ABSTRACT
Amber contains numerous well-preserved adult aquatic insects (e.g., aquatic beetles – Coleoptera, water bugs – Heteroptera, dragonflies – Odonata, caddisflies – Trichoptera, mayflies – Ephemeroptera, stone flies – Plecoptera). Since amber is fossilised resin of terrestrial conifer trees, it is an enigma how aquatic insects have ended up in the resin. Based on field studies in a Hungarian forest along a freshwater creek we suggest that tree resin traps water-seeking flying polarotactic aquatic insects because of its property to polarise reflected light. The sticky tree resin was modelled by a water-proof, transparent, colourless insect-monitoring glue laid on vertical and horizontal fallen tree trunks next to the creek. Adults of various polarotactic aquatic insect species were trapped only by the horizontal sticky trunk. In earlier field experiments we showed that these insects find water by means of the horizontal polarisation of water-reflected light, and therefore are attracted to and land on all surfaces which reflect horizontally polarised light. Using imaging polarimetry, we revealed the criterion of polarisation-based trapping by resiny tree trunks. According to our observations, flying aquatic insects can be trapped by sticky (resiny) regions of fallen tree trunks that reflect horizontally polarised light and thus attract polarotactic species. The resin continues to flow out of the trees even when fallen over or fractured in a storm. Our findings support and complement an earlier hypothesis, according to which amber-preserved adult aquatic insects have been trapped by resiny bark when they dispersed over land.
Introduction
Fossil remains of many different insect species have been found in amber (Poinar Citation1993; Grimaldi Citation1996; Weitschat and Wichard Citation2002; Perkovsky et al. Citation2003, Citation2007). About one quarter of all insects in amber are insects with aquatic larvae (Poinar and Poinar Citation1999; Poinar and Milki Citation2001; Kaddumi Citation2007; Kraemer Citation2007; Kraemer Citation2010; Perkovsky et al. Citation2010; Weitschat and Wichard Citation2010). Since amber is fossilised resin of terrestrial conifer trees, it is an enigma how primary and secondary aquatic insects got into resin. The larvae and adults of primary aquatic insects live in water (e.g. water beetles – Coleoptera), while in the case of secondary water insects only the larvae live in water and the adults are terrestrial (e.g. dragonflies – Odonata, crane flies – Tipulidae, horseflies – Tabanidae). The origin of adult aquatic insects in amber have been explained with the following hypotheses: (1) Resin flowed into water-filled treeholes and the aquatic insects living in these tiny water bodies were trapped by resin (Poinar et al. Citation1993; Schöborn et al. Citation1999; Miller Citation2003). (2) Tree resin flowed down from the bark to the forest floor and contacted with water bodies or flowed directly into water. Aquatic insects became attached to the surface of liquid resin and struggled deeper into it, or were covered by new resin, or were enclosed in small water drops in resin (Schmidt and Dilcher Citation2007). (3) Wind blew carcases of dead aquatic insects onto resin outflows (Jazdzewski and Kulicka Citation2000; Keyser and Weitschat Citation2005). (4) Insects associated with water were trapped for whatever reason when they dispersed over land (Poinar Citation1992; Wichard and Weitschat Citation1996).
Until now only hypothesis 3 has been supported by field studies in a recent swamp forest performed by Schmidt and Dilcher (Citation2007). In this work we concentrate on hypothesis 4, assuming an active flight of aquatic insects over land. It is, however, unclear why these dispersing aquatic insects alight on resiny bark. It has been supposed that they were attracted to the resin of conifer trees, because the resin glittered and sparkled like water (Poinar Citation1992; Wichard and Weitschat Citation1996). Here we present experimental evidence for hypothesis 4 showing that water-seeking flying polarotactic aquatic insects do indeed alight on sticky bark based on the attractiveness of water-imitating reflection-polarisation rather than lured simply by the sparkling glitter of the resin.
The receptor-physiological and behavioural basis of our finding is the fact that flying aquatic insects find water-bodies by seeing the horizontal polarisation of light reflected from the water surface (Schwind Citation1991; Kriska et al. Citation1998, Citation2009). Water insects detect horizontally polarised light with the ventral region of their compound eyes possessing specialised polarisation-sensitive photoreceptors (Horváth and Csabai Citation2014). In numerous experiments it has been shown that more than 350 aquatic insect species are attracted to horizontally polarised light even if its intensity is much smaller than that of unpolarised light (reviewed by Horváth and Varjú Citation2004). It was also proven that in the detection of water bodies the primary cue is the horizontal polarisation of water-reflected light, and other cues (e.g. colour, glittering, smell, taste, sound, temperature) play a secondary role (reviewed by Horváth and Csabai Citation2014).
