0.001) as well.
Figure 1. TAPSE/PASP ratios positively correlated with LVEF and negatively correlated with E/a and E/e’ values.
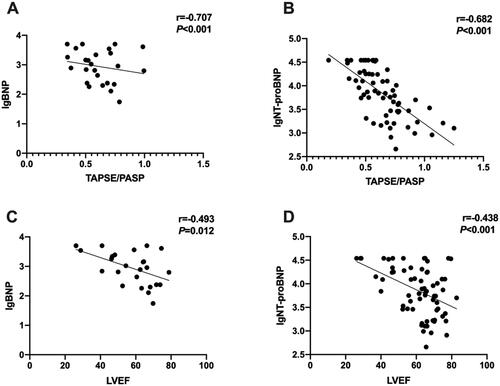
TAPSE/PASP ratios negatively correlated with BNP and NT-proBNP (r= −0.613, p < 0.001; r= −0.659, p < 0.001, respectively), as LVEF negatively correlated with BNP and NT-proBNP (r= −0.462, p = 0.020; r=-0.446, p < 0.001, respectively) as well. showed the logarithm of BNP or NT-proBNP and the value of TAPSE/PASP or LVEF.
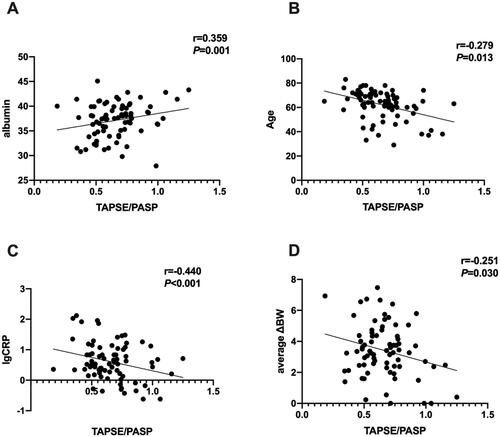
TAPSE/PASP ratios positively correlated with albumin (r = 0.359, p = 0.001), and negatively correlated with age, CRP and average ΔBW (r= −0.279, p = 0.013; r= −0.254, p = 0.026; r= −0.251, p = 0.030, respectively) ().
3.3. RV-PA uncoupling and prognosis
Logistic regression analysis, inducing the TAPSE/PASP ratio as a continuous variable (per 0.1 mm/mmHg increase), identified that the TAPSE/PASP ratio was associated with CVD events odds (Odds ratio (OR) 0.386 [95% CI 0.231–0.645], p < 0.001) and frequent IDH odds (OR 0.571 [95% CI 0.397–0.820], p = 0.002).
illustrates the survival curve of CVD events according to TAPSE/PASP <0.65 mm/mmHg (Log-rank p < 0.001), and illustrates the survival curve composite outcomes including both CVD events and death according to TAPSE/PASP < 0.65 mm/mmHg (Log-rank p < 0.001). In addition, Cox regression analysis, inducing the TAPSE/PASP ratio as a continuous variable (per 0.1 mm/mmHg increase), also identified that the TAPSE/PASP ratio was associated with CVD events (HR 0.530 [95% CI 0.397–0.709], p < 0.001). The LVEF, E/e’, and BNP (HR 0.959 [95% CI 0.925–0.991], p = 0.013; HR 1.063 [95% CI 1.005–1.123], p = 0.032; HR 1.003 [95% CI 1.000–1.007], p = 0.035, respectively) were also significantly associated with the CVD events through an univariable Cox regression model, and TAPSE/PASP ratio was proven to be significantly related to CVD events even after multivariable adjustment (adjusted HR 0.539 [95% CI 0.391–0.743], p < 0.001) (). No significant association between echocardiography variables and all-cause mortality odds was found in the Logistic and Cox regression analysis.
Figure 4. Kaplan–Meier Survival curves for CVD events and composite outcome including both CVD events and death based on median TAPSE/PASP value (0.65 mm/mmHg).
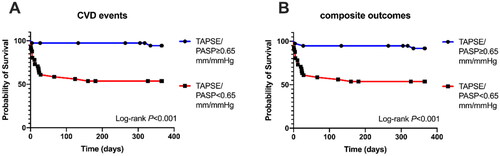
Table 3. Cox regression model for prognostic prediction of CVD events.
