Abstract
Dental wear facets on the occlusal surface of premolars and molars are traces of their main function, the mastication and therefore reflect masticatory movements and also paramasticatory (i.e. non-dietary use of teeth) behavior. Here we present the Modular Wear Facet Nomenclature applicable to most mammalian dentitions. Topographic positions of wear facets in relation to the major cusps and crests of the teeth are used to designate the areas of the occlusal surface the facets occupy (e.g. their mesial, distal, lingual, or buccal position). Previous published systems for labeling wear facets have been inconsistent with each other. Therefore, we provide a synoptic review of the most widely-used terminologies, and introduce the alternative Modular Wear Facet Nomenclature. This nomenclature aims to overcome the difficulties caused by the existing inconsistent wear facet terminologies. Our new approach is applicable to dentitions where the occlusal morphology does not change significantly for most of the lifetime of the animal. In those dentitions, the primary occlusal surfaces are not significantly modified as wear facets become more extensive with wearing. This appears to be a common pattern in pre-tribosphenic, tribosphenic molars, and the teeth derived from tribosphenic precursors (e.g. bunodont molar morphologies). In teeth where the secondary occlusal surface is functionally intensely modified (i.e. high-crowned and evergrowing teeth with large areas of dentine exposed) any facet labeling system appears to be challenging, since the identification of individual facets is blurred and their spatial position may be indeterminable.
Introduction
The post-eruptive shaping of teeth occurs through chewing activity (e.g. mastication) and occlusal relationships of antagonistic crowns, which results in characteristic complementary wear facets on the occlusal surface of opposing teeth. Wear facets usually develop during force-fit processes between antagonistic surfaces either due to an attritional tooth-to-tooth guidance with maybe a minimum number of trapped abrasives between both surfaces, or through abrasional tooth-substrate-tooth contacts engaging a resistant alimentary bolus (Maier & Schneck Citation1981). A facet pattern starts to develop as soon as the crowns are erupted and antagonists begin to occlude. Fortelius (Citation1985) differentiated between primary and secondary occlusal surfaces. Facets on primary surfaces develop gradually within the enamel cover and later expose the dentine. Facets on a secondary surface expose the dentine after a relatively short period of wear and result in an occlusal surface composed of enamel and dentine fields, which remains constant for a relatively long period of time. These secondary surfaces are specialized wear facets that are mostly developed in taxa with a horizontal power stroke movement, independently from the direction (Koenigswald Citation2016, this volume). These surfaces are formed by complicated arrangements of enamel crests, and dentine fields that require initial wear to become fully functional. It therefore becomes very difficult to identify individual facets.
Ever since Cope (Citation1883) and Osborn (Citation1888) developed the model of dental-cusp nomenclature, dental researchers suggested various ways to describe features of teeth to have a common language for discussion (see also Ungar Citation2010), and as Butler noted adequately ‘Language is for communication’ (Butler Citation1978, p. 451). During the last century, various attempts were undertaken to refine the nomenclature in order to better reflect mammalian dental evolution (e.g. Vandebroek Citation1967; Crompton & Jenkins Citation1968; Szalay Citation1969; Hershkovitz Citation1971; Butler Citation1978; Maier & Schneck Citation1981; Van Valen Citation1982). However, describing the occlusal surface in detail is a challenging task, because various factors influence their formation and there is a wide variation among different taxa. It was frequently discussed that ontogeny (Winkler & Kaiser Citation2011), ingested diet (Fortelius Citation1987), habitat (Kaiser & Schulz Citation2006; Schulz & Kaiser Citation2013), and even tooth position (Kullmer et al. Citation2009; Taylor et al. Citation2013) have an influence on the formation of the occlusal surface and its characteristic features. A consistent nomenclature is highly desirable as a labeling system to address broader evolutionary and ecological questions in various disciplines. It would enable discussion of specific structural elements on the surface and their functional-adaptive interpretation in the context of, for example, evolutionary stages or biomechanical changes. In 2008 a group of researchers (Research Unit 771 of the German Research Foundation) from various disciplines (e.g. palaeontology, biology, anthropology, agricultural and engineering science) encountered the difficulties in communicating occlusal surface characteristics and attempted to find a common language. With the goal to develop a consistent nomenclature that can also accommodate new structures from new fossil findings, an effort has been made to identify common ground for better communication of occlusal surface characteristics. During numerous intense discussions and meetings between 2008 and 2014 we discussed the new modular system to name wear facets to facilitate the communication between disciplines – and we found this system to be flexible and open to a wide range of future applications. Many others of the DFG Research Unit 771 have contributed to these discussions. We thank the following individuals: Ulrike Anders, Janka Brinkkötter, Romina Hielscher, Kai Jäger, Anne Schubert, Achim Schwermann, Leonie Schwermann (all University of Bonn); Elehna Bethune, Caroline Braune, Ivan Calandra, Juan Pablo Gailer, Volker Hallay, Christina Landwehr, Mirella Skiba (all University of Hamburg); Jürgen Hummel (University of Göttingen); Pascal Brehm, Laura Hauser, Ericson Hölzchen, Sarah Urban (all Senckenberg Research Institute and Natural History Museum Frankfurt).
History of wear facet analyses and labeling concepts
Butler (Citation1952) and Mills (Citation1955) first documented the correspondence of wear facet patterns on the cusps of antagonistic upper and lower molars. Mills (Citation1955) described facets as flat, highly polished areas on the molar cusps which are visible under low-power magnification but also macroscopically under oblique light. Butler (Citation1973) restricted the term facet to those areas where wear is produced through contacts between opposing teeth. He described the facet areas as typically flat, reflecting light, and having striations (Figure ); according to his functional interpretation facets are indicative of relative occlusal movements. Fortelius (Citation1985) followed the functional interpretations of Butler (Citation1973) and corroborated the idea that facets are wear-dependent, and that their orientation is determined by the interactive wear against one or several other facets. Generally, wear is described as a mechanical and/or chemical process resulting in material loss (Williams Citation2005).