If in flight water insects perceive horizontally polarised reflected light coming from below, they are attracted to this light source and land on it or touch it. Once they locate a water surface, primary aquatic insects (i.e. larvae and adults of species living in the water, e.g. water beetles – Coleoptera) land on the water surface or take the plunge into water. On the other hand, secondary aquatic insects (the larvae of which live in water, but whose adults are terrestrial, e.g. dragonflies – Odonata, mayflies – Ephemeroptera, or tabanid flies – Tabanidae) sporadically land on or plunge into the water, they usually only touch the water surface when laying eggs into the water, or they test the quality of the water, drink, or bathe in it for thermoregulation. All these water-specific reactions of aquatic insects require at least one contact with the water surface. Yet just this one contact can be lethal for them, namely if the reflecting surface is not water but a shiny, horizontally polarising, resiny surface, which is sticky and not likely to allow an insect that has touched it to escape from it.
To support our explanation, we performed field experiments and carried out observations along a Hungarian forest creek known to be home to many different aquatic insect species. We modelled the reflection-polarisation characteristics of resiny trees using sticky tree trunks of different colourations. We measured their optical characteristics with imaging polarimetry, and photodocumented the insects captured by these sticky traps. We showed that (1) horizontally polarising sticky bark does attract water-seeking flying aquatic insects, and (2) many different insects (both aquatic and terrestrial) can be trapped by certain sticky tree trunks in the vicinity of a creek. These two findings satisfy the main prerequisite of our explanation: visual attraction of flying polarotactic aquatic insects to horizontally polarised light reflected from certain fallen tree trunks with patches of oozed-out resin.
Materials and methods
Field experiments
We performed four field experiments and observation campaigns in June 2018 from 17:00 to 21:00 (= UTC + 2 hours) on warm days (with minimum and maximum air temperatures 20 and 34 °C, respectively) in the North Hungarian Mountains close to the village of Dömörkapu along an asphalt road running parallel to the Bükkös Creek (47° 41ʹ 45” North, 18° 59ʹ 50” East). The study area was a Hungarian nature reserve rich in flora and fauna including numerous terrestrial and aquatic insect species. At the time of our observation several species of mayfly as well as other aquatic and terrestrial insect species swarmed and flew around. Experiment 1 was performed on 11 and 14 June 2018 between 17:00 and 21:00 (= UTC + 2 hours) and the captured water insects were collected at the end of the test. Experiments 2, 3 and 4 were carried out between 4 and 10 June 2018. The trapped insects were photographed, collected and identified daily. In the case of dragonflies and chironomids, males and females were distinguished, and for mayflies, besides sex, subimagoes and imagoes were also identified.
In experiment 1 we deployed four plastic trays (50 cm × 50 cm × 3 cm) on the asphalt road along a straight line 1 m apart from each other (Supplementary Fig. S1). The bottom of the plastic trays was covered with a single layer (~ 6 mm) of pine bark. The bark pieces were fixed to the plastic bottom with a universal glue called Palmatex (Henkel Hungary Ltd., Budapest, www.pattex.com). Each tray and its bark layer were painted to the same colour (black, light brown, dark brown, white) with common spray paints. Into each tray a transparent, yellowish cooking oil (Vénusz® sunflower grain oil, Bunge Ltd., Martfű, Hungary) was filled up to the half mark (3 mm) of the height (6 mm) of the bark layer. These trays modelled the horizontal sticky (resiny) regions of fallen tree trunks with the mentioned colours. All insects which touched the oily bark were trapped. The order of these insect traps was randomised in 30 min intervals. The temperature of the traps was measured with a contact thermometer (GAO Digital Multitester EM392B 06554H, EverFlourish Europe Gmbh., Friedrichsthal, Germany) with nominal precision of ±1 °C. The experimental site was surrounded by trees, thus the surfaces were illuminated mainly by the down-welling skylight and light reflected from the green vegetation.
In experiment 2 dark brown, black and white tree trunk groups were laid on the dark grey asphalt road along a straight line 1 m apart from each other (Supplementary Fig. S2). Each group (60 cm × 60 cm × 15 cm) consisted of four half wood pieces (30 cm × 30 cm × 15 cm), each piece being half of a cylindrical clog (diameter: 30 cm, height: 30 cm). The clogs originated from a dry walnut-tree, and their originally grey bark surface was painted with the same spray paints as in experiment 1. The painted bark surfaces were covered by a transparent, colourless, odourless, weather-proof, insect-monitoring glue (BabolnaBio®, Hungary).
