Abstract
The adherence of four Candida species to human buccal epithelial cells (BECs) following treatment with the most commonly consumed dietary carbohydrates was investigated in vitro. Adhesion of C. albicans, C. tropicalis, C. glabrata and C. parapsilosis was significantly promoted by incubation in minimal medium containing a high concentration (500 mM) of fructose, galactose, glucose, maltose, sorbitol or sucrose (p < 0.001). C. albicans grown in galactose elicited maximal increase in adhesion followed by glucose and sucrose. Maltose and fructose also promoted adherence of Candida spp. but to a lesser extent than galactose and glucose, while no statistical difference in adherence was observed when Candida spp. were grown in the presence of lactose and trehalose. Xylitol significantly reduced adherence of Candida spp. to BECs. No statistically significant difference in the adherence capabilities of different growth phases of C. albicans was noted, and the effect of galactose and glucose persisted irrespective of the phase of growth used. The dietary carbohydrates, therefore, might represent a risk factor for oral candidosis. The limitation of their consumption by substituting xylitol could be of value in the control of oral Candida colonization and infection.
Introduction
Oral candidosis is a common opportunistic infection in cancer patients and currently ranks as the most common human fungal disease. Candidaalbicans, the major human pathogen of the genus Candida, colonizes human mucosal surfaces, particularly those within the oral cavity and vagina, and may become haematogenously disseminated in immunocompromised persons Citation[1], Citation[2]. Adherence of Candida spp. to host surfaces is thought to be a crucial step in the pathogenic process and a prerequisite for colonization of these surfaces Citation[2]. This adhesion occurs by the interaction between yeast and epithelial cell receptors, and a variety of mechanisms have been proposed Citation[2], Citation[3]. Yeast cells bind to galactoside-containing receptors on epithelial cells Citation[4], express a mannoprotein adhesin that recognizes fucosyl determinants of epithelial cell membrane glycosides Citation[5], and carry surface carbohydrates important for binding to epithelial cells Citation[6]. C. albicans adheres also to extracellular matrix proteins Citation[7], Citation[8], possibly with the involvement of adhesins that mimic the complement receptors CR2 and CR3 Citation[9]. Moreover, it has been shown that C. albicans binds to salivary components, including mucins Citation[10], proteoglycan Citation[11], and proline-rich protein Citation[12], suggesting that C. albicans has multiple mechanisms for adherence in the oral cavity. It is well known that C. albicans adhesion to mucosal and artificial surfaces in the mouth is enhanced by several factors such as germ-tube production, phospholipase, protease, other extracellular enzymatic activities, carbohydrates, pH and temperature Citation[2], Citation[3].
Dietary sugars, such as glucose and sucrose commonly consumed, may be of importance in the pathogenesis of C. albicans in the oral cavity, given the effect of such sugars on the adherence of the yeast in vitroCitation[13]. C. albicans cells grown in defined medium supplemented with a high concentration (500 mM) of dietary carbohydrates exhibited increased adherence to acrylic surfaces Citation[14], Citation[15]. Samaranayake et al. Citation[14] showed that yeasts incubated in sucrose and glucose had a better adhesion to acrylic strips than controls grown in sugar-free media. Growth in the presence of lactose and xylitol showed no significant difference when compared with the control yeasts Citation[15]. Pre-incubation of C. albicans in the presence of a range of sucrose concentrations (50–500 mM) gave a significant positive correlation between the number of yeasts adherent to acrylic surfaces or epithelial cells and the sucrose concentration Citation[14], Citation[15]. Similarly, Douglas and co-workers Citation[16–18] showed that adherence of C. albicans to acrylic surfaces, as well as epithelial cells, was enhanced significantly after growth of the yeast to the stationary phase in media containing high concentrations of various sugars as the carbon source. Galactose was the most effective sugar tested and fructose the least Citation[17]. Electron microscopy revealed that incubation in galactose or sucrose results in a greater production of the outer fibrillar-floccular layer of the cell wall Citation[17], Citation[18]. This layer, composed primarily of mannoproteins, has been recognized as being responsible for the increased adherence to acrylics Citation[19], Citation[20]. Candida adherence to epithelial surfaces appeared to be similarly promoted, but relatively few investigations compared the adhesion of Candida cells after growth in various carbohydrates Citation[14–18]. The effects of a number of carbohydrates, such as fructose, lactose, maltose and xylitol, are also poorly understood, and there are no data, to the best of our knowledge, on the adhesion of sorbitol- and trehalose-grown yeasts. Furthermore, most of the reports currently available deal with C. albicans, thus there is little information on the epithelial adhesion of non-albicans species such as C. tropicalis, C. glabrata, C. parapsilosis and C. krusei, which have emerged as significant pathogens in immunocompromised patients Citation[1], Citation[2]. Based on the above, it was the aim of this study to investigate the effects of the most commonly consumed dietary carbohydrates on the in vitro adhesion of C. albicans, C. tropicalis, C. glabrata and C. parapsilosis to buccal epithelial cells.