Figure 1. Light reflecting dental wear facets on the occlusal surface of different selected mammalian taxa (fossil and extant). (A) Wear facet extended along the protoloph (PRL-m), the metaloph (MTL-m) and the parastyl (PAS-dl) of Lophiodon, Perissodactyla (specimen HLMD-Ro 2, collection Hessisches Landesmuseum Darmstadt, Germany). (B) Wear facets on the lingual side of the cusps (l1-l; l2-l) of the lower post-canine teeth of Kayentatherium, Tritylodontidae (cast of specimen USNM 317213, United States National Museum collection Washington DC, USA). (C) Wear facets in the parastyle (PAS-l), the protocone (PR-m) and hypocone (HY-m) of a stem equid from Europe, Perissodactyla (specimen STIPB M7410, University of Bonn, Steinmann-Institute teaching collection, Germany). (D) Wear surface within the guiding rail between the cusps (b3-l; b4-l) of the lower molar of Neoplagiaulax, Multituberculata (SEM image of specimen NHMB CY870, collection Naturhistorisches Museum Basel, Switzerland). (E) Wear facet on the mesial side of an upper molar (PAC-d) and on the mesial side of a lower molar (pacd-m) of Henkelotherium, Dryolestoidea (specimens GuiMam 1109 and GuiMam 1100, currently housed in the Steinmann-Institute, University of Bonn, Germany). F+G) Facets of the metacone (ME-ml) and paracone (PA-ml) in the upper M2 and on the metaconid (md-db) of the m1 and the protoconid (pr-db) of the m3 in the dentition of Pongo, Primates (specimen SMF-Schoch-1975, collection Senckenberg Research Institute and Natural History Museum Frankfurt, Germany). Orientation: ∟ indicates buccal (upward) and mesial (left or right) for each example.
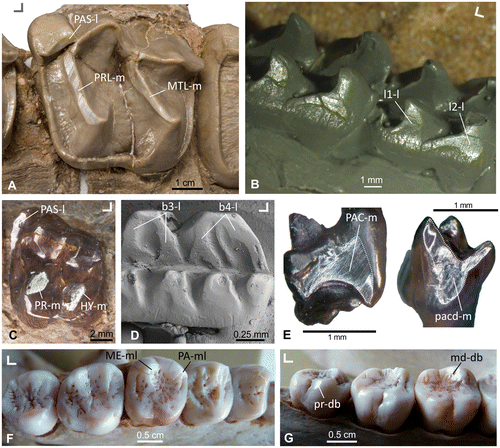
Figure 2. (A, B) Schematic illustration of the wear facets on the upper and lower first molar surfaces of a stem equid after Butler (Citation1952). (C, D) The same facet pattern translated into the new modular facet system and coloration of the matching antagonistic facet pairs. (E) Circle of directions for the positional module of the Modular Wear Facet Nomenclature.
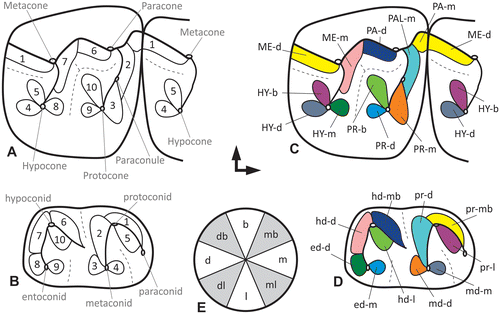
Butler (Citation1952) established a numbering system for facets on upper and lower molars considering the occlusal surface of two succeeding molars as one functional unit [FU] by labeling the basins that form the distal part of one molar and the mesial part of the next molar. For the antagonistic facet pairs on upper and lower molars Butler used the same numbers in his system. Facets 1 to 5 are located at the trigonid structure of the lower molars and the ‘amphicyclix’-basin of the upper molars, whereas facets 6–10 are found at the talonid basin of the lower molars and the ‘mesocyclix’-basin of the upper molar. Butler’s (Citation1952) nomenclature clearly infers a functional correlation between upper and lower teeth. Mills (Citation1955) mapped facets of primate teeth without numbering them, and referred instead to the power stroke phase of mastication in which they are functioning. Later in his comprehensive study on the evolution of the mammalian dentition, Crompton (Citation1971) identified homologous facets and proposed a labeling system in the order of their appearance in evolution. A number of similar systems and nomenclatures followed in order to elucidate the evolutionary development of new functional elements and homologous structures. Gingerich (Citation1974) used Crompton’s (Citation1971) nomenclature for his facet description in Plesiadapis dentitions, but added a buccal-phase (B) and a lingual-phase (L) to the numbers. This is in accordance with Mills (Citation1955) who distinguished also two types of wear facets, as ‘buccal-’ and ‘lingual-phase-facets’. Gingerich (Citation1974) defined following Mills (Citation1955) the buccal-phase-facets as a result of an upward, medial, and slightly forward movement of the lower jaw into centric occlusion on the working side. The lingual-phase-facets are formed during movement of the mandible from centric occlusion, in medial, mesial and slightly downward movement, until disclusion. These movements were termed as ‘Phase I’ (the buccal-phase facets are in contact) and ‘Phase II’ (the lingual-phase facets are in contact) of the power stroke by Kay and Hiiemäe (Citation1974). In addition, Kay and Hiiemäe (Citation1974) expanded Crompton’s (Citation1971) numbering system. While Crompton (Citation1971) defined a maximum of six wear facets on the molars of Didelphodus, Kay and Hiiemäe (Citation1974) – working with primate molars – recognized four additional facets (7–10). On several wear facets of the upper molars, ‘a’ and ‘b’ are appended to the numbering if a facet is split into two areas, even if being formed during the same movement by the same antagonistic structure. For example, if facet 1 of a lower molar shears along the mesio-lingual part of the upper molar paracone, it generates facet 1a and additionally facet 1b during its contact to the mesio-lingual side of the preparaconule crista. Later Kay (Citation1977) described 11 wear facets for cercopithecid molars. In the same work, he described facet 7n which arises on the posthypocrista matching with the premetacristid. A homologous wear facet in Aegyptopithecus was numbered 7 earlier by Kay and Hiiemäe (Citation1974) based on the assumption that it was the same as facet 7 of more primitive primates, which is not the case as shown by Kay (Citation1977). The ‘n’ was added for this ‘new’ facet. Kay and Hiiemäe (Citation1974) also described a facet 10n. In addition, Kay (Citation1977) indicated a wear facet 9 on the distobuccal slope of the protocone and a wear facet X (a mesial extension of facet 9) between the mesiobuccal slope of the protocone and the distolingual slope of the protoconid. Maier (Citation1980) did not use ‘a’ and ‘b’ in his study on prosimian molar facets. He distinguished 12 facets, and numbered Kay’s (Citation1977) facet X as 11 and facet 10n as 12. Additionally, Maier and Schneck (Citation1981, 1982) described a new facet 13 only occurring on hominoid molars. It is positioned on the distal slope of the protocone oblique cristid in upper molars. The corresponding facet on the lower molars occurs on the mesial side of the hypoconulid between the prehypoconulid-cristid and the entohypoconulid-cristid.
