Abstract
The continuous increase in the prevalence of asthma poses a threat to human health. Despites numerous researches, the understanding of asthma development still remain elusive, hindering the development of effective treatment. Here, we explored the role of lncRNA RP5-857K21.7 (RP5-857K21.7) in the development of asthma and its potential molecular mechanism of regulation. Airway smooth muscle cells (ASMCs) were isolated and cultured after which some of the cells were induced with PDGF-BB to build an asthma cell model, and then, qRT-PCR analysis was used to measure the expression level of RP5-857K21.7 in the cell model. Result shows that the RP5-857K21.7 is significantly downregulated in PDGF-BB-induced ASMCs cells. Through CCK-8, transwell, and flow cytometry assay, we examined the functional impact of RP5-857K21.7 on the proliferation, migration, and apoptosis of the ASMCs, respectively, and found that the overexpression of RP5-857K21.7 markedly inhibit PDGF-BB-induced ASMCs cell proliferation, migration and induce apoptosis. Bioinformatics analysis predicted that the RP5-857K21.7 could sponge miR-508-3p and result was validated through a dual-luciferase reporter assay, biotinylated RNA pull-down assay, and RIP-qRT-PCR analysis. Mechanistically, RP5-857K21.7 regulates the PI3K/AKT/mTOR pathway by endogenously sponging miR-508-3p to inhibit PDGF-BB-induced ASMCs cell proliferation, migration and induce apoptosis. The current research suggests that the RP5-857K21.7 and its associated molecular pathway (miR-508-3p/PI3K/AKT/mTOR axis) might be a useful therapeutic target for the treatment of asthma disease.
Keywords:
Introduction
As a well-known lung disease, asthma is presented as a chronic and heterogenic disease of the respiratory system. It is characterized by underlying pathologies such as chronic inflammatory process and airway remodelling mediated by several cells and cytokines [Citation1]. Several structural changes to the airway have been observed in asthma and these include increased smooth muscle mass, gland enlargement, neovascularization, sub-epithelial fibrosis and epithelial alterations [Citation2]. Controversial airway remodelling has been associated with an underlying chronic inflammatory process. Poor clinical outcome among patients is an attribute of airway remodelling and these changes from remodelled airway, enhance thickening of airway walls leading to airway narrowing, airway oedema, bronchial hyperresponsiveness, and mucous hypersecretion [Citation2]. Without equivocation, an in-depth understanding of the molecular mechanism of airway remodelling will engender a more effective therapeutic target for asthma [Citation3]. Moreover, several evidences showed that many inflammatory mediators are crucial to the development of airway remodelling in asthma [Citation4]. For instance, platelet-derived growth factor-BB (PDGF-BB) has been identified as one of the inflammatory mediators that is sufficiently upregulated in asthmatic tissues [Citation1]. PDGF-BB overexpression has been attributed to hyperresponsiveness, reduced lung compliance, decrease in the genes encoding contractile protein and high number of airway smooth muscle cells [Citation5]. In the same vein, in vivo study in mice reveals that PDGF-BB stimulated ASM hyperplasia and transformation in lung mechanics [Citation5].
Both miRNA and lncRNA have been identified as transcriptional regulators [Citation6] and accumulating research reports shows that both molecules interact with each other, which further modulates their influence on the transcriptome [Citation6]. These interactions may result into competition between the lncRNA and miRNA for the similar mRNA targets, miRNA-triggered RNA decay, generation of miRNA from lncRNA and lncRNA acting as a bait for miRNAs [Citation6]. LncRNA that sponges miRNA (competitive endogenous RNAs) and inhibits their interaction with their target mRNA has been regarded as the most dominant form of interaction observed by several researchers [Citation7]. In most cases, lncRNA binds miRNA making use of the regions close to their 3′ end called mRNA response element [Citation8]. Notably, the lncRNAs have been implicated in several diseases including asthma and has been reported to play a role in modulating the pathological progress of asthma [Citation9]. Several studies have revealed the role of lncRNA in the regulation, progression and migration of ASMCs [Citation3]. For instance, lncRNA PVT1 modulates the proliferation and migration of ASMCs in asthma [Citation10]. In the same vein, miRNAs have also been implicated in several biological processes. Altered expression of miR-223 has been observed to play an important role in the obstruction of lung disease in both asthma and COPD [Citation11] and miR-223-3p and miR-142-3p has been associated with airway obstruction [Citation12]. Specifically, the miR-508-3p has been reported to be abnormally expressed in a variety of tumours and diseases [Citation13–15], but its expression in asthma has not been studied yet. Although RP5-857K21.7 (ENSG00000229344) has been reported to be lowly expressed in asthma patients, its specific molecular function and mechanism of regulation in asthma has never been reported.