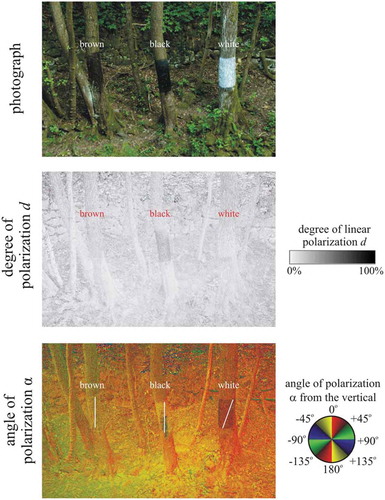
In experiment 3 we selected three neighbouring maple trees on the shore of the creek. Two of their vertical trunks were painted black and white (with the spray paints used in experiment 1) between 1 and 2 m height from the ground (). The painted bark areas faced towards the creek and were covered with insect-monitoring glue used in experiment 2. The third trunk was simply covered with the adhesive. The insects trapped by these sticky trunk regions were photographed, collected and identified.
Figure 1. Photographs and patterns of the degree d and angle α of linear polarisation of the sticky real brown, painted black and painted white vertical maple tree trunk pieces measured with imaging polarimetry in the green (550 nm) spectral range in field experiment 3. The polarimeter’s optical axis was horizontal. The trunks were in the shade of trees and thus illuminated by light from the clear sky and the surrounding vegetation. In the α-pattern the white bars show the local direction of polarisation of the painted bark
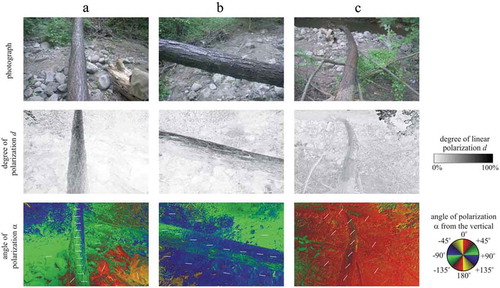
In experiment 4 we selected three fallen horizontal brown maple tree trunks which overarched the creek and covered the upper half of their bark surface with the insect-monitoring glue used in experiment 2 (). The insects trapped by these sticky trunk areas were photographed, collected and identified.
Figure 2. Photographs and patterns of the degree d and angle α of linear polarisation of the sticky region of two fallen horizontal brown maple tree trunks (a and b are the same but seen from two different viewing directions) overarching the mountain creek used in experiment 4 measured with imaging polarimetry in the green (550 nm) spectral range. The tilt angle of the polarimeter’s optical axis was −35° from the horizontal. In the α-pattern the white bars show the local direction of polarisation of the sticky bark region. In case a and b the scene was in the shade of trees and illuminated by light from the clear sky and the surrounding vegetation. In case c, beyond skylight, the scene was illuminated from the left side by direct light of the setting sun
Imaging polarimetry
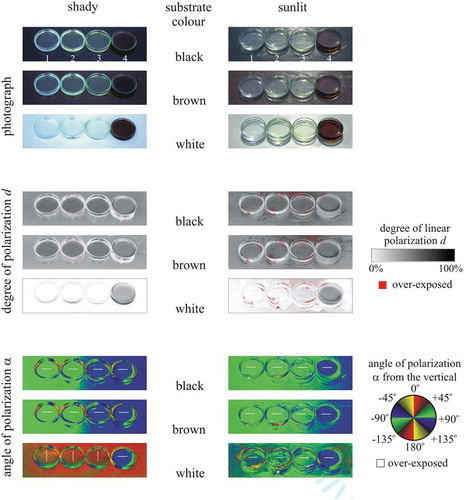
Both primary and secondary aquatic insects are polarotactic, i.e., they are attracted to (among other cues) the horizontal polarisation of water-reflected light when looking for water bodies (Horváth Citation2014). Therefore, we measured the reflection-polarisation characteristics of the sticky targets used in our field experiments with imaging polarimetry (Horváth and Varjú Citation1997, Citation2004) in the red (650 nm), green (550 nm) and blue (450 nm) parts of the spectrum. The targets were in the shade of trees and illuminated by light from the clear sky and the surrounding vegetation. We also measured the reflection-polarisation characteristics of the colourless insect-monitoring glue used in experiments 2–4, the yellowish cooking oil used in experiment 1, a yellowish Canada balsam, and a brown pine resin dissolved in isopropyl alcohol under shady and sunlit conditions when the Petri dishes containing these sticky liquids were on a black, brown and white substrate. The colour of these four sticky liquids model well that of fresh tree resins which can range from translucent white through light yellow to brown/reddish (Henwood Citation1993; Grimaldi Citation1996).