Maier and Schneck (Citation1981) also described a facet 4′ for hominoid molars. It develops during Phase I in the mastication cycle, when the buccal slope of the hypoconulid meets with the entometacrista. In their illustration (Citation1981, p. 132, Figure 2) 4′ is marked along the mesiolingual slope of the entometacrista, whereas in their Figure it is positioned at the distolingual slope of the entometacrista. Both represent two different spatial orientations and thus positions in relation to the morphological structure. It is therefore problematic to label them with the same facet number. The former 4′ of their description should rather be labeled as a sub-facet of facet 4, such as facet 4.1, because it is oriented in an almost similar direction as facet 4 and lies on the same morphological area of the tooth surface. As a consequence, only the latter should be labeled facet 4′ as it is positioned directly adjacent to facet 2 in the upper molar different from 4.1, but pointing in similar functional direction. Later Kullmer et al. (Citation2009, p. 601, Figure 1) relabeled facet 4′ of Maier and Schneck (Citation1981) as facet 2.1, because of its functional position and its relation to facet 2.
Figure 3. The Modular Wear Facet Nomenclature applied to the upper and lower tooth rows of a stem equid. (A) 3D surface model of the upper molars 1–3 based on specimen USNM 522988 (United States National Museum collection Washington DC, USA). (B) 3D surface model of the lower molars 1–3 based on specimen STIPB M 6593 (University of Bonn, Steinmann-Institute teaching collection, Germany). Mesial to the right, buccal to the top.
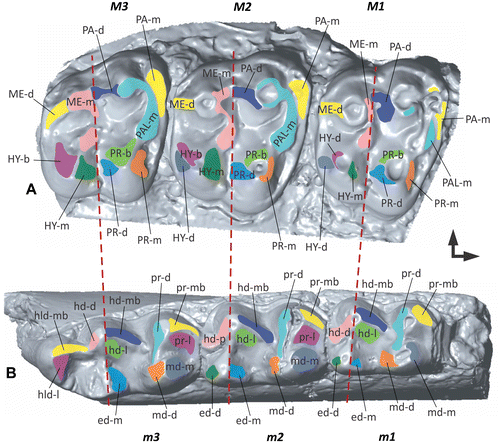
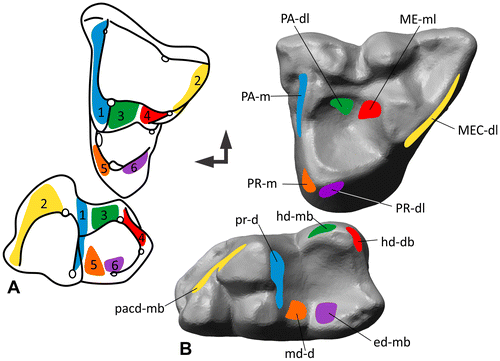
Figure 4. Homologous facets 1–7 after Crompton (Citation1971) translated into the modular dental wear facet system. (A) Matching facet pairs 1–7 on the upper and lower molar of Didelphodus modified from Crompton (Citation1971). (B) Facets found on M2/m2 of Didelphis traced on the polygonal 3D models of the occlusal surface.
Positional relationships of cusps and facets are used as a criterion of homology, but as mentioned above recognition of homologies between groups is severely hindered by the confusing facet nomenclature (Kay Citation1977; Fortelius Citation1985). Butler (Citation1978) proposed a concept of indirect homology for the cusps of mammalian molars. The basic principle is assigning cusps the same name in a specific pattern, based on the topographical position in relation to other elements on the crown and on the functional correspondence with the structures of the antagonistic tooth during occlusion. However, especially in early mammaliaforms the identification of homologous structures is difficult, because of the more or less sudden occurrence of several morphofunctional dental traits. Therefore, some authors erected additional facet nomenclatures for teeth of those groups where structures are difficult to be put in relation to that of tribosphenic mammals, e.g. tritylodonts, haramiyids, multituberculates, mammals with pseudotribosphenic molars (Kermack et al. Citation1965; Crompton & Jenkins Citation1967, 1968; Crompton Citation1972; Gingerich Citation1973; Chow & Rich Citation1982; Butler & Macintyre Citation1994; Wang et al. Citation1998). In most of these studies the authors use a numbering system for the detected facets as well, implying a homology of structures, which is difficult to prove. Numerical systems are easily misleading because the same numbers were used for facets in different positions. In Table a synopsis of the traditional and most commonly used numbering systems is provided. A similar synopsis is found in Hunter and Fortelius (Citation1994), but they did not include the systems of Gingerich (Citation1974), Maier (Citation1980), and Maier and Schneck (Citation1981).
Table 1. Summary of existing occlusal wear-facet nomenclature from cited literature in comparison to the Modular Wear Facet Nomenclature.
Here we propose a modular terminology in which the wear facets are identified and named according to their topographic relationships to the primary and evolutionarily conserved cusps and crests on the teeth. We advocate that the Modular Wear Facet Nomenclature can be more widely applicable across various taxa with disparate dental morphology derived from the tribosphenic precursors, than the previously inconsistent numerical nomenclatures. In contrast to the traditional numbering of functional units that implies their homology, the terminology proposed here identifies the individual facets by their topographic position and inclination. This allows discussion of their functional context, their homology, and their changes during ontogeny and phylogeny more freely. The knowledge of structure and function of the mammalian dentition increased significantly in the last decades by new fossil findings, detailed functional studies on the mammal teeth, and the application of 3D methods. However, we are well aware that establishing a new nomenclature is still a challenge.