Thus, the current study aims to elucidate the role of RP5-857K21.7 in the pathophysiology of Platelet- derived growth factors (PDGF) induced airway smooth muscle cells (ASMCs) and the molecular mechanism involved. Bioinformatic prediction revealed miR-508-3p as RP5-857K21.7 target miRNA and the PI3K/AKT/mTOR axis was shown as the molecular signalling pathway through which RP5-857K21.7 regulates the proliferation, migration, and apoptosis of ASMCs by sponging miR-508-3p. Altogether, our data, for the first time, shows the role and regulatory mechanism of RP5-857K21.7 in ASMCs. The RP5-857K21.7 could be an effective therapeutic target for the treatment of asthma.
Materials and methods
Culture and induction of ASMCs
ASMC cell lines (cat. no. 3400; Sciencell Research Laboratories, Carlsbad, CA, USA) were maintained in the Dulbecco’s Modified Eagle’s Medium- DMEM (Gibco, USA) consisting of 20% foetal bovine serum (FBS, Gibco, USA), penicillin (100 U/mL; Invitrogen, USA) and streptomycin (100 μg/mL; Invitrogen, USA) in an incubator with 5% CO2 at 37 °C. Medium changing was done every 72 h until confluence was formed and trypsin-EDTA (1 mM EDTA, trypsin (0.25%) in HBSS; Gibco, USA) solution was used for passaging. ASMCs at passage 4–8 were grown with the addition of 25 ng/mL PDGF-BB (Sigma-Aldrich, Merck KGaA, Darmstadt, Germany), acting as a full mitogen that promotes DNA synthesis and cell proliferation, for 24 h [Citation10]. An inverted light microscope (Olympus, Japan) was used to identify ASMCc through morphological observation.
Cell transfection
RP5-857K21.7 full-length obtained through polymerase chain reaction (PCR) was placed into the vector pcDNA3.1 (Sangon, China) and subsequently referred to as pcDNA- RP5-857K21.7. miR-508-3p mimic and miR-NC (equivalent control) were synthesized by Shanghai GenePharm (China). On six-well plates, ASMCs were grown and at 60% confluence, transfection was done using Lipofectamine 3000 system (Invitrogen, USA) following the producer’s guide. After 48 h of transfection, cells were harvested for other experiments.
Cell proliferation analysis
The proliferation ability of ASMCs was evaluated using the cell counting kit (Beyotime, China). The cells (1 × 106) were added into each well of 48-well plates with each group done in triplicate. Subsequently, CCK-8 reagent (10 ul) was added and then incubated 24 h at 37 °C. After incubation, at the different groups, the cell proliferation was detected with ELISA BrdU kit (Roche, UK) and absorbance was taken at 450 nm.
Quantitative real-time PCR (qRT-PCR) analysis
ASMCs total RNA isolation was done with Trizol reagent (Invitrogen, USA) and reverse- transcribe following the manufacturer’s instruction (TaKaRa, China). qRT-PCR was done with SYBR Prime Script RT-PCR kit (TaKaRa, China) to identify the indicated gene expression. The levels of miRNAs and lncRNAs using GADPH as the endogenous control were calculated with the 2−ΔΔCt method [Citation16]. All assays were done in triplicate.
Cell migration assay
Transwell chamber of 8-um pore size (Corning, USA) was employed to estimate ASMCs migration capability. Concisely, resuspension of the cells (1 × 105) in DMEM (serum-free, 200 μl Gibco, USA) was done and later placed into the upper layer of the transwell, while the lower layer was occupied with DMEM (Gibco, USA) consisting of FBS (20%) with or without PDGF-BB stimulation. After 24 h of incubation =, removal of the non-migrated ASMCs from the upper chamber of the membrane was performed, and methanol (Sigma Chemical, USA) was utilized for fixing cells on the lower chamber. Cells were stained with crystal violet (0.4%; (Shanghai, China) and five optical fields were snapped at random with a light microscope (Olympus, USA). the experiment was done in triplicate.
Flow cytometry assay for cell apoptosis
Annexin V-fluorescein isothiocyanate (Annexin V-FITC)/propidium iodide (PI) Apoptosis Detection Kit (Yeasen Biotechnology, China) was used to evaluate the apoptotic cells after 48 h transfection following the producer’s manual. Concisely, transfected cells were re-suspended and then, Annexin V-FITC and PI (5 μL each) were stirred slightly with the cell suspension. After, 15-min incubation was done at room temperature and flow cytometry (BD Biosciences, USA) was employed to monitor the apoptotic cells. Annexin V positive cells characterized as apoptotic cells.
Dual-luciferase reporter assay
In constructing the vectors for dual-luciferase reporter, pGL-3 basic vector (Progma, USA) was used to clone the predicted sequences of miR-508-3p binding sites in wildtype RP5-857K21.7 (RP5-857K21.7 wt) and the mutated binding sequences (RP5-857K21.7 mut). For the luciferase assay, miR-508-3p mimics or miR-NC co-transfection with the recombinant vectors was done. Renilla reporter system transfection into each group was performed to standardize the efficiency of the transfection.