3.4. ROC curve analysis for TAPSE/PASP, NT-proBNP and PASP in predicting CVD events and frequent IDH
The ROC curve was plotted to analyze the application of the reciprocal of TAPSE/PASP, NT-proBNP and PASP in predicting CVD events or frequent IDH. On predicting CVD events, the reciprocal of TAPSE/PASP (AUC = 0.816, p < 0.001) exhibited a better effect than PASP (AUC = 0.769, p = 0.001), but a worse effect than NT-proBNP (AUC = 0.898, p < 0.001). On predicting frequent IDH, the reciprocal of TAPSE/PASP (AUC = 0.726, p = 0.004) also exhibited a better effect than PASP (AUC = 0.652, p = 0.049), and a similar effect to NT-proBNP (AUC = 0.726, p = 0.003) (Supplementary material). The cutoff values, sensitivity and 1-specificity were summarized in .
Table 4. ROC curve analysis for related indicators of CVD events and frequent IDH.
4. Discussion
Combining RV systolic function with pulmonary circulation pressure, the TAPSE/PASP ratio is a noninvasive easy-to-use echocardiography-based assessment approach. This ratio may provide a novel perspective for evaluating RV function. Our results revealed significant correlations between the TAPSE/PASP ratios and LV systolic and diastolic function, BNP, NT-proBNP, albumin, age, CRP and interdialytic weight gain. Additionally, TAPSE/PASP ratios were associated with the risk of CVD events, and frequent IDH as well. It was the first report of the assessment of the noninvasive RV–PA coupling in patients with MHD.
In the echocardiography variables assay of the current study, TAPSE/PASP ratios positively correlated with LVEF and negatively correlated with E/A and E/e’ values, while LVEF negatively correlated with E/e’ values with a lower correlation coefficient, indicating that RV function is more relevant with diastolic function and RVD might occur before LVD in patients with MHD. Similarly, a study of patients with HF with preserved ejection fraction (HFpEF) revealed that a lower TAPSE/PASP ratio was independently associated with adverse outcomes [Citation21]. Moreover, it has been documented that impaired RV function would result in more severely impaired LV function [Citation28], all indicating that RV function would be of much concern in patients with MHD.
In the clinical parameters assay of the current study, TAPSE/PASP ratios positively correlated with albumin, and negatively correlated with age CRP and interdialytic weight gain, indicating worse nutritional status, aging, chronic inflammation conditions and relatively high interdialytic weight gains may impair RV function. On the one hand, malnutrition is one of the complications of chronic kidney disease (CKD). A study by Chien proved that malnutrition defined as decreased albumin concentrations, was related to ventricle remodeling [Citation29]. Otaki also assumed that low albumin levels in patients with CKD and HF were associated with a worse prognosis [Citation30]. On the other hand, higher interdialytic weight gains were remarkably significantly associated with higher mortality risk and cardiovascular hospitalization, as well as rapid renal urea clearance decline [Citation31,Citation32]. Thus, our results indicate a way to improve RV function and prognosis of patients with MHD by improving their nutritional status and extensive volume control.
In regression analysis of the current study, TAPSE/PASP ratios were identified as associated with odds of CVD events and frequent IDH. On the one hand, Nakagawa et al. reported that RV–PA uncoupling, defined as TAPSE/PASP ratio <0.48 mm/mmHg, was associated with a 2.75-fold increased risk of the composite endpoint of all-cause death or HF hospitalization in a large cohort of patients with HFpEF [Citation21]. Similarly, patients in our study with a worse TAPSE/PASP ratio (<0.65 mm/mmHg) presented higher BNP and NT-proBNP levels, and worse LV systolic and diastolic function, suggesting a more severe cardiac involvement. On the other hand, IDH is one of the common complications of hemodialysis and is associated with all-cause and cardiovascular mortality and hospitalization odds [Citation33]. Previous studies revealed that changes in the septal E/e’ ratio correlated with increased LV filling pressure and were the major contributing factors to IDH [Citation34]. Taking the correlation of TAPSE/PASP and E/e’ into consideration, improving RV function may decrease the risk of IDH as well.
Several limitations of this study should be remarked upon. First, due to the assessment of RV function being a newly developed item in echocardiography in our center, the data was relatively limited, however, we still got those significant results, indicating that RVD in patients with hemodialysis would be worthwhile to investigate further. Second, the study was performed using echocardiography, whereas cardiac magnetic resonance imaging is considered the gold standard for right ventricular functional assessment.
5. Conclusion
The noninvasive RV-PA coupling, evaluated using the TAPSE/PASP ratio, in patients with MHD was found to be associated with increased CVD events and frequent IDH. Therefore, it is crucial for patients with MHD to undertake regular evaluations of RVD in routine practice.
Authors’ contributions
Chen Wang and Xu-Yang Cheng designed and planned the study. Chen wang handled the selection of suitable patients for the study, arranged the collection of clinical data, performed data analysis and drafted the manuscript. Li Meng participated the collection of clinical data. Yu-Qing Chen guided the study as a senior author. All the authors contributed to data interpretation and manuscript revision. The author(s) read and approved the final manuscript.