Statistical analysis
For comparing the numbers of trapped insects in a given experiment, we used Χ2 tests of homogeneity with the use of the R statistical package v3.6.0 (R Core Team Citation2013).
Results
Reflection-polarisation characteristics of sticky tree trunks
The black, dark brown, light brown and white horizontal oil traps laid out on the asphalt road in experiment 1 reflected horizontally (angle of polarisation from the vertical α ≈ 90°) polarised light (Supplementary Figs. S1, S3). The black and the two brown traps reflected light with high degrees of linear polarisation d > 80% at the Brewster’s angle [= arctan (n = 1.5) = 56.3° from the vertical for refractive index n = 1.5 of oil and pine resin, Ribeiro and Bernardo-Gil (Citation1990)], while the white trap reflected only weakly polarised light with d < 30% (Supplementary Figs. S1, S3). The highly and horizontally polarised signal of the black and brown traps was attractive to water-seeking polarotactic aquatic insects, while the weakly and horizontally polarised signal of the white trap was unattractive.
The sticky dark brown and black horizontal tree trunk pieces used in experiment 2 reflected light with high degrees of horizontal polarisation (d > 80%) at the Brewster’s angle, while the sticky white horizontal tree trunk pieces reflected only weakly (d < 15%) and not always horizontally, but also obliquely and vertically polarised light (Supplementary Fig. S2). The highly and horizontally polarised light from the black and brown trunk pieces was attractive to water-seeking aquatic insects, while the weakly and not horizontally polarised light from the white trunk pieces was practically unattractive.
The sticky dark brown, black and white vertical tree trunk pieces in experiment 3 reflected obliquely or vertically polarised light (). The black trunk was most (d < 50%) and the white trunk least polarising (d < 15%), wile the brown trunk was inbetween (d < 30%). Due to the non-horizontal direction of polarisation of trunk-reflected light, all three vertical trunks were unattractive to water-seeking polarotactic aquatic insects.
The sticky regions of the two nearly horizontal brown fallen tree trunks overarching the creek in experiment 4 reflected highly polarised light (d > 60%) at the Brewster’s angle (). When the trunks were illuminated by light from the clear sky and the surrounding vegetation the trunk-reflected light was horizontally polarised (). When, however, the trunk was lit by direct light of the setting sun, the trunk-reflected light was oliquely polarised (). Thus, in the former two cases the sticky trunks were attractive to aquatic insects, while in the latter case the sticky trunk was unattractive.
shows the patterns of the degree and angle of polarisation of the colourless insect-monitoring glue used in experiments 2–4, the yellowish cooking oil used in experiment 1, a yellowish Canada balsam, and a brown pine resin dissolved in isopropyl alcohol measured with imaging polarimetry in the green (550 nm) spectral range under shady and sunlit conditions when the Petri dishes containing these liquids were on a black, brown and white substrate. On dark (black, brown) substrates there are no significant differences in the reflection-polarisation characteristics of these four liquids reflecting light with medium (d < 55%) degrees of horizontal polarisation. However, on bright (white) substrates, bright (colourless whitish or light yellowish) resins can reflect light with low (d < 10%) degrees of vertical (in shade) or horizontal (in sunshine) polarisation. Only dark (brown) resins can reflect light with medium (d < 55%) horizontal polarisation on bright substrates.
Figure 3. Photographs and patterns of the degree d and angle α of linear polarisation of the colourless insect-monitoring glue used in experiments 2–4 (1), the yellowish cooking oil used in experiment 1 (2), a yellowish Canada balsam (3), and a brown pine resin dissolved in isopropyl alcohol (4) measured with imaging polarimetry in the green (550 nm) spectral range under shady (left column) and sunlit (right column, when the sun shone frontwise) conditions when the Petri dishes containing the sticky liquids were on a black, brown and white substrate. The tilt angle of the polarimeter’s optical axis was −65° from the horizontal. In the α-patterns the white bars show the local direction of polarisation of the liquid surface
Insects captured by the sticky traps and tree trunks
In experiment 1, the black, dark brown, light brown and white oil traps captured 989, 461, 273 and 5 chironomids, respectively (). According to χ2 test of homogeneity, these differences are statistically significant (χ2 = 1200.7, df = 3, p < 0.0001). Hence, the darker the trap, the more attractive it was to polarotactic chironomids, which detect water by polarotaxis (Lerner et al. Citation2008; Horváth et al. Citation2011). In experiment 2, only 5 and 11 mayflies were trapped by the dark brown and black sticky tree trunks, respectively.