Biotin RNA pull‑down assay
To investigate the coupling ability of RP5-857K21.7 and miR-508-3, the ASMCs lysates were acquired and incubated with synthesized biotin-labelled anti-sense DNA probes for 3 h. Subsequently, streptavidin-coupled agarose beads (Invitrogen, USA) were added to the reaction mixture for RNA complex isolation and then incubated. Finally, we determined the expression level of miR-508-3 in the BioRP5-857K21.7 WT and MUT type group, and also the control group through qRT-PCR analysis.
RNA-binding protein immunoprecipitation (RIP) assay
The RIP assay on ASMSCs was done with EZ-Magna RIP kit (Millipore, USA) in accordance with producer’s instruction which was done 48-h post-transfection with the miR-508-3p overexpression construct. Magnetic beads preparation for immunoprecipitation was performed, and the cells were lysed. After, conjugation of the cell lysates to the magnetic beads in RIP immunoprecipitation buffer was done at 4 °C overnight using human anti-Ago2 antibody (Millipore, USA) and control IgG (Millipore, USA). Then, beads washing and incubation of samples with proteinase K buffer to separate the purified immunoprecipitated RNA. Lastly, immunoprecipitated RNA was evaluated with qRT-PCR to examine the RP5-857K21.7 enrichment.
Western blot assay
For western blot assay, primary antibodies for PI3K, AKT, p-mTOR, mTOR and GAPDH were obtained from Abcam (Cambridge, USA). In brief, RIPA lysis buffer was utilized to extract proteins from cultured cells and later separated using 12% SDS-PAGE and moved to polyvinylidene difluoride (PVDF) membrane (Invitrogen, USA) blocked using skim milk (5%) at room temperature for 1 h, and then the incubation with the primary antibodies (1:1000) was done at 4 °C overnight, followed by the incubation with the HRP-conjugated secondary antibody (1:1000) (Abcam, USA) at room temperature for 1 h. After, detection of the blots with an enhanced chemiluminescence kit (Pierce, USA), and blots integrated density quantification was done using Image-Pro Plus 6.0 software (Media Cybernetics, USA).
Target gene prediction
Prediction of RP5-857K21.7 target miRNA (miR-508-3p) was done with miRcode online database http://www.mircode.org/.
Statistical analysis
All data estimated in this experiment were shown as mean ± SD. The statistical analysis was done using SPSS 17.0 statistical software (IBM, USA) and Student’s t-test was employed to evaluate mean values significance between two groups, while a one-way ANOVA was done for multiple groups comparison.
Results
RP5-857K21.7 is significantly downregulated while miR-508-3p upregulated in PDGF-BB-induced ASMCs
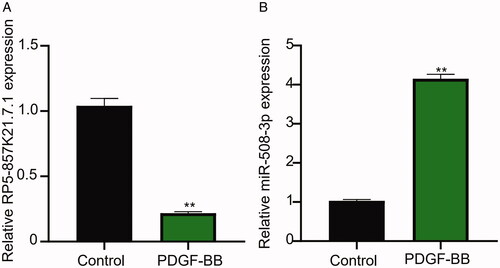
At the start of this study, we confirmed the effect of PDGF-BB induction on the expression level of RP5-857K21.7 and miR-508-3p in ASMCs through qRT-PCR analysis. As shown in , the stimulation of ASMCs with PDGF-BB significantly inhibited RP5-857K21.7 mRNA expression level when compared to the blank control group, implying that the aberrant expression of RP5-857K21.7 could regulate the biological effect of PDGF-BB on ASMCs. In contrast, miR-508-3p was markedly upregulated in PDGF-BB-induced ASMCs (), indicating that abnormal miR-508-3p expression may play a role in PDGF-BB-induced ASMCs.
Figure 1. The lncRNA RP5-857K21.7 is significantly downregulated while miR-508-3p upregulated in PDGF-BB-induced ASMCs. (A) QRT-PCR analysis shows that the stimulation of ASMCs with PDGF-BB significantly inhibited RP5-857K21.7 mRNA expression level compared to the blank control group, suggesting that RP5-857K21.7 expression could regulate the biological effect of PDGF-BB on ASMCs. (B) QRT-PCR analysis confirmed that miR-508-3p was markedly upregulated in PDGF-BB-induced ASMCs, indicating that abnormal miR-508-3p expression may play a role in PDGF-BB-induced ASMCs. All experimental data are shown as the mean ± SD of at least three independent experiments with a significance level of p < .05 (**p < .01).