Materials and methods
Organisms
Four species of Candida were used in this study: C. albicans, C. tropicalis, C. glabrata, and C. parapsilosis. One strain each of C. albicans, C. tropicalis, C. glabrata, and C. parapsilosis was isolated from cancer patients with oral candidosis. Methods of isolation, identification and antifungal susceptibility testing have been described elsewhere Citation[21]. Four strains from the American Type Culture Collection (C. albicans ATCC 36082, C. tropicalis ATCC 750, C. glabrata ATCC 22553 and C. parapsilosis ATCC 22019) were also used. All organisms were stored in Sabouraud dextrose agar (SDA) medium (Difco Laboratories, Detroit, MI, USA), kept at 4°C and subcultured routinely.
Growth conditions
Overnight cultures of various Candida spp. were grown at 37°C in yeast nitrogen base medium (Difco) supplemented with 2.5% glucose. Flasks containing 50 ml of minimal media (ammonium sulphate 1 g/l and monopotassium sulphate 1 g/l, adjusted to pH 6.0) and supplemented with 50 mM glucose, or 500 mM fructose, galactose, glucose, lactose, maltose, sorbitol, sucrose, trehalose and xylitol, were inoculated with 1 ml of the overnight culture and some were grown for 12 h (exponential phase), while others were grown for 24 h (stationary phase) in a shaking water bath (160 rpm) at 37°C. Yeast cells were harvested by centrifugation at 1200 g for 10 min and the pellet was washed twice, with 10 ml of Hanks's balanced salts solution (HBSS), pH 7.0. A final yeast suspension of 1×107 cells/ml was prepared by appropriate dilution in HBSS followed by haemocytometer counting. These cells were used in the adherence assay.
Preparation of buccal epithelial cells for adherence assays
Buccal epithelial cells (BECs) were collected during the early morning from six healthy adult fasting male volunteers by gentle rubbing of the mucosal surface of the cheeks with a sterile tongue depressor. BECs were washed twice with HBSS and collected by centrifugation (500 g, 10 min). This step was intended to wash away saliva and other contaminating oral secretions. These cells were then used to study the adhesion of Candida spp. to BECs following exposure of yeast to dietary carbohydrates. A final suspension of 2×105 BECs/ml was prepared by appropriate dilution in HBSS after haemocytometer counting. Only freshly prepared samples of BECs were used in adherence assays.
Adherence assay
The candidal adhesion assay was performed as described previously Citation[21]. Briefly, a mixture of equal volumes of BECs (2×105 BECs/ml) and yeast cells (1×107 yeast/ml), treated as described above, was incubated at 37°C for 2 h in a shaking bath at 180 rpm. Cells were filtered through a 20 µm pore size filter (Retsch, Idar-Oberstein, Haan, Germany) to remove non-adherent yeast cells. The epithelial cells on the filter were washed twice with 5 ml portions of HBSS and finally suspended in 5 ml of the same buffer. A drop of this suspension was mounted on a glass slide, air-dried, heat-fixed and stained with crystal violet for 1 min and adherence assayed microscopically at a magnification of ×400. The number of yeast cells adhering to every BEC was counted for 100 BECs taken at random and only uniform, unfolded epithelial cells were included. Each Candida species was assayed twice for adhesion to BECs on two separate occasions.
Cells of C. albicans ATCC 36082 as well as BECs suspended in HBSS were pretreated separately with galactose and glucose (500 mM). The mixtures were incubated for 30 min at 37°C on an orbital shaker. The cells were harvested, washed twice with HBSS, resuspended in this buffer, then standardized following haemocytometer counting and their adherence was assayed as described above.
