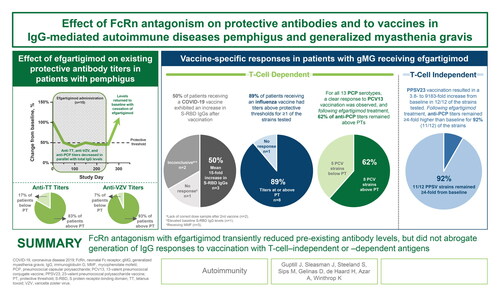
Abstract
Antagonism of the neonatal Fc receptor (FcRn) by efgartigimod has been studied in several autoimmune diseases mediated by immunoglobulin G (IgG) as a therapeutic approach to remove pathogenic IgGs. Whereas reduction of pathogenic titres has demonstrated efficacy in multiple autoimmune diseases, reducing total IgG could potentially increase infection risk in patients receiving FcRn antagonists. The objective of this study was to analyse the effect of FcRn antagonism with efgartigimod on existing protective antibody titres and the ability to mount an immune response after vaccine challenge. Serum levels of total IgG and protective antibodies against tetanus toxoid (TT), varicella zoster virus (VZV), and pneumococcal capsular polysaccharide (PCP) were measured in all patients enrolled in an open-label trial of efgartigimod for the treatment of pemphigus. Vaccine specific-responses were assessed by measuring changes in IgG titres in patients with generalised myasthenia gravis (gMG) who were treated with efgartigimod and who received influenza, pneumococcal, or coronavirus disease 2019 (COVID-19) vaccines during participation in the double-blind trial ADAPT or open-label extension, ADAPT+ (n = 17). FcRn antagonism reduced levels of protective anti-TT, anti-VZV, and anti-PCP antibodies and total IgG to a similar extent; anti-TT and anti-VZV titres remained above minimally protective thresholds for the majority of patients, (10/12) 83% and (14/15) 93% respectively. Protective antibodies returned to baseline values upon treatment cessation. Antigen-specific IgG responses to influenza, pneumococcal, and COVID-19 immunisation were detected in patients with gMG who received these vaccines while undergoing therapy with efgartigimod. In conclusion, FcRn antagonism with efgartigimod did not hamper generation of IgG responses but did transiently reduce IgG titres of all specificities.
Graphical Abstract
Introduction
Immunoglobulin G (IgG)–mediated autoimmune diseases, such as pemphigus and generalised myasthenia gravis (gMG), represent a continual and increasing health burden [Citation1]. Pemphigus is characterised by the presence of autoantibodies targeting epidermal desmoglein (Dsg)-3 and/or Dsg-1 and comprises two main subtypes, pemphigus vulgaris (PV) and pemphigus foliaceus (PF). Clinical manifestations include mucosal and skin lesions that may become life-threatening (primarily due to secondary infections) if left untreated [Citation2–4]. gMG is a rare chronic disease that causes debilitating and potentially life-threatening muscle weakness. Approximately 85% of patients with gMG have IgG autoantibodies against skeletal muscle nicotinic acetylcholine receptor (AChR), muscle-specific tyrosine kinase (MuSK), and low-density lipoprotein receptor-related protein 4 (LRP4); of these, AChR antibodies are the most common [Citation5–7].
Both conditions are associated with increased risk of infection—often due to immunological abnormalities and the immunosuppressive therapies used to treat them. Due to the immunocompromised status and baseline bulbar and respiratory muscle weakness, patients with gMG are at an increased risk of morbidity and mortality from respiratory diseases, including infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the cause of coronavirus disease 2019 (COVID-19) [Citation8,Citation9]. Long-term corticosteroid use and rituximab therapy were also predictive of severe COVID-19 infections in patients with gMG [Citation10]. Likewise in pemphigus, data from an ongoing French study suggest immunosuppression with rituximab in this population may be a major risk factor for COVID-19 [Citation11].