Overexpression of RP5-857K21.7 inhibits PDGF-BB-induced proliferation and migration of ASMCs and promotes its apoptosis
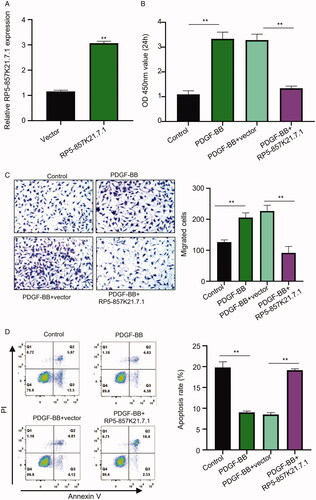
To further understand the role of RP5-857K21.7 in the proliferation, migration, and apoptosis of ASMCs, we transfected the cells with the generated RP5-857K21.7 overexpression vector (pcDNA- RP5-857K21.7) and assess the functional impact of this on the cells. confirms the efficacy of the constructed overexpression plasmid as there was a significant upregulation of RP5-857K21.7 mRNA expression in the cells transfected with RP5-857K21.7 plasmid vector compared to those transfected with an empty vector. Subsequent colony formation assay experiment conducted showed a significant increase in the proliferation of ASMCs after induction with PDGF-BB compared to the control group. On the contrary, the overexpression of RP5-857K21 significantly reduced the proliferative ability of the PDGF-BB-induced ASMCs as compared to the PDGF-BB + vector group (, p < .01). Furthermore, through transwell assay, we showed that PDGF-BB-induction significantly increased the migration of the ASMCs compared to the control group, while transfecting the PDGF-BB-induced ASMCs with RP5-857K21.7 overexpression vector notably reduced the migrative ability of the cell compared to transfecting with an empty vector (, p < .01). Besides, the rate of apoptosis in ASMCs was observed to be higher in the PDGF-BB + RP5-857K21 group in relation to the PDGF-BB + vector group (, p < .01). Altogether, these data suggest that the RP5-857K21 plays an important role in the development of asthma and its overexpression could inhibit the progression of PDGF-BB-induced ASMCs and promotes it apoptosis.
Figure 2. The overexpression of RP5-857K21.7 inhibits PDGF-BB-induced proliferation and migration of ASMCs and promotes its apoptosis (A) The efficacy of the constructed RP5-857K21.7 overexpression plasmid confirmed through qRT-PCR analysis. RP5-857K21.7 mRNA expression was significantly upregulated in the cells transfected with the RP5-857K21.7 plasmid vector compared to those transfected with an empty vector. (B) CCK8 assay revealed a significant increase in the proliferation of ASMCs after induction with PDGF-BB compared to the control group while the overexpression of RP5-857K21 significantly reduced the proliferative ability of the PDGF-BB-induced ASMCs when compared to the PDGF-BB + vector group. (C) Transwell assay showed that PDGF-BB-induction significantly increased the migration of the ASMCs compared to the control group, while RP5-857K21.7 overexpression notably reduced the migrative ability of the cell. (D) Flow cytometry assay showed that the rate of apoptosis in the PDGF-BB-induced ASMCs was higher after overexpression with RP5-857K21 compared to the empty vector group. All experiments were conducted in triplicates and the experimental data are presented as the mean ± SD of the experiments with the significance level defined as p < .05.
RP5-857K21.7 directly targets miR-508-3p
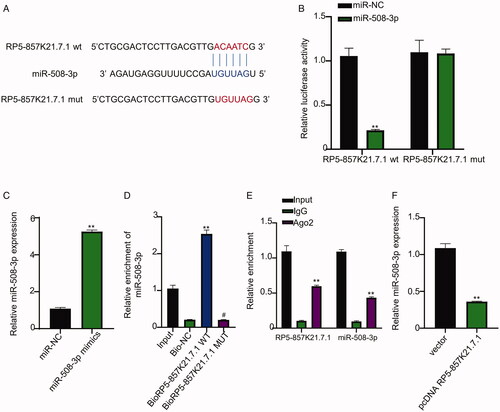
Further, we sought to determine the mechanism through which RP5-857K21.7 regulate the proliferation and migration of ASMCs. First, we conducted a bioinformatics prediction of RP5-857K21.7 target miRNA using miRcode online database (http://www.mircode.org/). As shown in , RP5-857K21.7 was predicted to have nucleotide sequences complimentary to the one found in the binding site of miR-508-3p, suggesting that RP5-857K21.7 could sponge miR-508-3p. To confirm this result, we conducted a luciferase reporter gene experiment which revealed that miR-508-3p mimics significantly inhibited the luciferase activity of cells transfected with the RP5-857K21.7 wild-type (wt) vector but had no significant inhibiting impact on the luciferase activity of ASMCs transfected with the RP5-857K21.7 mutant-type (mut) vector when compared to miR-NC (, p < .01). Furthermore, we confirmed the efficiency of the miR-508-3p mimics employed in this study. As shown in , miR-508-3p was significantly upregulated in the ASMCs transfected with miR-508-3p mimics when compared to the those transfected with miR-NC (p < .01). Besides, through biotinylated RNA pull-down assay, we found that more miR-508-3p were significantly enriched in the ASMCs transfected with the biotin-labelled RP5-857K21.7 wild-type (WT) probe compared to the mutant-type (MUT), confirming that RP5-857K21.7 can directly interact with miR-508-3p sequence in in ASMCs cells (, p < .01). Through an RIP-qRT-PCR experiment, we also established evidence that supports the ability of the RP5-857K21.7 to directly interact with the miR-508-3p sequence in ASMCs cells through the Ago2 complex as more RP5-857K21.7 and miR-508-3p sequence were found to be significantly enriched in precipitated Ago2 compared to the IgG (, p < .01). Moreover, we observed that the overexpression of RP5-857K21.7 significantly increased the expression level of miR-508-3p in ASMCs, further validating the regulative ability of RP5-857K21.7 (, p < .01). Finally, we measured the expression level of miR-508-3p in ASMCs after induction with PDGF-BB. Our result revealed that miR-508-3p was significantly upregulated in PDGF-BB-induced ASMCs compared to the control group, suggesting that the miR-508-3p could also be significantly involved in the regulation of PDGF-BB in ASMCs (, p < .01).