Statistical analysis
To evaluate the differences in the adherence value among Candida species, the Student's t test was used to determine significant differences using statistical analysis software (STATISTICA for Windows, version 5.0, StatSoft Inc., Oklahoma City, OK, USA). Data are expressed as the mean±SE, and a p value of ≤0.05 was considered to be statistically significant.
Results
The effects of carbohydrates on adherence of C. albicans, C. tropicalis, C. glabrata and C. parapsilosis to BECs are shown in . Results from adhesion assays are expressed as means (±standard error) of two independent experiments and on two different occasions with duplicate determinations. The adhesion of Candida spp. grown in carbohydrates at 500 mM (test organisms) was also compared with that of organisms grown in relatively low concentrations of glucose (50 mM; control organisms) and expressed as relative adherence.
Table I. The effect of growth of various Candida spp. in saccharide-containing media on adherence to buccal epithelial cells (BECs).
Fructose, galactose, glucose, maltose, sorbitol and sucrose significantly enhanced adhesion of Candida spp. to BECs to different extents according to the carbohydrate used (p<0.05 to 0.001). Galactose was the most effective carbohydrate of those tested, and galactose-grown yeasts elicited more than twofold enhancement in adhesion (relative adherence = 2.59 for C. albicans) (). A similar pattern of enhancement was observed for C. tropicalis, C. glabrata and C. parapsilosis (). Lactose and trehalose also increased C. albicans adhesion, but the difference in adherence values between test and control organisms did not reach statistical significance (p > 0.05). On the other hand, C. albicans grown in 500 mM xylitol demonstrated a significant reduction in adherence (p < 0.001, relative adherence = 0.80) ().
When comparing the mean number of adherent yeasts after growth in 500 mM galactose, C. albicans demonstrated the greatest degree of adhesion in comparison with C. tropicalis, C. glabrata and C. parapsilosis. Thus, the pattern of increased adherence was C. albicans > C. glabrata > C. tropicalis > C. parapsilosis. However, lactose and trehalose were observed to be the least favourable, while galactose, glucose and sucrose at 500 mM were the most favourable for adherence among the sugars tested.
No significant difference was observed between the adherence of control exponential and stationary phase grown cells of C. albicans to BECs (p>0.05). Growing of C. albicans in the presence of 500 mM galactose and glucose for 12 h (exponential phase) or for 24 h (stationary phase) enhanced the adherence of this yeast to BEC (p<0.05) (). Inclusion of galactose or glucose in the assay medium produced a reduction in the observed enhancement of adherence of C. albicans grown in the presence of these sugars (). Furthermore, no significant difference was observed between the adherence to BECs of exponential grown cells of C. albicans in the presence of 500 mM glucose and inclusion of 500 mM glucose in the assay medium (p>0.05).
Figure 1. Effect of galactose and glucose on the adherence of C. albicans ATCC 36082 to buccal epithelial cells (BECs). (A) Yeasts grown for 24 h (stationary phase) or 12 h (exponential phase) without sugars ( control). (B) Yeasts grown for 24 h (stationary phase) or 12 h (exponential phase) in the presence of sugars (500 mM). (C) Sugars (500 mM final concentration) included in the assay medium.
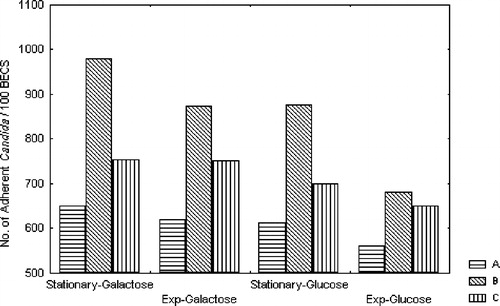
The effects of pretreatment of yeast and/or buccal cells for 30 min with 500 mM galactose or glucose before assay are presented in . It is apparent that the adherence was substantially enhanced in both cases as compared with the control. However, the relative adherence of 1.77 and 1.35 was obtained when both types of cells, i.e. yeast cells and BECs, were pretreated at the same time with galactose and glucose, respectively.
Table II. Effect of pretreatment of yeast cells and/or BECs with galactose and glucose on the adherence of C. albicans ATCC 36082 to BECs.