Antagonism of the neonatal Fc receptor (FcRn) results in a reduction in IgG levels and is being evaluated as a treatment approach for pemphigus and gMG, among other conditions. Efgartigimod is a human IgG1 antibody Fc-fragment, a natural ligand of FcRn, that has been engineered using ABDEG technology to have increased affinity to FcRn compared to endogenous IgG while retaining the characteristic pH dependence [Citation12]. While the molecules containing ABDEG mutations (M252Y/S254T/T256E/H433K/N434F) increase the affinity to FcRn, these mutations have been shown to have diminished binding to FcγRs [Citation13]. Efgartigimod, bound to FcRn, outcompetes endogenous IgGs from binding FcRn, thereby leading to their degradation in lysosome and reducing their half-life. In clinical trials involving patients with pemphigus and gMG, efgartigimod reduced concentrations of all IgG subclasses to similar extent including pathogenic IgG autoantibodies, without affecting levels of other immunoglobulins or albumin [Citation12, Citation14–16]. The reduction of IgG was transient and incomplete and preclinical models have also shown that IgG production is not impaired [Citation17]. Thus, efgartigimod treatment should not preclude the potential for patients to mount an IgG immune response.
In this study, we sought to investigate the impact of efgartigimod treatment on humoral immune responses in pemphigus and gMG. In patients with pemphigus treated with efgartigimod for up to 34 weeks, we examined levels of protective antibodies associated with prior vaccinations or infection. In patients with gMG who participated in ADAPT and the corresponding open-label extension (ADAPT+), we analysed humoral responses among those vaccinated to influenza, pneumococcus, or SARS-CoV-2 during the trial.
Methods
Study designs
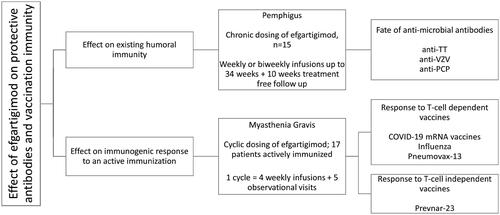
This study consisted of two posthoc analyses of data from a phase 2, open-label trial of efgartigimod in patients with pemphigus (NCT03334058) and from ADAPT, a phase 3, randomised, double-blind, placebo-controlled trial of efgartigimod in gMG (NCT03669588) and its open-label extension (ADAPT+; NCT03770403), as illustrated in . These trials were conducted in accordance with Good Clinical Practice guidelines and conformed to the ethical principles of the Declaration of Helsinki and relevant country-specific laws, with appropriate review and approval of study protocols and forms by the relevant ethics committees or institutional review boards and collection of written, informed consent from all trial participants [Citation15,Citation16].
Figure 1. Schematic illustration of pemphigus and gMG trials, trial dosing, and post-hoc analysis conducted.
Pemphigus trial
The pemphigus phase 2 open-label study was an adaptive study to learn the conditions of use of efgartigimod in pemphigus. The present analysis involved 15 patients participating in cohort 4 of the phase 2 pemphigus trial (Table S3). In earlier cohorts, dosing every 4 weeks was insufficient to maintain autoantibody suppression and disease activity, while with weekly and alternate-week dosing, sufficient autoantibody-suppression to afford clinical benefit was observed. Patients treated in cohort 4, were dosed with weekly efgartigimod until reaching end of consolidation (EoC, the time at which approximately 80% of blisters have healed and no new blisters developed for at least two weeks) and then dosed on alternative weeks up to 34 weeks. These patients exhibited the strongest and the longest IgG suppression, thus represent the most relevant group for vaccination immunity assessment. All patients in this group had a confirmed diagnosis of PV or PF (by positive direct immunofluorescence showing IgG deposits on the keratinocytes cell surface, positive indirect immunofluorescence on monkey oesophagus, and/or positive Dsg-1/3 enzyme linked immunosorbent assay) and mild to moderate disease defined by a pemphigus disease area index (PDAI) <45 at baseline. Newly diagnosed as well as relapsing patients were eligible. These participants received intravenous efgartigimod 25 mg/kg with frequency as described above. Newly diagnosed patients, and relapsing patients who were off-therapy, also received prednisone 20 mg/day at baseline; those already taking prednisone continued receiving the tapered dose at which relapse occurred. Oral prednisone could be tapered from EoC. Full design details and study results have been published [Citation15]. History of vaccination within the last 4 weeks prior to baseline visit, or with a planned vaccination during the study, with the exception of seasonal vaccination (e.g. influenza vaccine), were an exclusion criteria. Serum titres of protective vaccine antibodies against tetanus toxoid, varicella zoster virus and pneumococcal capsular polysaccharide were exploratory endpoints.