Challenges for comparative studies
One general problem of working with post-canine dentitions is that frequently molar facets from different taxa above the genus level are described with different nomenclatures by morphological reasons, by historical precedence, or simply by author’s idiosyncratic preference.
In the paper by Pinto-Llona (Citation2013) the issue of using different labels for the same facets is illustrated. The author reconstructed the palaeodiet of Pleistocene cave bears from Spain and labeled the facets used for his 2D macro- and microwear analyses with two alternative numbering systems, the first of which is Butler’s system (Citation1952) followed by that of Kay (Citation1977) in parentheses. The same method was used by Hunter and Fortelius (Citation1994). New quantitative 2D (Kaiser & Brinkmann Citation2006) and 3D methods for dental occlusion analysis (Kullmer et al. Citation2009, 2012; Fiorenza et al. Citation2011; Benazzi et al. Citation2013) and the analysis of masticatory biomechanics (Schulz & Kaiser Citation2010; Calandra et al. Citation2012; Schulz et al. Citation2013) offer the opportunity to characterize facets in more detail. Kaiser and Brinkmann (Citation2006) introduced a labeling system of occlusal enamel ridge patterns in bovids and equids as a strictly function-orientated system that numbers facets (adopted from Janis Citation1990). This system refers to the sequence of the antagonistic contact of facets, and in addition indicates the longitudinal position of the facet in relation to the cusp. Schulz and Kaiser (Citation2010) applied the labeling system of Kaiser and Brinkmann (Citation2006) to facets of upper and lower teeth that allow 3D surface texture measurements to infer oral behavior (e.g. diet reconstruction and chewing function) based on microscopic wear features. Schulz and Kaiser (Citation2010) stated that facets on the mesial/distal as well as buccal/lingual sides of the cusp tips can be described regarding their functional as well as dietary traits (as indicated by their surface textures). The advantage of the labeling system by Kaiser and Brinkmann (Citation2006) as well as Schulz and Kaiser (Citation2010) is that it can be distinctly differentiated between the two enamel ridges of one side of a cusp in ruminants. However, the system has not been transferred to any other mammal group and thus the potential of its application to different tooth morphologies was not subsequently tested.
Two main issues arise in search of an adequate labeling system: (1) how to refer to already existing nomenclatures, and (2) how to develop a universal wear facet nomenclature that is applicable to a wide range of extinct and extant tooth morphologies. The different labeling systems for homologous facets (see Table ) can impede discussion of functional questions, and lead to misinterpretations. Thus, a labeling system that is applicable to a wide range of tooth morphologies in fossil and extant mammals is of great advantage for future discussion of the evolution of the mammalian dentition. Butler himself (Citation1978, p. 451) stated: ‘Let those who are contemplating the introduction of new names pause to consider whether in so doing they are advancing the subject or making it more difficult to understand’. We aim for a broader comparative nomenclature that helps to describe facets in as many groups as possible to foster quantitative research. The primary purpose is to advance the communication about mammalian molar structures and therefore the communication about the evolution of mammalian mastication.
Description of the new modular system
The Modular Wear Facet Nomenclature for mammalian premolars and molars is based on well-established terms for the evolutionarily conserved landmarks (cusps, crests, basins), although the homology hypotheses of these structures are always subject to further tests with new phylogenetic analyses, or findings of new fossils. In the proposed Modular Wear Facet Nomenclature, cusps and crests are the primary elements for facet naming: (1) the location of the facet on the tooth, and (2) the incident angle of the facet (orientation). The system proposed here combines standard structures, but is flexible and can be expanded with additional information depending on the aim of the study the nomenclature will be used for, e.g. the phase of the chewing cycle (I for ‘Phase I’; II for ‘Phase II’).
We use the classic tribosphenic molar model as an example to illustrate the potential of the new Modular Wear Facet Nomenclature. The first module of the facet name consists of an acronym of the cusp or crest with which the facet is associated. In case of the protocone this would be ‘PR’ following the conventional usage of capital letters for the upper teeth. In case of the protoconid this would be ‘pr’ following the convention of using lowercase letters for the lower teeth (see Table for proposed acronyms). The second module describes the position of the facet in relation to the structure of the first module. We distinguish five main incident angles depicting the facet’s position on the cusp or crest. Identifiers for these angles are ‘m’ for mesial, ‘d’ for distal, ‘b’ for buccal, ‘l’ for lingual and ‘h’ for horizontal. We follow the standard terminology of anatomical orientations in fossil vertebrate dentitions of Smith and Dodson (Citation2003). These identifiers can be combined in order to precisely specify the orientation of the facet. For example, ‘mb’ describes a facet facing in mesiobuccal direction and ‘db’ describes a facet in distobuccal direction (Figure (E)). The first letter thus characterizes the primary incident angle of the facet, the second letter the secondary incident angle, which means that the order is weighted. In combination for the protoconid this would result in ‘pr-m’ as facet term, for the protocone this would result is ‘PR-d’ as facet term. If a user wishes to describe the facet’s incident angle in relation to a crest, the first module is the acronym of the crest name. This can be also expanded to larger elements on the occlusal surface like lophs, fossettes, styles or cingula depending on the terms the user prefers to work with. With increasing molar use, wear facets are often not restricted only to the enamel but can extend into the dentine or even exclusively be on the dentine in later ontogenetic stages. If a user wishes to emphasize the type of tissue bearing the wear facet, an ‘e’- or ‘de’-indicator module can be added. In addition, we propose to color- or pattern-code antagonistic facet pairs for better recognition which parts occlude (Figure ).
Table 2. Short list of proposed abbreviations for the most common structures on mammalian molars.