Discussion
We have investigated the effects of a number of dietary carbohydrates on the in vitro adherence of four different Candida spp. to BECs. The most commonly consumed dietary carbohydrates, i.e. fructose, galactose, glucose, lactose, maltose and sucrose, together with sorbitol, trehalose and xylitol, were selected for the adhesion assays. Dietary carbohydrates, such as glucose and sucrose, may be of importance in the pathogenesis of C. albicans in the oral cavity, given the effect of such sugars on the adherence of the yeast in vitro. Results from adhesion assays indicated that incubation in fructose, galactose, glucose, maltose and sucrose significantly promotes adherence of various Candida spp. to BECs. In contrast, yeast cells grown in 500 mM of lactose and trehalose show no significant effect on the adherence to BECs, which is consistent with published data on the relationship between intra-oral concentration of these carbohydrates, candidal carriage and candidosis Citation[2], Citation[13]. McCourtie and Douglas Citation[17] showed that the adherence of yeast to acrylic surfaces in vitro was increased after growth of the yeast in a medium containing high levels of different sugars, particularly galactose. Samaranayake and MacFarlane Citation[15] showed that various strains of C. albicans pre-incubated in a medium containing glucose, sucrose, galactose, xylitol or maltose, exhibited enhanced adherence to HeLa epithelial monolayers and to BECs. The most effective sugar in this respect was maltose and the least effective was glucose Citation[15]. In these studies it was noted that adhesion was directly proportional to the sugar concentration. McCourtie and Douglas Citation[17] found that the C. albicans grown on 500 mM galactose adhered to acrylic surfaces at a maximal linear rate throughout an incubation period of 1 h, whereas non-linear adhesion rates were observed with cells grown on 500 mM sucrose, 50 mM glucose or 50 mM galactose Citation[17]. However, these studies concentrated mainly on a single strain of C. albicans isolated from a patient with denture stomatitis, and the disparity could also be due to such an origin. Our results indicated that the adherence-promoting effect of sucrose was observed for C. tropicalis, C. glabrata and C. parapsilosis; maximal adhesion to BECs occurred after incubation in galactose and glucose as shown in . As far as we know, there is no information available on the modulating effect of sucrose or glucose on epithelial adhesion of C. tropicalis and C. parapsilosis. However, it is conceivable that the enhanced adhesion is due to the production of a fibrillar-floccular layer, as reported for C. albicansCitation[19]. Glucose can also promote acid production and lower pH, with consequent activation of acid proteinases and extracellular phospholipases, factors involved in yeast adhesion Citation[22], Citation[23].
Yeasts incubated in media containing lactose showed no significant difference in adherence to BECs when compared with control yeasts Citation[14]. Moreover, Samaranayake and MacFarlane Citation[15] found that the xylitol-grown C. albicans cells are up to two times more adherent to epithelial surfaces than control-grown cells. Our results indicated that Candida spp. incubated in 500 mM xylitol exhibited a significant inhibition in adhesion. Xylitol is incorporated in toothpaste and mouth rinse formulations, as well as in chewing-gum, for its anti-cariogenic effect. This alditol is not metabolized by oral bacteria and lower levels of Streptococcus mutans are found in plaque and saliva of subjects consuming such sugar substitute Citation[24]. Xylitol does not increase the in vitro growth of C. albicans, being metabolized poorly, if at all Citation[25]. The dietary intake of xylitol was reported to reduce oral candidal carriage Citation[26], and inhibit colonization and invasion of the gastrointestinal tract in a neutropenic mouse model Citation[27]. Furthermore, giant cell production by C. albicans cultured in xylitol was also described Citation[28], and it has been explained by the inability of yeast cells to catabolize or excrete the xylitol products that accumulate in the cytoplasm and induce an increased osmotic strength and cell swelling. Such conditions could account for reduced adherence through a poor production of an additional fibrillar-floccular layer on the yeast cell surface Citation[18], Citation[19].
No statistical difference in the adherence ability of different growth phases of C. albicans was observed in this study. Although stationary phase yeast showed greater adherence than exponential phase cells (650±22 compared to 612±26 yeast adhering to 100 BECs, respectively), this was not high enough to be statistically significant (p>0.5). These findings agree with those reported by Ghannoum and Abu-Elteen Citation[29]. King et al. Citation[30] reported that stationary phase yeasts attached to vaginal epithelial cells in greater numbers than exponential phase organisms, although prolonged incubation of the culture (for more than 24 h) did not significantly enhance adhesion. In contrast, Segal et al. Citation[31] reported a higher rate of adhesion with exponential phase yeasts. The reason for this difference is not immediately evident, although it may be attributable to the different growth media used by the three different groups. In addition, King et al. Citation[30] and Segal et al. Citation[31] used vaginal epithelial cells, while BECs were used in the present study and in the study by Ghannoum and Abu-Elteen Citation[29]. The effect of galactose and glucose on adherence of C. albicans as reported above persisted, irrespective of the growth phase.