ADAPT
In ADAPT, 167 patients with gMG who on a stable dose of at least one MG therapy (acetylcholinesterase inhibitor, steroid, and/or non-steroidal immunosuppressant) were randomised 1:1 to efgartigimod IV 10 mg/kg or placebo. A full list of the inclusion and exclusion criteria can be found in the supplementary appendix of the primary publication [Citation16]. Treatments were administered as once weekly intravenous infusions for 4 weeks followed by a treatment-free period of at least 5 weeks. Additional treatment cycles were initiated based on an individualised dosing schedule according to clinical evaluation. After week 26, all participants could rollover into the ongoing, open-label extension (ADAPT+), during which they received efgartigimod according to an individualised cycle-based treatment schedule, including a minimum treatment-free period of 4 weeks. Significantly more patients in the efgartigimod group than in the placebo group had clinically meaningful improvements in the clinical patient-reported Myasthenia Gravis Activities of Daily Living and physician-reported Quantitative Myasthenia Gravis scores. The primary and some secondary endpoints required patients to have a clinically meaningful improvement in the associated outcome measure, and for this improvement to persist for at least 4 weeks. In AChR-Ab seropositive patients, there was a mean maximum reductions of 61.3% (SD 0.9) in total IgG, 1 week after the fourth infusion in cycle 1 and levels returned to baseline by week 12. The reductions observed in AChR-Ab levels were similar and followed the same time course, with a mean maximal reduction of 57.6% (SD 1.3) obtained 1 week after the fourth infusion in cycle 1 as shown in the overlay of total IgG and anti-AChR-Ab levels in Figure S2. Full trial results have previously been published [Citation16].
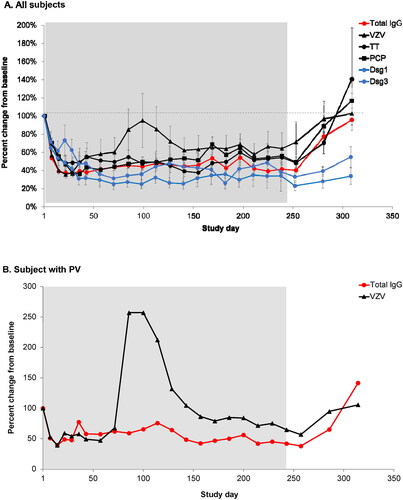
Figure 2. (A) Mean percent change from baseline for anti-varicella zoster virus (VZV), anti-tetanus toxin (TT), anti–pneumococcal capsular polysaccharide (PCP), anti-desmoglein 1 (Dsg1), anti-desmoglein 3 (Dsg3), and total immunoglobulin G (tIgG) antibodies in 15 patients treated for up to 238 days (34 weeks) with efgartigimod. For anti-Dsg 1/3 antibodies, data is plotted for patients who presented with positive anti-Dsg1/3 autoantibody levels ≥ 20 U/mL at baseline (n = 13 for anti-Dsg1, n = 8 for anti-Dsg3), excluding one patient who presented with positive anti-Dsg3 autoantibody levels only on day 235 when relapse occured. Error bars represent standard error of the mean (SEM). (B) Percent change from baseline for anti-VZV and tIgG in a patient with pemphigus vulgaris (PV) exhibiting a transient increase in anti-VZV IgG titres. Treatment period is indicated by background shading.