Figure 3. RP5-857K21.7 directly targets miR-508-3p (A) Bioinformatics prediction of RP5-857K21.7 target miRNA using miRcode online database (B) Confirmation of prediction result through dual-luciferase reporter gene assay. MiR-508-3p mimics significantly inhibited the luciferase activity of cells transfected with the RP5-857K21.7 wild-type (wt) vector but had no significant inhibiting impact on the luciferase activity of ASMCs transfected with the RP5-857K21.7 mutant-type (mut) vector when compared to miR-NC. (C) QRT-PCR analysis of miR-508-3p expression in ASMCs. MiR-508-3p mimics significantly upregulated miR-508-3p expression in ASMCs compared to miR-NC. (D) Biotinylated RNA pull-down assay showed that more miR-508-3p was significantly enriched in the ASMCs transfected with the biotin-labelled RP5-857K21.7 wild-type (WT) probe compared to those transfected with the mutant-type (MUT). RP5-857K21.7 can directly interact with miR-508-3p sequence in in ASMCs cells (E) RIP-qRT-PCR revealed that more RP5-857K21.7 and miR-508-3p sequence were found to be significantly enriched in precipitated Ago2 compared to the IgG. (F) QRT-PCR indicated that the overexpression of RP5-857K21.7 significantly increased the expression level of miR-508-3p in ASMCs, validating the regulative ability of RP5-857K21.7. The experimental data are presented as the mean ± SD of at least three independent experiments and the significance level is defined as p < .05.
RP5-857K21.7 can regulate the PI3K/AKT/mTOR pathway by sponging miR-508-3p in ASMCs cells
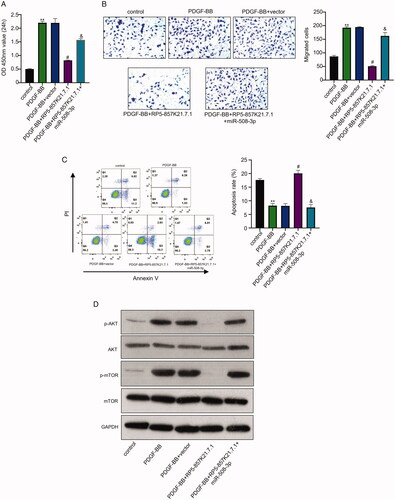
To determine if RP5-857K21.7 regulates the progression of asthma, we overexpress RP5-857K21.7 and miR-508-3p in PDGF-BB-induced ASMCs and examined their effect on the function of the cells. Through western blot analysis, we also determine if the PI3K/AKT/mTOR pathway, which has been widely reported to regulate cell cycle and consequently involved in the proliferation and progression of cancer cells [Citation17], is involved in the progression of the PDGF-BB-induced ASMCs. As shown in , co-transfecting the PDGF-BB-induced ASMCs with RP5-857K21.7 overexpression plasmid markedly reduced the proliferation of the cell compared to the PDGF-BB + vector experimental group (p < .01). This was, however, restored after co-transfecting the cell with miR-508-3p mimics (i.e. PDGF-BB + RP5-857K21.7 + miR-508-3p) (, p < .01). Furthermore, we found that co-transfecting the PDGF-BB-induced ASMCs with the RP5-857K21.7 overexpression plasmid and miR-508-3p mimics restored the migrative ability of the cells which was subsequently inhibited in the PDGF-BB + RP5-857K21 cell group suggesting that the overexpression of miR-508-3p could contribute to the progression of PDGF-BB in ASMCs (, p < .01). We also examined the effect of overexpressing RP5-857K21.7 and miR-508-3p on the apoptosis rate of the PDGF-BB-induced ASMCs. From the result, we observed that overexpressing RP5-857K21.7 in PDGF-BB-induced ASMCs significantly increased the rate of apoptosis in the cell which later reduced after co-transfection with miR-508-3p mimics (, p < .01). Lastly, western blot analysis showed that the PI3K and p-mTOR protein levels in the ASMCs after PDGF-BB induction markedly increased compared to the control group, while the expression level of the AKT and mTOR protein remain unchanged (). Our result demonstrated that co-transfecting the PDGF-BB-induced ASMCs with RP5-857K21.7 overexpression plasmid and miR-508-3p mimics notably restored the inhibited PI3K and p-mTOR protein expression level observed in the DGF-BB + RP5-857K21.7 group, suggesting that the RP5-857K21.7 could regulate the PI3K/AKT/mTOR pathway by sponging miR-508-3p.