Ethics approval and consent to participate
This study protocol adhered to the Declaration of Helsinki and was approved by the Ethics Committee of Peking University First Hospital. Written patient-informed consent was obtained from all participants.
Supplemental Material
Download MS Word (3.3 MB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author.
Additional information
Funding
References
- Thurlow JS, Joshi M, Yan G, et al. Global epidemiology of end-stage kidney disease and disparities in kidney replacement therapy. Am J Nephrol. 2021;52(2):1–9. doi: 10.1159/000514550.
- Di Lullo L, House A, Gorini A, et al. Chronic kidney disease and cardiovascular complications. Heart Fail Rev. 2015;20(3):259–272. doi: 10.1007/s10741-014-9460-9.
- McCullough PA, Chan CT, Weinhandl ED, et al. Intensive hemodialysis, left ventricular hypertrophy, and cardiovascular disease. Am J Kidney Dis. 2016;68(5S1):S5–s14. doi: 10.1053/j.ajkd.2016.05.025.
- Fathi R, Isbel N, Haluska B, et al. Correlates of subclinical left ventricular dysfunction in ESRD. Am J Kidney Dis. 2003;41(5):1016–1025. doi: 10.1016/s0272-6386(03)00199-9.
- Romejko K, Rymarz A, Szamotulska K, et al. Left ventricular diastolic dysfunction in chronic kidney disease patients not treated with dialysis. Nutrients. 2022;14(21):4664. doi: 10.3390/nu14214664.
- Sood MM, Pauly RP, Rigatto C, et al. Left ventricular dysfunction in the haemodialysis population. NDT Plus. 2008;1(4):199–205. doi: 10.1093/ndtplus/sfn074.
- Singh S, Aggarwal V, Pandey UK, et al. Study of left ventricular systolic dysfunction, left ventricular diastolic dysfunction and pulmonary hypertension in CKD 3b-5ND patients-A single centre cross-sectional study. Nefrologia (Engl Ed). 2023;43(5):596–605. doi: 10.1016/j.nefroe.2022.06.005.
- Paneni F, Gregori M, Ciavarella GM, et al. Right ventricular dysfunction in patients with end-stage renal disease. Am J Nephrol. 2010;32(5):432–438. doi: 10.1159/000320755.
- Daralammouri Y, Qaddumi J, Ayoub K, et al. Pathological right ventricular changes in synthesized electrocardiogram in end-stage renal disease patients and their association with mortality and cardiac hospitalization: a cohort study. BMC Nephrol. 2022;23(1):79. doi: 10.1186/s12882-022-02707-9.
- Said K, Hassan M, Baligh E, et al. Ventricular function in patients with end-stage renal disease starting dialysis therapy: a tissue Doppler imaging study. Echocardiography. 2012;29(9):1054–1059. doi: 10.1111/j.1540-8175.2012.01749.x.
- Demirci DE, Demirci D, İnci A. Long-term impacts of different dialysis modalities on right ventricular function in patients with end-stage renal disease. Echocardiography. 2022;39(10):1316–1323. doi: 10.1111/echo.15455.
- Mehta SR, Eikelboom JW, Natarajan MK, et al. Impact of right ventricular involvement on mortality and morbidity in patients with inferior myocardial infarction. J Am Coll Cardiol. 2001;37(1):37–43. doi: 10.1016/s0735-1097(00)01089-5.
- Zornoff LA, Skali H, Pfeffer MA, et al. Right ventricular dysfunction and risk of heart failure and mortality after myocardial infarction. J Am Coll Cardiol. 2002;39(9):1450–1455. doi: 10.1016/s0735-1097(02)01804-1.
- Houston BA, Brittain EL, Tedford RJ. Right ventricular failure. N Engl J Med. 2023;388(12):1111–1125. doi: 10.1056/NEJMra2207410.
- Guazzi M, Bandera F, Pelissero G, et al. Tricuspid annular plane systolic excursion and pulmonary arterial systolic pressure relationship in heart failure: an index of right ventricular contractile function and prognosis. Am J Physiol Heart Circ Physiol. 2013;305(9):H1373–1381. doi: 10.1152/ajpheart.00157.2013.
- Bosch L, Lam CSP, Gong L, et al. Right ventricular dysfunction in left-sided heart failure with preserved versus reduced ejection fraction. Eur J Heart Fail. 2017;19(12):1664–1671. doi: 10.1002/ejhf.873.
- Santas E, Palau P, Guazzi M, et al. Usefulness of right ventricular to pulmonary circulation coupling as an indicator of risk for recurrent admissions in heart failure with preserved ejection fraction. Am J Cardiol. 2019;124(4):567–572. doi: 10.1016/j.amjcard.2019.05.024.