The present analysis includes ADAPT participants who received an influenza or a pneumococcal vaccination during the 26-week double-blind treatment period or the ongoing open-label extension of the trial (ADAPT+) by the cut-off date of 10 September 2020 or who received a COVID-19 vaccine during ADAPT + prior to the cut-off date of 14 June 2021. The ADAPT protocol permitted vaccinations including inactivated or live-attenuated viruses if immunizations were given at least 48 h before or 48 h after efgartigimod administrations. No designated samples were collected to investigate established protective vaccine titres.
Immunoglobulin assays
Pemphigus trial
Serum titres of protective vaccine antibodies against tetanus toxoid (TT), varicella zoster virus (VZV), and serotypes of Streptococcus pneumoniae capsular polysaccharide were evaluated for all 15 patients enrolled in cohort 4 of the open-label study of efgartigimod in pemphigus. Blood samples used in IgG assays were taken during each study visit from baseline through week 34 of the treatment period and continuing through the 10-week, treatment-free follow-up period. Total serum IgG was assessed using enzyme-linked immunosorbent assay (ELISA). Vaccine-specific serum IgG levels were measured using indirect enzyme immunoassay (EIA; Virotech, Rüsselsheim am Main, Germany) for TT (assay range 0.01-10 IU/mL), chemiluminescence immunoassay (CLIA; DiaSorin, Saluggia, Italy) for VZV (assay range 10-4000 mIU/mL), and EIA (The Binding Site Group, Birmingham, UK) for pneumococcal serotypes (assay range 3.3-270 mg/L).
Based on previous studies, protective titre thresholds were defined for anti-TT as >0.1 IU/mL [Citation18] and anti-VZV as >150 IU/L [Citation19,Citation20]. Protective antibody thresholds for pneumococcal IgG have not been previously established for assays that utilise the 23-valent pneumococcal polysaccharide vaccine (PPSV23) antigen, and interlaboratory standardisation of the PPSV23 assays has not been established. However, a 2- to 4-fold rise in IgG titres or levels >1.3 µg/mL for >50% to 70% of the serotypes one month after immunisation with PPSV23 is generally considered a satisfactory post-vaccine (30 days) serologic response to pneumococcal polysaccharide vaccines [Citation21,Citation22].
ADAPT
In ADAPT, pharmacodynamic analyses were conducted weekly for 8 weeks after initiation of each cycle and then every 2 weeks for 26 weeks during the double-blind phase as well as in a similar pattern for the first year in ADAPT+. Serum samples used for vaccine-specific IgG analysis were taken from each vaccinated patient before vaccination, approximately 4 weeks after vaccination, and between 56 and 239 days after vaccination. Total IgG was assessed using ELISA (SGS, Poitiers, France) and pathogenic AChR IgG antibodies were determined in AChR-Ab seropositive patients using a validated RIA assay (SGS, Poitiers, France). IgGs specific to the capsular polysaccharide of each of the 13 serotypes in the 13-valent pneumococcal conjugate vaccine (PCV13) were quantified with a Multiplex Luminex® assay (Covance/Labcorp, Princeton, NJ) and the same assay was used to assess the response to the 12 serotypes PPSV23 contains in common with PCV13. The World Health Organisation recommends using an IgG antibody concentration of ≥350 ng/mL as the threshold of protection against pneumococcal serotypes included in pneumococcal conjugate vaccines [Citation23–25].