Our findings on the adherence-promoting effect caused by the pretreatment of either the yeast cells, the epithelial cells, or both, by 500 mM galactose or glucose generally support the mechanism by which carbohydrates enhance adherence, which appears to be by the production of an additional fibrillar-floccular layer that mediates Candida adhesion. Tokunaga et al. Citation[32] showed that the adhesion of Candida cells to BECs corresponded with increased density of the fibrillar structure at the outermost Candida cell wall. Another study associated adherence with the condensation or disposal of this layer, facilitating contact between epithelial cells and the deeper layer of the fungal wall Citation[18], Citation[19], Citation[33].
In conclusion, our results indicate that epithelial adhesion of Candida spp. is affected to different extents by dietary carbohydrates. However, sorbitol was observed as the least favourable and galactose as the most favourable sugar for adherence among those tested. Maltose and fructose promoted adherence to a lesser extent than galactose, glucose and sucrose. Significant inhibition was observed after incubation in xylitol. Lactose and trehalose did not appear to significantly affect adhesion. These findings imply that the frequent consumption of dietary carbohydrate such as galactose, sucrose, glucose, maltose or fructose, especially in association with poor oral hygiene, might represent a risk factor for oral candidal colonization and infection. Interestingly, a high local glucose concentration leads to an increased incidence of Candida paronychia in sugar cane workers Citation[34] and rinsing with sucrose leads to Candida-induced stomatitis in humans Citation[35]. A propagating role of a carbohydrate-rich diet in relapsing Candida vulvovaginitis was proposed in a study involving 240 women Citation[36]. A limited intake of these carbohydrates by substituting xylitol could be of value in the management of oral candidosis in denture wearers, diabetics and patients undergoing topical steroid therapy or prolonged antibiotics, as well as in the control of oral Candida colonization in patients at high risk of developing candidosis.
The author would like to thank Dr R.M. Abu-Alteen for his encouragement and support. This work was supported by a research grant from the Hashemite University, College of Graduate Studies and Scientific Research.
References
- Akpan A, Morgan R. Oral candidiasis: a review. Postgrad Med J 2002; 78: 455–9
- Sitheeque MAM, Samaranayake LP. Chronic hyperplastic candidosis/candidiasis (Candidal leukoplakia). Crit Rev Oral Biol Med 2003; 14: 253–67
- Ghannoum MA, Abu-Elteen KH. Pathogenicity determinants of Candida. Mycoses 1990; 33: 265–82
- Brassart D, Woltz A, Golliard M, Neeser JR. In vitro inhibition of adhesion of Candida albicans clinical isolates to human buccal epithelial cells by Fucα1→2Galβ-bearing complex carbohydrates. Infect Immun 1991; 59: 1605–13
- Tosh FD, Douglas IJ. Characterization of a fucoside-binding adhesin of Candida albicans. Infect Immun 1992; 60: 4734–9
- Miyakawa Y, Kuribayashi T, Kagaya K, Suzuki M. Role of specific determinants in mannan of Candida albicans serotype A in adherence to buccal epithelial cells. Infect Immun 1992; 60: 2493–9
- Klotz SA, Hein RC, Smith RL, Rouse JB. The fibronectin adhesin of Candida albicans. Infect Immun 1994; 62: 4679–81
- Lopez-Ribot JL, Chaffin WL. Binding of the extracellular matrix component entactin to Candida albicans. Infect Immun 1994; 62: 4564–71
- Gustafson KS, Vercelloti GM, Bendel CM, Hostetter MK. Molecular mimicry in Candida albicans. Role of an integrin analogue in adhesion of yeast to human endothelium. J Clin Invest 1991; 87: 1896–902
- Hoffman MP, Haidaris CG. Analysis of Candida albicans adhesion to salivary mucin. Infect Immun 1993; 61: 1940–9
- Hoffman MP, Haidaris CG. Identification and characterization of a Candida albicans-binding proteoglycan secreted from rat submandibular salivary glands. Infect Immun 1994; 62: 828–36