Table 1. Number of the chironomid flies trapped by the black, brown, light brown and white trays padded with bark and filled with cooking oil in experiment 1. χ2 test of homogeneity yielded significant differences among the sums (χ2 = 1200.7, df = 3, p < 0.0001)
In experiments 3 and 4 the number of specimens of various aquatic insect taxa trapped by the sticky trunk areas () are given in . The brown, black and white vertical sticky barks trapped 10, 9 and 9 different non-aquatic taxa, respectively. The total number of trapped taxa on these sticky vertical trunks was 13, while the horizontal trunk catched 14 aquatic insect taxa. Regarding the numbers of trapped individuals, it is clear that the vertical brown and black barks captured nearly the same amount of specimens (Nbrown = 26, Nblack = 23) and the white one was approximately half as attractive (Nwhite = 11). According to χ2 tests performed with equal expected catch values, there were significant differences in the trapping efficiencies of these three vertical surfaces (χ2 = 6.3, df = 2, p < 0.05). The white trunk trapped significantly fewer aquatic insects than the brown and black ones (white versus brown: χ2 = 6.1, df = 1, p < 0.05; white versus black: χ2 = 4.2, df = 1, p < 0.05; brown versus black: χ2 = 0.18, df = 1, p = 0.67). The total number of insects found on the sticky horizontal brown fallen trunk was Nhorizontal = 60. Taking into account that the sticky area on the fallen trunk was two times greater than the corresponding area on the vertical barks, it is clear that the surface density of the trapped insects was very close to that of the brown vertical sticky bark.
Table 2. Numbers of trapped aquatic insects on the three vertical (real brown, painted black, painted white) and one horizontal (real brown) sticky trunk surfaces. Females and males are indicated by ‘f’ and ‘m’ in brackets, respectively. In the case of mayflies (Ephemeroptera), ‘s’ and ‘i’ denote subimago and imago. χ2 test of homogeneity yieldedsignificant differences among the sums of specimens (χ2 = 6.3, df = 2, p < 0.05)
Figure 4. Mayflies trapped by the real brown (a–b), painted black (c–d) and painted white (e–f) vertical sticky barks in experiment 3. (a, c) Female Ephemera danica subimagoes. (b–d) Female Rhithrogena semicolorata subimagoes. (e) Male Ephemera danica subimago. (f) Male Epeorus silvicola imago

Figure 5. Mayflies trapped by the horizontal sticky bark in experiment 4. (a–b) Male and female Ephemera danica imagoes. (c) Male Epeorus silvicola. (d) Female R. semicolorata with her egg-batch

Figure 6. (a–f) Aquatic insects trapped by the painted black vertical sticky bark in experiment 3. (a) Beautiful demoiselle (Calopteryx virgo) female. (b) Female C. virgo exhausted during emergence. (c–d) Stoneflies (Plecoptera). (c) Common forestfly (Nemoura cinerea). (d) Sallfly (Chloroperla sp.). (e) Caddisfly (Rhyacophilidae). (f) Crane fly (Tipulidae)

Figure 7. Aquatic insects trapped by the real brown vertical sticky bark in experiment 3. (a) Beautiful demoiselle (Calopteryx virgo) male. (b) Male C. virgo exhausted during emergence. (c–d) Stoneflies (Plecoptera). (c) Common forestfly (Nemoura cinerea). (d) Nymphal skin of Perla abdominalis. (e) Alderfly (Sialis sp.). (f–g) Caddisflies. (f) Philopotamus sp. (g) Hydropsychidae. (h) Crane fly (Tipulidae)

Figure 8. Aquatic insects trapped by the painted white vertical sticky bark in experiment 3. (a) Nymphal skin of Calopteryx virgo. (b–c) Stoneflies (Plecoptera). (b) Common forestfly (Nemoura cinerea). (c) Isoperla tripartita. (d–e) Caddisflies (Trichoptera). (d) Rhyacophilidae. (e) Limnephilidae. (f) Crane fly (Tipulidae)

Figure 9. Aquatic insects trapped by the real brown horizontal sticky bark in experiment 4. (a) Beautiful demoiselle (Calopteryx virgo) male. (b–d) Stoneflies (Plecoptera). (b) Common forestfly (Nemoura cinerea). (c) Isoperla tripartita. (d) Sallfly (Chloroperla sp.). (e) Caddisfly (Limnephilidae). (f) Diving beetle (Colymbetes fuscus). (g) Female non-biting midge (Chironomidae). (f) Crane fly (Tipulidae). (h) Horsefly (Tabanidae)

In the case of mayflies, additional information is given in . Except for one single imago ()), all the 14 mayflies trapped by the vertical trunks were poorly flying subimagoes (). The subimago state occurs in the case of the development of males and females after which another moult leads to the final imago state, thus only immature mayflies were trapped by the vertical trunks. The horizontal sticky trunk trapped both sexes of mayflies and only imagines (, ). Laid egg-batches were often found next to the trapped females ().