Receptor-binding domain (RBD) IgGs specific for SARS-CoV-2 S-protein were assessed using a 5-plex Luminex Assay (performed at Q2 Solutions, Morrisville, NC). Serum concentrations of the IgG antibodies specific to a panel of five different antigens used as capture molecules: Spike Protein S2 (S2), Nucleocapsid (N), Spike Protein S1 (S1), Full-length Protein S (S1 + S2 extra cellular domain), and Receptor binding domain (RBD) were evaluated in a multiplex bead assay. Each of the recombinant antigens were covalently coupled to one of five distinct Luminex magnetic colour-coded microspheres (“bead-conjugates”). Each microsphere can be recognised and quantified by dual-laser based fluorescent flow cytometry. A cut-off value for SARS-CoV-2 IgG antibody titres that defines clinical immunity has not yet been established [Citation26,Citation27]. However, convalescent plasma was used for validation and samples at high, medium, and low concentrations (titres ranging from 620.2 to 4029.2 AU/ml) were evaluated to serve as reference values (Supplemental Table S1). A standard curve for each of the SARS-CoV-2 antigens was calculated using a 4-parameter logistic curve fit. Since the absolute antibody concentration in the serum cannot be quantified, the reference pooled human serum was used to calculate relative titres, which were reported in arbitrary units per millilitre (AU/mL). As only RBD IgG levels are relevant in the context of vaccination against SARS-CoV-2, only these levels were reported. The lower limit of quantification (LLOQ) was 23.6 AU/mL.
Hemagglutination inhibition assay
Anti-influenza vaccine titres were determined using the hemagglutination inhibition assay (HI; Covance/Labcorp, Princeton, NJ). The reported neutralising titre is the reciprocal of the highest dilution of serum that inhibits hemagglutination for a particular strain. A titre of ≥1:40 is considered seroprotective in adults [Citation28,Citation29]. HI titres were evaluated against the influenza strains included in the 2019-2020 influenza season vaccines: A/Brisbane/02/2018 (H1N1)pdm09-like virus, A/Kansas/14/2017 (H3N2)-like virus, and B/Colorado/06/2017-like virus (B/Victoria/2/87 lineage) in the trivalent formulation and these three strains plus B/Phuket/3073/2013-like virus (B/Yamagata lineage) in the quadrivalent formulation [Citation30].
Data analysis
Total serum IgG and protective antibody levels were summarised as mean percent change from baseline for each Ig measured or reported in units and plotted graphically by study days.
Results
Total and specific IgG levels during long-term efgartigimod therapy in patients with pemphigus
Results from the phase 2 trial of efgartigimod in pemphigus have been previously reported [Citation15, Citation31]. Briefly, disease control was achieved in 28 of 31 participants (90%) and complete clinical remission assessed only in cohort 3 and 4, was achieved in 14 of 22 participants (64%). Clinical improvements were strongly associated with the pharmacodynamic effect of efgartigimod. Over 34 weeks of efgartigimod treatment and 10 weeks of post-treatment follow-up, patients with pemphigus exhibited a median 49% reduction from baseline in total serum IgG level after the first infusion and a median 66% reduction on Day 29 after 4 weekly infusions (). Serum levels of anti-Dsg-1 and anti-Dsg-3 autoantibodies reached 50-60% reduction as long as efgartigimod was dosed. Anti-VZV and anti-PCP antibodies reached nadir between Days 22 and 36, whereas anti-TT IgG exhibited a somewhat slower decline. Total IgG and protective titres remained stably suppressed for as long as patients were dosed with biweekly efgartigimod. Following the last infusion (Day 238), a gradual return of total IgG and protective antibodies to baseline levels was observed. Pathogenic anti-Dsg autoantibodies showed more prolonged suppression as compared to total IgG and protective antibodies.
Twelve of 15 patients had baseline anti-TT IgG titres >0.1 IU/mL. Of those, two patients exhibited a transient decline of titres below 0.1 IU/mL, whereas 10 patients remained in the protective range throughout the study. We observed a transient and incidental drop in VZV-specific titres below the threshold of 150 IU/L in 1 subject whose baseline titre was 273 IU/L. No clinical signs of infection at times of maximal antibody suppression were observed.
During a period of stable total IgG suppression, an increase in anti-VZV antibodies was observed in two patients starting at approximately Day 36 for one patient and at Day 71 for the second patient (), possibly due to an exposure to varicella or subclinical reactivation of latent VZV. No clinical signs of varicella infection were reported for either patient.