- Cannon RD, Nand AK, Jenkinson HF. Adherence of Candida albicans to human salivary components adsorbed to hydroxylapatite. Microbiology 1995; 141: 213–19
- Olsen I. Oral adhesion of yeast. Acta Odontol Scand 1990; 48: 45–56
- Samaranayake LP, McCourtie J, MacFarlane TW. Factors affecting the in vitro adherence of Candida albicans to acrylic surfaces. Arch Oral Biol 1980; 25: 611–15
- Samaranayake LP, MacFarlane TW. The effect of dietary carbohydrates on the in vitro adhesion of Candida albicans to epithelial cells. J Med Microbiol 1982; 15: 511–17
- Douglas LJ, Houston JG, McCourtie J. Adherence of Candida albicans to human buccal epithelial cells after growth on different carbon sources. FEMS Microbiol Lett 1981; 12: 241–3
- McCourtie J, Douglas LJ. Relationship between cell surface composition of Candida albicans and adherence to acrylic after growth on different carbon sources. Infect Immun 1981; 32: 1234–41
- McCourtie J, Douglas LJ. Relationship between cell surface composition, adherence, and virulence of Candida albicans. Infect Immun 1984; 45: 6–12
- Tronchin G, Poulain D, Vernes A. Cytochemical and ultrastructural studies of Candida albicans: III. Evidence for modifications of the cell wall coat during adherence to human buccal epithelial cells. Arch Microbiol 1984; 139: 221–4
- McCourtie J, Douglas LJ. Extracellular polymer of Candida albicans: isolation, analysis and role in adhesion. J Gen Microbiol 1985; 131: 495–503
- Al-Abeid HM, Abu-Elteen KH, Elkarmi AZ, Hamad MA. Isolation and characterization of Candida spp. in Jordanian cancer patients: prevalence, pathogenic determinants, and antifungal sensitivity. Jpn J Infect Dis 2004; 57: 279–84
- Abu-Elteen KH, Elkarmi AZ, Hamad MA. Characterization of phenotype-based pathogenic determinants of various Candida albicans strains in Jordan. Jpn J Infect Dis 2001; 54: 229–36
- Samaranayake LP, Hughes A, Weetman DA, MacFarlane TW. Growth and acid production of Candida species in human saliva supplemented with glucose. J Oral Pathol 1986; 15: 251–4
- Marsh P, Martin M. Oral microbiology3rd edn. Chapman and Hall, London 1992
- Makinen KK, Ojanotko A, Vidgren H. Effect of xylitol on the growth of three oral strains of Candida albicans. J Dent Res 1975; 54: 1239
- Larmas M, Makinen KK, Scheinin A. Turku sugar studies. VIII. Principal microbiological findings. Acta Odontol Scand 1976; 34: 285–328
- Vargas SL, Patrick CC, Ayers GD, Hughes WT. Modulating effect of dietary carbohydrate supplementation on Candida albicans colonization and invasion in a neutropenic mouse model. Infect Immun 1993; 61: 619–26
- Ameglio F, Di Giorgio C, Terzaroli P, Gandolfo GM. “Giant cells” production by Candida albicans cultured in xylitol. Microbiologica 1990; 13: 343–6
- Ghannoum MA, Abu-Elteen K. Effect of growth of Candida spp. in the presence of various glucocorticoids on the adherence to human buccal epithelial cells. Mycopathologia 1987; 98: 171–8
- King RD, Lee JC, Morris AL. Adherence of Candida albicans and other Candida species to mucosal epithelial cells. Infect Immun 1980; 27: 667–74
- Segal E, Lehrer N, Ofek I. Adherence of Candida albicans to human vaginal epithelial cells: inhibition by amino sugars. Exp Cell Biol 1982; 50: 13–17
- Tokunaga, M, Niimi, M, Koike, H. Electron microscopic observation of adherence of Candida albicans to human buccal epithelial cells using rapid freezing technique. In: X Congress International Society for Human and Animal Mycology, Barcelona, 1988, Poster No. 122.
- Kennedy MJ. Adhesion and association mechanisms of Candida albicans. Curr Top Med Mycol 1988; 2: 73–169
- Cormane RH, Goslings RO. Factors influencing the growth of Candida albicans. Sabouraudia 1963; 3: 52–63
- Olsen I, Birkeland JM. Initiation and aggravation of denture stomatitis by sucrose rinses. Scand J Dent Res 1976; 84: 94–7
- Reed BD, Slattery ML, French TK. The association between dietary intake and reported history of Candida vulvovaginitis. J Fam Pract 1989; 29: 509–15