Discussion
Based on the fact that insect imagines with an obligate aquatic larval stage were frequently preserved in amber (Wichard and Weitschat Citation1996; Poinar and Poinar Citation1999; Weitschat and Wichard Citation2002), it can be assumed that ponds, lakes and creeks must have been abundant in amber forests. About 50% of modern tree resin solidifies on the bark (Henwood Citation1993). Thus, the origin of aquatic insect fossils in amber can be explained with the assumption that the tree resin oozed out onto the bark trapping flying water-seeking insects in what we now call amber forests (Poinar Citation1992; Wichard and Weitschat Citation1996).
The notion expressed by Poinar (Citation1992) and Wichard and Weitschat (Citation1996) that water-seeking insects when they dispersed over land were attracted to the glistening resin of trees, is supported by our observations, but these authors failed to explain what exactly it was that aquatic insects found so irresistable with regard to the tree resin. Until now there had not been any experimental and observational evidence for the reality of the suggested trapping mechanism. To reveal the possibility that tree resins entrapped aquatic insect imagines because of the resin’s appearance in reflected light, we studied the extent to which resin-imitating adhesives on tree trunks would lure and trap water-seeking flying limnetic insects. Our studies were conducted in a forest along a creek where flying aquatic insects were abundant. After covering the bark of some vertical trees and horizontal tree trunks with a colourless, transparent, odourless and weather-proof glue, we were able to investigate attractiveness and efficiency in trapping aquatic insects of the various sticky barks.
The result shows that only horizontally polarising sticky bark areas could trap aquatic insects. If the sticky bark reflected vertically or obliquely polarised light, no water insects were attracted, thus no such insects could become stuck in the resin-like glue. This was revealed by imaging polarimetry with which we measured the reflection-polarisation patterns of the sticky tree trunks used in our field experiments. The main reason for the differences in the degree of polarisation of light reflected from differently coloured tree trunks was the colour itself. According to the rule of Umow (Citation1905), the darker a surface in a given spectral range, the higher is the degree of polarisation d of surface-reflected light. Therefore, the black tree trunks reflected light with the highest d, brown trunks were less polarising, and white trunks reflected light with the lowest d as clearly seen in and Supplementary Figs. S1-S3.
Considering the trapping data of experiment 1, we can say that the aquatic insects trapped by the vertical and horizontal sticky test surfaces () represent the local fauna well (Andrikovics Citation1991). The total number of captured insects by the vertical sticky trunks was quite low, furthermore it is clear that the surface brightness had a relevant effect, and the brightest (white) surfaces trapped the smallest number of specimens.
On the other hand, the low catches on these vertical trunks indicate that these surfaces did not elicit such reaction that could lead to the mass trapping of aquatic insects. Although both vertical and horizontal sticky barks trapped most of the aquatic insect taxa being abundant at the experimental site, the order Ephemeroptera showed the most interesting behaviour, because the vertical surfaces trapped only subimagoes, while the horizontal trunk caught exclusively imagines ().
We suggest that the horizontally polarised light reflected from the sticky fallen tree trunk was the main reason for this result, because horizontally polarised light reflected from any surfaces can deceive and attract polarotactic aquatic insects like mayflies (Egri et al. Citation2017). The sticky vertical barks reflected light with directions of polarisation far from the horizontal, thus these surfaces were not attractive to water-seeking aquatic insects. After emergence, mayfly subimagoes usually search any kind of nearby object to land on and moult into an imago. Consequently, their trapping on the vertical sticky trunks could happen incidentally without any motivation related to polarotaxis or seeking water surfaces. This case could be similar for the beautiful demoiselle (Calopteryx virgo) nymphs that crawled out from the creek directly onto the sticky surface and then become trapped in the middle of the act of emergence () just like it has been found in real amber (Bechly and Wichard Citation2008; Wichard et al. Citation2009).