Response of patients with gMG to T-cell dependent vaccines
COVID-19 vaccination
During the ADAPT open-label extension (ADAPT+), seven patients received two doses of a mRNA COVID-19 vaccine by the cut-off date of 14 June 2021 (Supplemental Table S2). Few blood samples were taken during ADAPT+, and no total IgG levels were analysed. Therefore, we assessed the humoral response by measuring S-RBD IgGs from the limited sample set, and no correlation with total IgG levels was made.
None of the vaccinated patients experienced a known COVID-19 infection prior to receiving the vaccine or before the last post-vaccination sample was taken. One patient was immunised with mRNA-1273 (Moderna); the other six received BNT162b2 (Comirnaty; Pfizer), with a dosage interval of 16-40 days (Supplemental Table S2).
Patient 17 was enrolled in ADAPT + but at the time samples were taken had not received efgartigimod treatment and was not receiving any immunosuppressant drugs. After receiving two doses of BNT162b2, the patient exhibited a 20-fold increase in S-RBD IgG levels (max titre of 301.78 AU/ml) of 21 days after receiving the second dose of vaccine ().
Figure 3. Levels of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) S protein receptor-binding domain (S-RBD) immunoglobulin G (IgG) before the first and after the second dose (Day 1) of BNT162b2 (A-F) or mRNA-1273 (G) in individual patients with generalised myasthenia gravis (gMG) who participated in ADAPT+. Shaded areas represent efgartigimod treatment cycles. The dashed line represents the lower limit of quantitation. The lowest total IgG levels are expected 1 week after the last efgartigimod infusion of each cycle (red arrows).
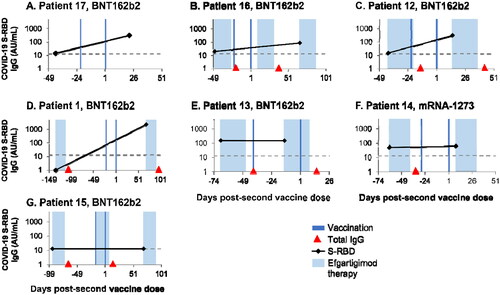
Patient 16 was vaccinated with BNT162b2 1 week after the fourth infusion in an efgartigimod cycle, when total IgG levels are expected to be maximally reduced (represented by the red arrows in ) [Citation16], and received another cycle of efgartigimod after the second dose of BNT162b2. This patient was not receiving any immunosuppressive therapy other than efgartigimod. When the post-vaccination sample was taken 66 days after the second BNT162b2 dose, a 4-fold increase in S-RBD IgG levels (max titre of 82.82 AU/ml) was observed ().
In addition to efgartigimod, Patient 12 was treated concomitantly with prednisolone (5 mg and 7.5 mg orally, each once daily on alternating days). This patient received BNT162b2 at the end of an efgartigimod treatment period and showed a 20-fold increase in S-RBD IgG levels (max titre of 299.56 AU/ml) 15 days after the second dose ().
Patient 1 (), who was not receiving any immunosuppressive therapy other than efgartigimod, was vaccinated with BNT162b2 92 days post-infusion, when total IgG levels are expected to be unaffected, and exhibited a 63-fold increase in post-vaccination S-RBD IgG levels (max titre of 2233.65 AU/ml) 69 days after the second dose.
In the other three patients, no increase in titres was observed (). Patient 15 had a body mass index of 47 kg/m2, was treated with high doses of mycophenolate mofetil (1500 mg twice daily) in addition to efgartigimod, and received both BNT162b2 doses in the middle of an efgartigimod treatment cycle. No increase in S-RBD IgG levels was noted after the second dose of BNT162b2. The two other patients (13 and 14) also received mycophenolate mofetil along with prednisolone (Supplemental Table 2), but post-vaccination samples were collected at incorrect timings (no sample after the second dose for patient 13 and sampling only 9 days after second dose for patient 14), and therefore are not directly comparable.
Influenza vaccination
By 23 January 2020, 11 patients with gMG participating in ADAPT had received an influenza vaccine, including two patients who were receiving placebo when they were vaccinated and nine patients who were receiving efgartigimod. Four of the efgartigimod recipients (Patients 5, 6, 7, and 8) received the quadrivalent influenza vaccine and the other five received an unknown influenza vaccine.