Figure 4. The RP5-857K21.7 regulates the PI3K/AKT/mTOR pathway by sponging miR-508-3p in ASMCs cells (A) and (B) CCK-8 and transwell assay shows that the overexpression of RP5-857K21.7 markedly reduced the proliferation and migration of the PDGF-BB-induced ASMCs compared to the empty vector while the co-transfection of miR-508-3p mimics significantly restored the cell proliferation and migration. (C) Flow cytometry detection of apoptosis rate of PDGF-BB-induced ASMCs showed that overexpressing RP5-857K21.7 significantly increased the rate of apoptosis in the cell which was then reduced after co-transfection with miR-508-3p mimics. (D) Western blot analysis revealed that the PI3K and p-mTOR protein expression level markedly increased in the ASMCs after PDGF-BB induction compared to the control group while that of AKT and mTOR protein remain unchanged. Data are presented as the mean ± SD of at least three independent experiments with a significance level of p < .05.
Discussion
Regarded as one of the most common lungs ailing health conditions, Asthma is a persistent and heterogenic disease that affects the respiratory system [Citation18]. It has been reported that airway remodelling plays a critical role in asthma pathology. Airway wall thickening culminates from the airway smooth muscle cell proliferation induced by growth factors [Citation19]. Our findings revealed that the expression of RP5-857K21.7 was significantly reduced in ASMCs induced by PDGF-BB in comparison with control group suggesting that the upregulation of RP5-857K21.7 could mediate the biological effect of PDGF-BB on ASMCs. Accumulating evidence showed that several growth factors such as epidermal growth factor, basic fibroblast growth factor, the vascular endothelial growth factor and the AA and BB isoform of the PDGF have been implicated in the pathogenesis of airway inflammation in asthma [Citation20]. PDGF-BB was detected as a potent biomarker of airway remodelling and vital mediator in asthma development and progress by increasing FeNO level [Citation21]. Furthermore, PDGF-BB reportedly induced significant increase in the proliferation and migration of asthmatic and non-asthmatic cells [Citation22]. Consistent with the findings above, our investigation revealed that there is an increased proliferation and migration of ASMCs after induction with PDGF-BB compared with control which further confirms that PDGF-BB plays a key role in the pathogenesis of ASMCs.
LncRNA has been implicated in many human health conditions. The effect of lncRNA on the pathogenesis of systemic inflammation of asthma has been studied [Citation23]. For instance, the overexpression of lncRNA-H19 has been reported to inhibit the proliferation and migration of ASMCs treated with PDGF-BB [Citation24]. Downregulating the differentially expressed lncRNA-AK149641 in OVA mice attenuated airway inflammatory response in OVA-induced asthma mouse model [Citation25]. Another study by Zhang et al., 2016 showed that the lncRNA BCYRN1 promotes ASMCs proliferation and migration in an asthma rat model [Citation26]. Strikingly, our investigation reveals that overexpression of the RP-5-857K21.7 sufficiently reduced the proliferative and migration ability of the PDGF-BB-stimulated ASMCs and increased apoptosis when compared with the control. This data suggested that RP-5-857K21.7 is critical to the development of asthma and its overexpression could inhibit the progression of ASMCs as well as promote its apoptosis. Over the years, miRNAs have been shown to play a critical role in lung development and the maintenance of diseased-free lungs [Citation27–29]. A study by Maes et al., 2016 revealed that there was an increased expression of miR-223-3p, miR-142-3p and miR-629-3p in the sputum of patients with severe asthma [Citation12]. For instance, miR-508-3p suppresses the proliferation and invasion of ovarian cancer cells by directly targeting the 3’UTR of cyclin A2 (CCNA2), and matrix metallo-proteinase-7 (MMP7), respectively [Citation30]. In a bid to understand the mechanism through which RP-5-857K21.7 promotes the proliferation and migration of ASMCs in asthma, bioinformatics analysis was conducted which revealed that RP-5-857K21.7 consist of nucleotides complimentary to the ones on the miR-508-3p’s binding region, suggesting that the RP-5-857K21.7 could endogenously bind to miR-508-3p and regulate its expression. A dual-luciferase reporter gene assay confirmed this prediction result. Furthermore, Biotinylated RNA pull-down assay and RIP-qRT-PCR analysis confirmed the ability of RP-5-857K21.7 to interact with miR-508-3p. Besides, we found that silencing RP-5-857K21.7 leaded to a significantly increase in the expression level of miR-508-3p. Cumulatively, our result revealed that the overexpression of miR-508-3p promotes the proliferation and migration of ASMCs induced by PDGF-BB which indicates that miR-508.3p could also be significantly involved in the regulation of PDGF-BB-induced ASMCs.