The taphonomy of flying polarotactic aquatic insects is the same as that of other flying terrestrial insects that get stuck on resin surfaces and are enclosed by successive resin outflows. However, the mechanism of attraction is different: aquatic insects are positively polarotactic, and thus are lured by the horizontally polarised resin-reflected light, rather than simply by the glittering of the shiny resin surface.
There are many different artificial surfaces and objects that mimic water by means of the horizontally polarised light reflected from them (Horváth et al. Citation2014): crude oil ponds, dark grey asphalt roads, black plastic sheets, glass surfaces, dark car bodies, black gravestones, solar panels: in fact all shiny dark surfaces that reflect horizontally polarised light. The attraction of polarotactic aquatic insects to these artificial surfaces has the negative consequence that the eggs laid onto these dry surfaces perish due to dehydration, and the polarised signal can frequently be so strong that the adults cannot escape from it and perish as well because of exhaustion or dehydration (Horváth and Kriska Citation2008). This visual phenomenon is called polarised light pollution (Horváth et al. Citation2009; Egri et al. Citation2017). Our explanation of the trapping of water-seeking aquatic insects by resin is essentially an ancient type of polarised light pollution with, however, minimal or no evolutionary consequences as aquatic insects still interpret horizontally polarised light with water.
Data accessibility
Our paper has the following electronic supporting materials: Supplementary Figures S1, S2, S3.
Authors’ contributions
Substantial contributions to conception and design: GH, GK, BM-R
Performing experiments and data acquisition: GH, GK, ÁE
Data analysis and interpretation: GH, GK, ÁE, BM-R
Drafting the article or revising it critically for important intellectual content: GH, GK, ÁE, BM-R
Ethical approval and informed consent
Our field studies did not need a permission or an approval.
_AmberPol_HistBiol-revised-supplement.doc
Download MS Word (1.6 MB)Acknowledgments
We are grateful for two anonymous reviewers for their constructive and valuable comments.
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary Material
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Andrikovics S. 1991. On the long-term changes of the invertebrate macrofauna in the creeks of the Pilis-Visegrádi mountains (Hungary). Verhandlungen der Internationalen Verein für Limnologie. 24:1969–1972.
- Bechly G, Wichard W. 2008. Damselfly and dragonfly nymphs in Eocene Baltic amber (Insecta: odonata), with aspects of their palaeobiology. Palaeodiversity. 1:37–73.
- Core Team R 2013. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna http://www.R-project.org/
- Egri Á, Pereszlényi Á, Farkas A, Horváth G, Penksza K, Kriska G. 2017. How can asphalt roads extend the range of in situ polarized light pollution? A complex ecological trap of Ephemera danica and a possible remedy. J Insect Behav. 30:374–384.
- Grimaldi DA. 1996. Amber: window to the past. New York: Abrams.
- Henwood A. 1993. Recent plant resins and the taphonomy of organisms in amber: a review. Mod Geol. 19:35–59.
- Horváth G, Kriska G. 2008. Polarization vision in aquatic insects and ecological traps for polarotactic insects. In: Lancaster J, Briers RA, editors. Aquatic insects: challenges to populations (Vol. Chapter 11). Wallingford (Oxon, UK): CAB International Publishing; p. 204–229.
- Horváth G, Kriska G, Robertson B. 2014. Anthropogenic polarization and polarized light pollution inducing polarized ecological traps. In: Horváth G, editor. Polarized light and polarization vision in animal sciences. Heidelberg: Springer; p. 443–513.
- Horváth G, Csabai Z. 2014. Polarization vision of aquatic insects. In: Horváth G, editor. Polarized light and polarization vision in animal sciences. Heidelberg: Springer; p. 113–145.
- Horváth G. ed. 2014. Polarized light and polarization vision in animal sciences. Heidelberg: Springer.
- Horváth G, Kriska G, Malik P, Robertson B. 2009. Polarized light pollution: a new kind of ecological photopollution. Front Ecol Environ. 7:317–325.
- Horváth G, Móra A, Bernáth B, Kriska G. 2011. Polarotaxis in non-biting midges: female chironomids are attracted to horizontally polarized light. Physiol Behav. 104:1010–1015.
- Horváth G, Varjú D. 1997. Polarization pattern of freshwater habitats recorded by video polarimetry in red, green and blue spectral ranges and its relevance for water detection by aquatic insects. J Exp Biol. 200:1155–1163.
- Horváth G, Varjú D. 2004. Polarized light in animal vision - polarization patterns in nature. Heidelberg: Springer.