The immune response in the two placebo recipients exceeded the protective threshold (≥1:40) for most strains (). In Patient 1, HI titres against A/H1N1 and A/H3N2 increased after vaccination, and those against B/Yamagata remained stable above the protective threshold (, left). In Patient 2 (, right), HI titres against A/H3N2 and B/Victoria rose above the protective threshold and remained there, while titres against B/Yamagata were stable at that level. Due to a missing sample, post-vaccination titres of A/H1N1 could not be analysed.
Figure 4. . Individual hemagglutination inhibition (HI) titers from 7 patients with generalized myasthenia gravis (gMG) vaccinated against influenza during the double-blind treatment period or open-label extension of ADAPT. (A) Patients who received only placebo. (B) Patients treated with efgartigimod and vaccinated between treatment cycles. (C) Patients treated with efgartigimod and vaccinated within a treatment cycle. (D) Efgartigimod-treated patient who was vaccinated but infected with influenza. PT, protection threshold; ULQ, upper limit of quantification. Solid black line indicates day of vaccination. Dotted black line indicates date of influenza infection.
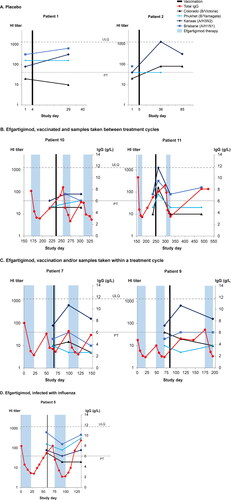
As previously reported [Citation16], total IgG and AChR-Ab IgG levels were significantly reduced in gMG patients treated with efgartigimod ( and , and Figure S2). Individual anti-AChR-Ab levels are not shown in the figures as they follow the same time course as total IgG levels (Figure S2). Patients 10 and 11 were vaccinated between efgartigimod treatment cycles (). In Patient 10 (left panel), titres against A/H3N2 and B/Yamagata increased ∼2-fold 3 weeks after vaccination, when total IgG levels were comparable to pre-vaccination levels. Total IgG decreased during the efgartigimod cycle occurring between measurement of the two post-vaccination HI titres, yet the titres for A/H1N1, A/H3N2, and B/Yamagata were at or above the protective threshold 80 days after vaccination. In Patient 11 (right panel), the pre- and first post-vaccination samples were collected during a treatment-free period when IgG levels were not substantially reduced, and there was a clear post-vaccination immune response against all tested strains. When efgartigimod treatment was initiated 7 weeks after vaccination, total IgG levels were reduced by 61.5% at nadir. Influenza titres followed the same kinetics, decreasing to levels at or just above pre-vaccination levels but remaining above the protective threshold for A/H1N1 and A/H3N2. On day 486, total IgG levels were normalised, and titres against A/H3N2 and A/H1N1 were 2-fold higher than those measured before vaccination.
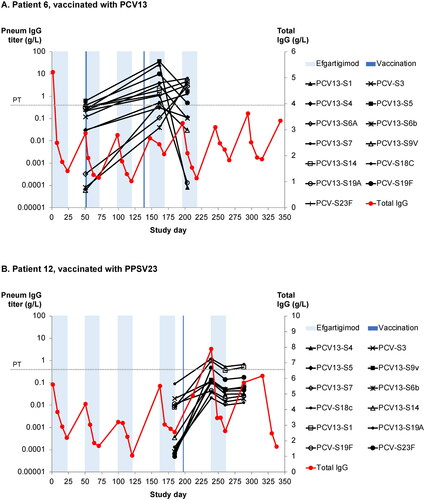
Figure 5. (A) Anti–pneumococcal capsular polysaccharide (PCP) titres against 13 PCP serotypes in an efgartigimod-treated patient after vaccination with 13-valent pneumococcal conjugate vaccine (PCV13). The protective threshold protecting against invasive pneumococcal infection is 350 ng/mL (dotted line). (B) Anti-PCP titres against 12 serotypes in an efgartigimod-treated patient after vaccination with 23-valent pneumococcal polysaccharide vaccine (PPSV13). Shaded areas represent efgartigimod treatment periods.