Furthermore, the PI3K/AKT/mTOR pathways has been implicated in the ASMC proliferation and migration. And several studies have demonstrated the important role of PI3K in asthma pathophysiology as its inhibition promotes therapeutically beneficial processes including blunt mucus production, prevention of mast cell degranulation, reduction in immune cell recruitment, and facilitation of bronchodilation [Citation31]. Also, the expression of α-smooth muscle actin has been shown to be regulated by miR-133a via the PI3K/AKT/mTOR signalling pathway by targeting IGF1R [Citation32]. In our study, an increased level of PI3K/AKT and p-mTOR protein was observed in PDGF-BB induced ASMCs in asthma compared to control group, whereas overexpression of RP-5-857K21.7 and miR-508-3p restored the inhibited PI3K and p-mTOR protein expression suggesting that RP-5-857K21.7 could regulate ASMCs in asthma via the PI3K/AKT/mTOR pathway by sponging miR-508-3p.
Conclusion
The RP5-857K21.7 is lowly expressed in PDGF-BB-induced ASMCs cells and its overexpression markedly inhibit PDGF-BB-induced ASMCs cell proliferation, migration and induce apoptosis by regulating the PI3K/AKT/mTOR pathway through sponging miR-508-3. RP5-857K21.7 could be a potential diagnostic tool or therapeutic target for treating asthma disease.
Author contributions
Xiaojun Wang and Lingfen Xu designed the study. Yong Yu performed the experiments, Yimin Fu analysed the data. The first draft of the manuscript was written by Xiaojun Wang and Yimin Fu, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Disclosure statement
The authors declare that they have no competing interests.
Data availability statement
All data generated or analysed during this study are included in this published article and its additional files.
References
- Kardas G, Daszyńska-Kardas A, Marynowski M, et al. Role of platelet-derived growth factor (PDGF) in asthma as an immunoregulatory factor mediating airway remodeling and possible pharmacological target. Front Pharmacol. 2020;11:47.
- Bergeron C, Tulic MK, Hamid Q. Airway remodelling in asthma: from benchside to clinical practice. Can Respir J. 2010;17(4):e85–e93.
- Lin L, Li Q, Hao W, et al. Upregulation of LncRNA Malat1 induced proliferation and migration of airway smooth muscle cells via miR-150-eIF4E/akt signaling. Front Physiol. 2019;10:1337.
- Clarke DL, Dakshinamurti S, Larsson AK, et al. Lipid metabolites as regulators of airway smooth muscle function. Pulm Pharmacol Ther. 2009;22(5):426–435.
- Hirota JA, Ask K, Farkas L, et al. In vivo role of platelet-derived growth factor-BB in airway smooth muscle proliferation in mouse lung. Am J Respir Cell Mol Biol. 2011;45(3):566–572.
- Lopez-Urrutia E, Bustamante Montes LP, Ladron de Guevara Cervantes D, et al. Crosstalk between long non-coding RNAs, Micro-RNAs and mRNAs: deciphering molecular mechanisms of master regulators in cancer. Front Oncol. 2019;9:669.
- Salmena L, Poliseno L, Tay Y, et al. A ceRNA hypothesis: the rosetta stone of a hidden RNA language? Cell. 2011;146(3):353–358.
- Jalali S, Bhartiya D, Lalwani MK, et al. Systematic transcriptome wide analysis of lncRNA-miRNA interactions. PLoS One. 2013;8(2):e53823,
- Zhu X, Wei Y, Dong J. Long noncoding RNAs in the regulation of asthma: current research and clinical implications. Front Pharmacol. 2020;11:532849.
- Wang WL, Luo XM, Zhang Q, et al. The lncRNA PVT1/miR-590-5p/FSTL1 axis modulates the proliferation and migration of airway smooth muscle cells in asthma. Autoimmunity. 2021;54(3):138–110.