Application example I (early horse)
In order to demonstrate that the proposed Modular Wear Facet Nomenclature is applicable to a broad variety of mammaliaform dentitions, we translate a classic bunodont example (early horse) that was used by Butler (Citation1952) into the new system (Figures and ).
Butler (Citation1952) identified ten facet pairs and numbered each of his functional wear facets accordingly. He illustrated how the wear facets correspond in different fossil perissodactyl taxa, including stem equids that Butler (Citation1952) assigned to the genus Hyracotherium. Today it is the common understanding that this genus is paraphyletic (MacFadden Citation2005), and consists of several different genera with only slight differences in their molar morphologies (Froehlich Citation2002). We therefore use the terms ‘stem equid’ or ‘early horse’. Butler (Citation1952, p. 800) described his findings as: ‘To each wear-facet on the upper molar there corresponds a wear-facet on the lower molar which occludes with it’. His basic findings can be summarized as follows:
Butler’s functional unit [FU] 1
A facet distal to the metacone on the upper molars and confluent with the facet mesial to the paracone of the following molar occludes with the mesiobuccal face of the protoconid. Translated to the modular system, this would be facet ME-d, which is contiguous with facet PA-m of the adjacent tooth in the upper molar, forming a functional unit. Both together match the facet pr-mb of the lower molar.
FU 2 and 3
The facet mesial to the paraconule lies next to the facet mesial to the protocone, and depending on the wear stage they can be confluent and form one facet. They occlude with the facet on the distal face of the protoconid, and with the facet on the distobuccal face of the metaconid. Translated into the modular system PAL-m matches pr-d, and PR-m matches md-m.
FU 4
The facet distal to the hypocone of the upper molars occludes with the facet mesial to the metaconid on the lower molars. Translated into the modular system HY-d matches md-m.
FU 5
On the buccal side of the hypocone on the upper molars lies the facet that matches the lingual facet of the protoconid. Translated into the modular system HY-b occludes with pr-l.
FU 6
Butler (Citation1952) further described a facet on the distal face on the paracone, which occludes with the facet on the mesiobuccal face of the hypoconid. Translated into the modular system the facet of the upper molar is PA-l, and hd-mb for the corresponding facet on the lower molar.
FU 7 and 8
The facet mesial to the metacone occludes with the facet on the distal face of the hypoconid. Depending on the wear stage facet pair 7 can be confluent with facet pair 8, which comprises the facet mesial to the hypocone that is matching the facet distal to the entoconid. Translated to the modular system the facets of pair 7 are ME-m and hd-d, and the facets of pair 8 are HY-m and ed-d.
FU 9
The distal facet on the protocone occludes with the facet on the mesial face of the entoconid. Translated into the modular system pair 9 is PR-d for the facet on the upper molar, and ed-m on the lower.
FU 10
Facet 10 on the upper molar lies on the buccal side of the protocone occluding with the lingual facet on the hypoconid of the lower molars. Translated into the modular system this pair is PR-b and hd-l.
Application example II (Didelphis)
Crompton (Citation1971) used a different approach than Butler (Citation1952). Rather than looking at facets as functional units he identified homologous facets and proposed a labeling system in the order of their appearance in evolution. His classic example for the demonstration of his homologous facet nomenclature is Didelphodus. In order to demonstrate the modular wear facet system, we use molars of Didelphis in comparison (Figure ).
Facet 1
Crompton (Citation1971) described one large facet on the mesial side of the paracone of the upper molars, extending along the paracrista in Didelphodus. This wear surface matches the facet extending along the metacristid of the lower molar, distal to the protoconid and metaconid. However, in our example of Didelphis facet 1 on the upper molar is smaller and lies mesial to the paracone, and the paracrista is very short. Translated to the modular system: PA-m matches mecd-d.
Facet 2
On the distal side of the metacone lies facet 2 extending along the metacrista with a distolingual orientation. Facet 2 occludes with the wear facet along the paracristid, mesial to the protoconid and the paraconid, of the lower molars. This wear surface is facing in an mesiobuccal direction. Translated to the modular system, this facet pair would be labeled MEC-dl for the upper and pacd-mb for the lower molar.
Facet 3
Distolingual to the paracone lies facet 3 that matches the wear surface mesiobuccal to the hypoconid. PA-dl and hd-mb according to the labeling system proposed here.
Facet 4
Mesiolingual to the metacone lies facet 4 that matches the wear surface distobuccal to the hypoconid. ME-ml and hd-db according to the labeling system proposed here. Because facet 4 is extended along the hypocristid, an alternative facet name is hyd-db in the lower molars, depending on the user’s intention.
Facet 5
Mesial to the protocone of the upper molar lies a wear surface that is named facet 5 after Crompton (Citation1971). It matches the wear surface distal to the metaconid of the lower molar. Translated to the modular system this facet pair is PR-m and md-d.
Facet 6
Distolingual to the protocone of the upper molars of Didelphis lies facet 6. It occludes with the facet mesiobuccal to the entoconid inside the talonid basin of the lower molars. Translated to the modular system this facet pair is PR-dl and ed-mb.
Discussion
The description and analysis of wear facets on mammalian premolars and molars had been hampered by an inconsistent nomenclature. The Modular Wear Facet Nomenclature is developed to overcome these difficulties by establishing a system of acronyms that are referencing the topographic position of the wear facets relative to the primary tooth crown landmarks with well-established homology. Combination of acronym modules allows the exact topographic characterization of wear facets independent of the taxa studied. The new system is applicable to premolars and molars of the basic tribosphenic pattern or moderately modified tribosphenic (bunodont) pattern, which largely retain the primary occlusal surface. These are the majority of Mesozoic mammaliaforms and mammals, Paleogene representatives of many modern therian groups, insectivorous marsupials and placentals, many rodents as well as primates. For highly modified teeth with a more uniform functional surface and a mainly grinding function (such as hypsodont molars of herbivores) the proposed modular nomenclatorial system is less suitable, because various tooth elements may be incorporated into a single functional surface (i.e. complex enamel bands, alternating dentine and enamel ridges, flat occlusal surface). In many highly-modified mammalian cheek-teeth, the individual elements of the primary occlusal surface of the ancestral tribosphenic pattern are no longer recognizable (e.g. elephantid molars).