- Brener MI, Lurz P, Hausleiter J, et al. Right ventricular-pulmonary arterial coupling and afterload reserve in patients undergoing transcatheter tricuspid valve repair. J Am Coll Cardiol. 2022;79(5):448–461. doi: 10.1016/j.jacc.2021.11.031.
- Guazzi M, Dixon D, Labate V, et al. RV contractile function and its coupling to pulmonary circulation in heart failure with preserved ejection fraction: stratification of clinical phenotypes and outcomes. JACC Cardiovasc Imaging. 2017;10(10 Pt B):1211–1221. doi: 10.1016/j.jcmg.2016.12.024.
- Brener MI, Grayburn P, Lindenfeld J, et al. Right ventricular-pulmonary arterial coupling in patients with HF secondary MR: analysis from the COAPT Trial. JACC Cardiovasc Interv. 2021;14(20):2231–2242. doi: 10.1016/j.jcin.2021.07.047.
- Nakagawa A, Yasumura Y, Yoshida C, et al. Prognostic importance of right ventricular-vascular uncoupling in acute decompensated heart failure with preserved ejection fraction. Circ Cardiovasc Imaging. 2020;13(11):e011430. doi: 10.1161/circimaging.120.011430.
- Shah AM, Cikes M, Prasad N, et al. Echocardiographic features of patients with heart failure and preserved left ventricular ejection fraction. J Am Coll Cardiol. 2019;74(23):2858–2873. doi: 10.1016/j.jacc.2019.09.063.
- Nagueh SF, Smiseth OA, Appleton CP, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2016;29(4):277–314. doi: 10.1016/j.echo.2016.01.011.
- Silbiger JJ. Pathophysiology and echocardiographic diagnosis of left ventricular diastolic dysfunction. J Am Soc Echocardiogr. 2019;32(2):216–232.e212. doi: 10.1016/j.echo.2018.11.011.
- Chen YK, Chu CS, Niu SW, et al. The prognostic value of URR equals that of Kt/V for all-cause mortality in Taiwan after 10-year follow-up. Sci Rep. 2023;13(1):8923. doi: 10.1038/s41598-023-35353-8.
- Flythe JE, Katsanos SL, Hu Y, et al. Predictors of 30-day hospital readmission among maintenance hemodialysis patients: a hospital’s perspective. Clin J Am Soc Nephrol. 2016;11(6):1005–1014. doi: 10.2215/cjn.11611115.
- Flythe JE, Xue H, Lynch KE, et al. Association of mortality risk with various definitions of intradialytic hypotension. J Am Soc Nephrol. 2015;26(3):724–734. doi: 10.1681/asn.2014020222.
- Paneni F, Gregori M, Ciavarella GM, et al. Relation between right and left ventricular function in patients undergoing chronic dialysis. J Cardiovasc Med (Hagerstown). 2013;14(4):289–295. doi: 10.2459/JCM.0b013e32834eacf0.
- Chien SC, Chandramouli C, Lo CI, et al. Associations of obesity and malnutrition with cardiac remodeling and cardiovascular outcomes in Asian adults: A cohort study. PLoS Med. 2021;18(6):e1003661. doi: 10.1371/journal.pmed.1003661.
- Otaki Y, Watanabe T, Takahashi H, et al. Comorbid renal tubular damage and hypoalbuminemia exacerbate cardiac prognosis in patients with chronic heart failure. Clin Res Cardiol. 2016;105(2):162–171. doi: 10.1007/s00392-015-0899-z.
- Miyasato Y, Hanna RM, Miyagi T, et al. Associations of interdialytic weight gain in the long intervals with mortality and residual kidney function decline. Hemodial Int. 2023;27(3):326–338. doi: 10.1111/hdi.13094.
- Zhang Y, Wang J, Xing Y, et al. Dynamics of cardiac autonomic responses during hemodialysis measured by heart rate variability and skin sympathetic nerve activity: the impact of interdialytic weight gain. Front Physiol. 2022;13:890536. doi: 10.3389/fphys.2022.890536.
- Kanbay M, Ertuglu LA, Afsar B, et al. An update review of intradialytic hypotension: concept, risk factors, clinical implications and management. Clin Kidney J. 2020;13(6):981–993. doi: 10.1093/ckj/sfaa078.
- Chen CY, Yang NI, Lee CC, et al. Dynamic echocardiographic assessments reveal septal e/e’ ratio as independent predictor of intradialytic hypotension in maintenance for hemodialysis patients with preserved ejection fraction. Diagnostics (Basel). 2021;11(12):2266. doi: 10.3390/diagnostics11122266.