- Jazdzewski K, Kulicka R. 2000. A note on amphipod crustaceans in a piece of Baltic amber. Ann Zool Fennici. 50:99–100.
- Kaddumi HF. 2007. Amber of Jordan. Amman: Eternal River Museum of Natural History.
- Keyser D, Weitschat W. 2005. First record of ostracods (Crustacea) in Baltic amber. Hydrobiologia. 538:107–114.
- Kraemer MMS. 2007. Systematic, palaeoecology, and palaeobiogeography of the insect fauna from Mexican amber. Palaeontographica Abt A. 282:1–133.
- Kraemer MMS. 2010. Mexican amber. In: Penney D, editor. Biodiversity of fossils in amber from the major world deposits. Manchester: Siri Scientific Press; p. 43–57.
- Kriska G, Bernáth B, Farkas R, Horváth G. 2009. Degrees of polarization of reflected light eliciting polarotaxis in dragonflies (Odonata), mayflies (Ephemeroptera) and tabanid flies (Tabanidae). J Insect Physiol. 55:1167–1173.
- Kriska G, Horváth G, Andrikovics S. 1998. Why do mayflies lay their eggs en masse on dry asphalt roads? Water-imitating polarized light reflected from asphalt attracts Ephemeroptera. J Exp Biol. 201:2273–2286.
- Lerner A, Meltser N, Sapir N, Erlick C, Shashar N, Broza M. 2008. Reflected polarization guides chironomid females to oviposition sites. J Exp Biol. 211:3536–3543.
- Miller KB. 2003. The unusual occurrence of aquatic beetles in amber, Copelatus aphroditae Balke, n. sp., and C. predaveterus Miller, n. sp. (Coleoptera: dytiscidae: copelatinae). Proc Entomol Soc Washington. 105:809–815.
- Perkovsky EE, Zosimovich VY, Vlaskin AP. 2010. Rovno amber. In: Penney D, editor. Biodiversity of fossils in amber from the major world deposits. Manchester: Siri Scientific Press; p. 116–136.
- Perkovsky EE, Rasnitsyn AP, Vlaskin AP, Taraschuk MV. 2007. A comparative analysis of the Baltic and Rovno amber arthropod faunas: representative samples. Afr Invertebr. 48:229–245.
- Perkovsky EE, Zosimovich VY, Vlaskin AY. 2003. Rovno amber insects: first results of analysis. Russ Entomol J. 12:119–126.
- Poinar GO Jr. 1992. Life in Amber. Stanford: Stanford University Press.
- Poinar GO Jr. 1993. Insects in amber. Annu Rev Entomol. 38:145–159.
- Poinar GO Jr, Milki R. 2001. Lebanese amber: the oldest insect ecosystem in fossilized resin. Corvallis: Oregon State Univ Press.
- Poinar GO Jr, Poinar R. 1999. The amber forest: a reconstruction of a vanished world. Princeton: Princeton University Press.
- Poinar GO Jr, Waggoner BM, Bauer UC. 1993. Terrestrial soft-bodied protists and other microorganisms in Triassic amber. Science. 259:222–224.
- Ribeiro A, Bernardo-Gil GG. 1990. Densities and refractive indices of components of pine resin. J Chem Eng Data. 35:204–206.
- Schmidt AR, Dilcher DL. 2007. Aquatic organisms as amber inclusions and examples from a modern swamp forest. Proc National Acad Sci USA. 104:16581–16585.
- Schöborn W, Dörfelt H, Foissner W, Krienitz L, Schäfer U. 1999. A fossilized microcenosis in Triassic amber. J Eukaryotic Microbiol. 46:571–584.
- Schwind R. 1991. Polarization vision in water insects and insects living on a moist substrate. J Comp Physiol A. 169:531–540.
- Umow N. 1905. Chromatische Depolarisation durch Lichtzerstreuung. Physikalische Zeitschrift. 6:674–676.
- Weitschat W, Wichard W. 2010. Baltic amber. In: Penney D, editor. Biodiversity of fossils in amber from the major world deposits. Manchester: Siri Scientific Press; p. 80–115.
- Weitschat W, Wichard W. 2002. Atlas of plants and animals in Baltic amber. München: Dr. Friedrich Pfeil Verlag.
- Wichard W, Gröhn C, Seredszus F. 2009. Aquatic insects in Baltic amber. Kessel: Remagen Verlag.
- Wichard W, Weitschat W. 1996. Wasserinsekten in Bernstein - eine paläobiologische Studie. Entomologische Mitteilungen von Löbbecke-Museum + Aquazoo (Düsseldorf). 4(Supplement):1–122.