The Modular Wear Facet Nomenclature does not imply a priori hypothesis of homology of wear facets. Rather, it is primarily descriptive, and conveys the topographic relationships to the structures with previously established homology, such as the primary cusps and their crests. Homology of wear facets is determined by the structures they occur on (e.g. cusps, basin surfaces, crests, lophs etc.). In this manner, the system (and the implicit homology of wear facets) can be further changed and re-adapted as the homology hypotheses of the primary structures. The modular system is highly flexible and can be expanded with additional information if necessary (e.g. phase of chewing cycle, I or II; enamel, ‘e’; dentine, ‘de’). If the homology is uncertain, specific terms can also be used (e.g. cusp numbering system in rodents). The high flexibility and simple modular system makes the new nomenclature highly adaptable to a wide array of occlusal surfaces and changes in interpretations of tooth crown structures.
Conclusion
We hereby propose the Modular Wear Facet Nomenclature that designates wear facets in topographic relation to primary landmarks. As such, this nomenclature is more broadly applicable to a wider variety of mammalian molar patterns. This system can be further adapted to other tooth positions than only cheek teeth. Given the different and inconsistent numerical systems to identify wear facets in the literature, the newly proposed topographic system offers an advantage for descriptive purposes, and is more conducive for comparisons, and it therefore can improve scientific communication. The Modular Wear Facet Nomenclature proposed here for the mammalian molar dentition can easily be expanded to the facets on incisors and canines. The location of the facet is based on conventionally accepted and well-established terms for tooth elements (e.g. cusps, crests, basins). If the homology of tooth elements is uncertain, specific terms can also be used (for example cusp numbering system in rodents). The tooth elements are indicated by acronyms, with capital letters for upper teeth and lower case letters for lowers teeth (see Table for proposed selected acronyms, and Table 3 for full list in the Supplementary Material). The topographic position of the facet relative to certain primary structure (cusp or crest) can also be used to define and name the wear facets for canines and incisors. Because the topographic wear facet nomenclature is modular, it can be expanded with additional information to convey the distinctive phases of masticatory movement (e.g. I for ‘Phase’ I; II for ‘Phase II’), or according to the tissue types of the wear facets (‘e’ for enamel; ‘de’ for dentine).
Funding
This work was supported by the Deutsche Forschungsgemeinschaft (DFG; German Research Foundation) and is publication No. 89 of the DFG Research Unit 771 ‘Function and performance enhancement in the mammalian dentition – phylogenetic and ontogenetic impact on the masticatory apparatus’.
Supplemental data
Supplemental data for this article can be accessed http://dx.doi.org/10.1080/08912963.2017.1302442.
Disclosure statement
No potential conflict of interest was reported by the authors.
FOR771_Table_3_extended_Schultz_etal_revised.docx
Download MS Word (49.9 KB)Acknowledgements
During the funding period of our Research Unit from 2008 to 2014, we enjoyed help and support of various curators that made valuable material accessible. We want to name particularly those related to the materials mentioned in this paper and thank the responsible curators of the following museums who allowed us to study dentitions of fossil and extant mammals under their care: G. Gruber (Hessisches Landesmuseum Darmstadt, Germany), Loïc Costeur (Naturhistorisches Museum Basel, Switzerland), David Bohaska (Smithsonian National Museum of Natural History Washington DC, USA). We are grateful to reviewers M. Fortelius, L. Holbrook, J. Hooker and Z.-X. Luo for their comments that helped to improve our manuscript.
References
- Averianov A, Lopatin A. 2005. Eocene lagomorphs (Mammalia) of Asia: 1. Aktashmys (Strenulagidae fam. nov.). Paleontol J. 39:81–90.
- Bärmann EV, Rößner GE. 2011. Dental nomenclature in Ruminantia: towards a standard terminological framework. Mamm Biol. 76:762–768.
- Benazzi S, Kullmer O, Schulz D, Gruppioni G, Weber GW. 2013. Technical note: individual tooth macrowear pattern guides the reconstruction of Sts 52 (Australopithecus africanus) Dental Arches. Am J Phys Anthropol. 150:324–329.10.1002/ajpa.v150.2
- Breda M. 2008. Palaeoecology and palaeoethology of the Plio-Pleistocene genus Cervalces (Cervidae, Mammalia) in Eurasia. J Vertebr Paleontol. 28:886–899.10.1671/0272-4634(2008)28[886:PAPOTP]2.0.CO;2
- Butler PM. 1939. Studies of the mammalian dentition – differentiation of the post-canine dentition. Proc Zool Soc London B. 109:1–36.
- Butler PM. 1952. The milk-molars of Perissodactyla with remarks on molar occlusion. Proc Zool Soc London B. 121:777–817.
- Butler PM. 1973. Molar wear facets of early tertiary North American primates. In: Symposia of the 4th International Congress of Primatology; Craniofacial Biology of Primates; 1972 Aug; Portland, OR: Karger, Basel. Vol 3; 1–27.
- Butler PM. 1978. Molar cusp nomenclature and homology. In: Butler PM, Joysey KA, editors. Studies in the development, function and evolution of teeth. London: Academic Press; p. 441–453.
- Butler PM. 2000. The evolution of tooth shape and tooth function in primates. In: Teaford M, Moya Smith M, Ferguson MWJ, editors. Development function and evolution of teeth. New York (NY): Cambridge Press; p. 201–211.10.1017/CBO9780511542626
- Butler PM, Macintyre GT. 1994. Review of the British Haramiyidae (?Mammalia, Allotheria), their molar occlusion and relationships. Philos Trans R Soc London B. 345:433–458.10.1098/rstb.1994.0119
- Calandra I, Schulz E, Pinnow M, Krohn S, Kaiser TM. 2012. Teasing apart the contributions of hard dietary items on 3D dental microtextures in primates. J Hum Evol. 63:85–98.10.1016/j.jhevol.2012.05.001
- Chow M, Rich TH. 1982. Shuotherium dongi, n. gen. and sp., a therian with pseudo-tribosphenic molars from the Jurassic of Sichuan. China Austral Mammal. 5:127–142.