- Roffel MP, Bracke KR, Heijink IH, et al. miR-223: a key regulator in the innate immune response in asthma and COPD. Front Med (Lausanne)). 2020;7:196.
- Maes T, Cobos FA, Schleich F, et al. Asthma inflammatory phenotypes show differential microRNA expression in sputum. J Allergy Clin Immunol. 2016;137(5):1433–1446.
- Huang T, Kang W, Zhang B, et al. miR-508-3p concordantly silences NFKB1 and RELA to inactivate canonical NF-κB signaling in gastric carcinogenesis . Mol Cancer. 2016;15:9.
- Zhai Q, Zhou L, Zhao C, et al. Identification of miR-508-3p and miR-509-3p that are associated with cell invasion and migration and involved in the apoptosis of renal cell carcinoma. Biochem Biophys Res Commun. 2012;419(4):621–626.
- Guo SJ, Zeng HX, Huang P, et al. MiR-508-3p inhibits cell invasion and epithelial-mesenchymal transition by targeting ZEB1 in triple-negative breast cancer. Eur Rev Med Pharmacol Sci. 2018;22:6379–6385.
- Yu X, Zhe Z, Tang B, et al. α-Asarone suppresses the proliferation and migration of ASMCs through targeting the lncRNA-PVT1/miR-203a/E2F3 signal pathway in RSV-infected rats. Acta Biochim Biophys Sin (Shanghai)). 2017;49(7):598–608.
- King D, Yeomanson D, Bryant HE. PI3King the lock: targeting the PI3K/akt/mTOR pathway as a novel therapeutic strategy in neuroblastoma. J Pediatr Hematol Oncol. 2015;37(4):245–251.
- Barros R, Moreira A, Padrao P, et al. Dietary patterns and asthma prevalence, incidence and control. Clin Exp Allergy. 2015;45(11):1673–1680.
- Wilkinson WB, Poswillo DE. Asymmetry in mandibulofacial dysostosis. J Craniofac Genet Dev Biol. 1991;11(1):41–47.
- Zou H, Fang QH, Ma YM, et al. Analysis of growth factors in serum and induced sputum from patients with asthma. Exp Ther Med. 2014;8(2):573–578.
- Brzozowska A, Majak P, Jerzyńska J, et al. Exhaled nitric oxide correlates with IL-2, MCP-1, PDGF-BB and TIMP-2 in exhaled breath condensate of children with refractory asthma. Pdia. 2015;2:107–113.
- Ambhore NS, Katragadda R, Raju Kalidhindi RS, et al. Estrogen receptor beta signaling inhibits PDGF induced human airway smooth muscle proliferation. Mol Cell Endocrinol. 2018;476:37–47.
- Zhu Y, Mao D, Gao W, et al. Analysis of lncRNA expression in patients with eosinophilic and neutrophilic asthma focusing on LNC_000127. Front Genet. 2019;10:141.
- Yu H, Qi N, Zhou Q. LncRNA H19 inhibits proliferation and migration of airway smooth muscle cells induced by PDGF-BB through miR-21/PTEN/akt axis. J Asthma Allergy. 2021;14:71–80.
- Zhang J, Zhou Y, Gu H, et al. LncRNA-AK149641 associated with airway inflammation in an OVA-induced asthma mouse model. J Bioenerg Biomembr. 2020;52(5):355–365.
- Zhang XY, Zhang LX, Tian CJ, et al. LncRNAs BCYRN1 promoted the proliferation and migration of rat airway smooth muscle cells in asthma via upregulating the expression of transient receptor potential 1. Am J Transl Res. 2016;8(8):3409–3418.
- Schickel R, Boyerinas B, Park SM, et al. MicroRNAs: key players in the immune system, differentiation, tumorigenesis and cell death. Oncogene. 2008;27(45):5959–5974.
- Sonkoly E, Wei T, Janson PC, et al. MicroRNAs: novel regulators involved in the pathogenesis of psoriasis? PLoS One. 2007;2(7):e610,
- Nana-Sinkam SP, Hunter MG, Nuovo GJ, et al. Integrating the MicroRNome into the study of lung disease. Am J Respir Crit Care Med. 2009;179(1):4–10.
- Guo F, Zhang K, Li M, et al. miR-508-3p suppresses the development of ovarian carcinoma by targeting CCNA2 and MMP7. Int J Oncol. 2020;57(1):264–276.
- Yoo EJ, Ojiaku CA, Sunder K, et al. Phosphoinositide 3-kinase in asthma: novel roles and therapeutic approaches. Am J Respir Cell Mol Biol. 2017;56(6):700–707.
- Shao Y, Chong L, Lin P, et al. MicroRNA-133a alleviates airway remodeling in asthma through PI3K/AKT/mTOR signaling pathway by targeting IGF1R. J Cell Physiol. 2019;234(4):4068–4080.