- Cope ED. 1883. On the trituberculate type of molar tooth in the Mammalia. Proc Am Philos Soc. 21:324–326.
- Crompton, AW. 1971. The origin of the tribosphenic molar. In: Kermack DM, Kermack KA, editors. Early mammals. Zool J Linn Soc. Academic Press. Vol. 50, supplement 1; p. 65–87.
- Crompton AW. 1972. Postcanine occlusion in cynodonts and tritylodontids. Bull Brit Mus Nat Hist Geology. 21:29–71.
- Crompton AW, Jenkins FA. 1967. American Jurassic symmetrodonts and Rhaetic “pantotheres”. Science. 155:1006–1009.10.1126/science.155.3765.1006
- Crompton AW, Jenkins FA. 1968. Molar occlusion in Late Triassic mammals. Biol Rev. 43:427–458.10.1111/brv.1968.43.issue-4
- Crompton AW, Hiiemäe K. 1970. Molar occlusion and mandibular movements during occlusion in the American opossum, Didelphis marsupialis L. Zool J Linn Soc. 49:21–47.
- Crompton AW, Kielan-Jaworowska Z. 1978. Molar structure and occlusion in Cretaceous therian mammals. In: Butler PM, Josey KA, editors. Studies in the development, function and evolution of the teeth. London: Academic Press; p. 249–287.
- Davis BM. 2012. Micro-computed tomography reveals a diversity of Peramuran mammals from the Purbeck Group (Berriasian) of England. Palaeontology. 55:789–817.10.1111/pala.2012.55.issue-4
- Fiorenza L, Benazzi S, Kullmer O. 2011. Para-masticatory wear facets and their functional significance in hunter–gatherer maxillary molars. J Archeol Sci. 38:2182–2189.10.1016/j.jas.2011.03.012
- Fortelius M. 1985. Ungulate cheek teeth: development, functional, and evolutionary interrelations. Acta Zool Fennica. 180:1–76.
- Fortelius M. 1987. A note on the scaling of dental wear. Evol Theor. 8:73–75.
- Freudenthal M, Martin-Suarez E, Bendala N. 2002. Estimating age through tooth wear. A pilot study on tooth abrasion in Apodemus (Rodentia, Mammalia). Mammalia. 66:275.
- Froehlich DJ. 2002. Quo vadis eohippus? The systematics and taxonomy of the early Eocene equids (Perissodactyla). Zool J Linn Soc. 134:141–256.10.1046/j.1096-3642.2002.00005.x
- Gingerich PD. 1973. Molar occlusion and function in the Jurassic mammal Docodon. J Mammal. 54:1008–1013.10.2307/1379107
- Gingerich PD. 1974. Dental function in the Palaeocene primate Plesiadapis. In: Martin RD, Doyle GA, Walker AC, editors. Prosimian biology. London: Duckworth; p. 531–541.
- Hershkovitz P. 1971. Basic crown patterns and cusp homologies of mammalian teeth. In: Dahlberg AA, editor. Dental morphology and evolution. Chicago, IL: University of Chicago Press; p. 95–150.
- Hooker JJ, Russell DE. 2012. Early Palaeogene Louisinidae (Macroscelidea, Mammalia), their relationships and north European diversity. Zool J Linn Soc. 164:856–936.10.1111/zoj.2012.164.issue-4
- Hunter JP, Fortelius M. 1994. Comparative dental occlusal morphology, facet development, and microwear in two sympatric species of Listriodon (Mammalia: Suidae) from the middle Miocene of western Anatolia (Turkey). J Vert Paleontol. 14:105–126.10.1080/02724634.1994.10011541
- Janis C. 1990. The correlation between diet and dental wear in herbivorous mammals, and its relationship to the determination of diets of extinct species. In: Boucot A, editor. Evolutionary paleobiology of behavior and coevolution. Amsterdam: Elsevier; p. 241–259.
- Kaiser TM, Brinkmann G. 2006. Measuring dental wear equilibriums – the use of industrial surface texture parameters to infer the diets of fossil mammals. Palaeogeogr Palaeoclim Palaeoecol. 239:221–240.10.1016/j.palaeo.2006.01.013
- Kaiser TM, Schulz E. 2006. Tooth wear gradients in zebras as an environmental proxy – a pilot study. Mitt Hamburg Zool Mus Inst. 103:187–210.
- Kay RF. 1977. The evolution of molar occlusion in the Cercopithecidae and early Catharines. Am J Phys Anthropol. 46:327–352.10.1002/(ISSN)1096-8644
- Kay RF, Hiiemäe KM. 1974. Jaw movement and tooth use in recent and fossil primates. Am J Phys Anthropol. 40:227–256.10.1002/(ISSN)1096-8644
- Kemp TS. 2005. The origin and evolution of mammals. Oxford: Oxford Univ Press.
- Kermack KA, Lees PM, Mussett F. 1965. Aegialodon dawsoni, a new Trituberculosectorial tooth from the Lower Wealden. Proc Roy Soc B. 162:535–554.10.1098/rspb.1965.0055
- Koenigswald WV. 2016. Specialized wear facets and late ontogeny in mammalian dentitions. Hist Biol. doi:10.1080/08912963.08912016.01256399.
- Kullmer O, Benazzi S, Fiorenza L, Schulz D, Bacso S, Winzen O. 2009. Technical note: occlusal fingerprint analysis: quantification of tooth wear pattern. Am J Phys Anthropol. 139:600–605.10.1002/ajpa.v139:4
- Kullmer O, Schulz D, Benazzi S. 2012. An experimental approach to evaluate the correspondence between wear facet position and occlusal movements. Anat Rec. 295:846–852.10.1002/ar.v295.5
- MacFadden BJ. 2005. Fossil horses-evidence for evolution. Science. 307:1728–1730.10.1126/science.1105458
- Maier W. 1980. Konstruktionsmorphologische Untersuchungen am Gebiß der rezenten Prosimiae (Primates) [Constructional and morphological investigations of the dentition of extant Prosimiae (Primates)]. Abh Senckenberg Naturforsch Gesellschaft. 538:1–158.
- Maier W, Schneck G. 1981. Konstruktionsmorphologische Untersuchungen am Gebiß der hominoiden Primaten [Constructional and morphological investigations of the hominoid dentition]. Zeitschr Morphol Anthropol. 72:127–169.
- Maier W, Schneck G. 1982. Functional morphology of hominoid dentitions. J Hum Evol. 11:693–696.10.1016/S0047-2484(82)80057-2
- Mihlbachler MC. 2008. Species taxonomy, phylogeny, and biogeography of the Brontotheriidae (Mammalia: Perissodactyla). Bull Am Mus Nat Hist. 311:1–475.10.1206/0003-0090(2008)501[1:STPABO]2.0.CO;2
- Mills JRE. 1955. Ideal dental occlusion in the primates. Dent Practitioner. 6:47–61.
- Mills JRE. 1966. The functional occlusion of the teeth of Insectivora. J Linn Soc London Zool. 46:1–25.10.1111/zoj.1966.46.issue-308
- Osborn HF. 1888. The evolution of mammalian molars to and from the tritubercular type. Am Nat. 22:1067–1079.10.1086/274831
- Osborn HF. 1897. Trituberculy: a review dedicated to the late professor cope. Am Nat. 31:993–1016.10.1086/276747
- Osborn HF. 1907. Evolution of the mammalian molar teeth. New York (NY): MacMillan.
- Patterson B. 1956. Early Cretaceous mammals and the evolution of mammalian molar teeth. Fieldiana. 13:1–105.
- Pérez ME, Vucetich MG, Kramarz AG. 2010. The first Eocardiidae (Rodentia) in the Colhuehuapian (early Miocene) of Bryn Gwyn (northern Chubut, Argentina) and the early evolution of the peculiar cavioid rodents. J Vert Paleontol. 30:528–534.10.1080/02724631003618223
- Pickford M. 1983. On the origins of Hippopotamidae together with descriptions of two new species, a new genus and a new subfamily from the Miocene of Kenya. Geobios. 16:193–217.10.1016/S0016-6995(83)80019-9
- Pinto-Llona AC. 2013. Macrowear and occlusal microwear on teeth of cave bears Ursus spelaeus and brown bears Ursus arctos: Inferences concerning diet. Palaeogeogr Palaeoclim Palaeoecol. 370:41–50.10.1016/j.palaeo.2012.11.017
- Radinsky LB. 1969. The early evolution of the Perissodactyla. Evolution. 23:308–328.10.2307/2406794
- Rincon AF, Bloch JI, Macfadden BJ, Jaramillo CA. 2013. First Central American record of Anthracotheriidae (Mammalia, Bothriodontinae) from the early Miocene of Panama. J Vert Paleontol. 33:421–433.10.1080/02724634.2013.722573
- Sánchez IM, Morales J. 2008. Micromeryx azanzae sp. nov. (Ruminantia: Moschidae) from the middle-upper Miocene of Spain, and the first description of the cranium of Micromeryx. J Vert Paleontol. 28:873–885.10.1671/0272-4634(2008)28[873:MASNRM]2.0.CO;2
- Schultz JA. 2012. Funktionelle Morphologie und Abnutzungsmuster prätribosphenischer Molaren am Beispiel der Dryolestida (Mammalia, Cladotheria) [ dissertation thesis]. Bonn: Rheinische Friedrich-Wilhelms-Universität Bonn.
- Schulz E, Calandra I, Kaiser TM. 2013. Feeding ecology and chewing mechanics in hoofed mammals: 3D tribology of enamel wear. Wear. 300:169–179.10.1016/j.wear.2013.01.115
- Schulz E, Kaiser TM. 2010. Applying tribology to teeth of hoofed mammals. Scanning. 32:162–182.10.1002/sca.v32:4
- Schulz E, Kaiser TM. 2013. Historical distribution, habitat requirements and feeding ecology of the genus Equus (Perissodactyla). Mamm Rev. 43:111–123.10.1111/mam.2013.43.issue-2
- Smith JB, Dodson P. 2003. A proposal for a standard terminology of anatomical notation and orientation in fossil vertebrate dentitions. J Vert Paleontol. 23:1–12.10.1671/0272-4634(2003)23[1:APFAST]2.0.CO;2
- Szalay FS. 1969. Origin and evolution of function of the mesonychid condylarth feeding mechanism. Evolution. 23:703–720.10.2307/2406864
- Thalmann U. 1994. Die Primaten aus dem eozänen Geiseltal bei Halle/Saale (Deutschland) [Eocene primates from Geiseltal near Halle/Saale (Germany)]. Cour Forsch-Inst Senckenberg. 175:1–161.
- Taylor LA, Kaiser TM, Schwitzer C, Müller DW, Codron D, Clauss M, Schulz E. 2013. Detecting inter-cusp and inter-tooth wear patterns in Rhinocerotids. PLoS One. 8:e80921.10.1371/journal.pone.0080921
- Thenius E. 1989. Zähne und Gebiss der Säugetiere. Berlin: de Gruyter.
- Ungar P. 2010. Mammal teeth. Baltimore (MD): The Johns Hopkins University Press.
- Vandebroek G. 1967. Origin of the cusps and crests of the tribosphenic molar. J Dent Res. 46:796–804.
- Van Valen LM. 1982. Homology and causes. J Morphol. 173:305–312.10.1002/(ISSN)1097-4687
- Wang Y-Q, Clemens WA, Hu Y-M, Li C-K. 1998. A probable pseudo-tribosphenic upper molar from the Late Jurassic of China and the early radiation of the Holotheria. J Vert Paleontol. 18:777–787.10.1080/02724634.1998.10011106
- Williams JA. 2005. Wear and wear particles – some fundamentals. Tribol Internat. 38:863–870.10.1016/j.triboint.2005.03.007
- Winkler DE, Kaiser TM. 2011. A case study of seasonal, sexual and ontogenetic divergence in the feeding behavior of the moose (Alces alces LINNÉ, 1758). Verhandl naturwiss Ver Hamburg. 46:331–348.