In Patients 7 and 9, the pre-vaccination samples were taken during an efgartigimod treatment cycle (). Patient 7 (left panel) received the influenza vaccine near the end of an efgartigimod cycle, close to the IgG nadir. Eighty Days after vaccination, when total IgG levels were 2 times higher than those at the time of vaccination, titres against A/H3N2 had increased 8-fold and remained above protective levels after an additional cycle of efgartigimod. Patient 9 (right panel) was vaccinated 2 weeks after an efgartigimod cycle while total IgG levels were still reduced. One month after vaccination, when total IgG levels were 2.5-fold higher than pre-vaccination levels, HI titres against A/H3N2 had increased 8-fold and those against A/H1N1 increased to the protective threshold; titres against both strains remained at or above protective levels throughout a subsequent efgartigimod cycle.
As shown in Figure S1, Patient 6 showed no response to any of the strains tested, whereas titres remained >40 (the protective threshold) for other efgartigimod-treated subjects (Patients 3, 4, and 8), despite efgartigimod-induced IgG reduction.
Of the influenza vaccine recipients, only Patient 5 reported influenza-like illness. Influenza was confirmed with nasal swab, and the grade of the outcome was severe. The patient was diagnosed with influenza at Day 127 in the study, which coincided with the timing of the second post-vaccination sample (). At this time point, the total IgG levels had returned to baseline, and influenza titres were above the protective threshold for all strains except B Victoria. Among ADAPT participants who did not receive a 2019-2020 seasonal influenza vaccine during the study, 2/81 (2.5%) efgartigimod-treated patients and 3/81 (3.7%) of those receiving placebo reported influenza as an adverse event. In the open-label extension, 2/133 (1.5%) unvaccinated patients reported influenza.
PCV13
In addition to the influenza vaccine, Patient 6 randomised in the ADAPT study received two doses of PCV13 based on the determination of the treating physician: the first during an efgartigimod treatment cycle and the second dose in a treatment-free period (). The pre-vaccination sample was collected at the start of the efgartigimod treatment cycle. A clear antibody response was seen towards all 13 strains. After the initiation of another treatment cycle, the titres decreased for some strains but remained above 350 ng/mL for 8 of 13 strains.
Response of patients with gMG to T-cell independent vaccine: PPSV23
Patient 12 was vaccinated with PPSV23 during the fourth dose in the cycle (). Anti-pneumococcal titres were assessed against 12 of 23 strains included in the vaccine, and pre- to post-vaccination titre increases were observed ranging from 3.8- to 9183-fold. After a subsequent cycle, when total IgG levels were maximally reduced, anti-pneumococcal titres remained higher than pre-vaccination levels for all tested strains, with at least 4-fold increase compared to baseline for 11 of 12 assessed strains within this one patient. In general, titres for the tested strains remained substantially increased from baseline levels even during periods of reduced total IgG levels.
Discussion
Antagonism of FcRn has been shown to be a potent therapeutic strategy to clear pathogenic autoantibodies in IgG-driven autoimmune disorders [Citation14–16, Citation32]; however, this study is the first to examine the effect on specific vaccine titres and humoral immune response to novel vaccine challenges in patients treated with the FcRn antagonist efgartigimod. Our data suggest that during efgartigimod treatment, patients can maintain the ability to mount an IgG response to antigen challenges and levels of protective antibody titres, whether induced naturally or by vaccines, closely follow total IgG reduction kinetics.
In the pemphigus population, reductions in protective antibody titres for TT, VZV, and PCP paralleled the reduction in total IgG levels () [Citation12, Citation14, Citation16, Citation32]. The subsequent return to normal levels upon cessation of efgartigimod treatment indicated no negative effect on long-lived plasma cell (LLPC) responses. Based on phase 1 data, IgA and IgM levels should not be impacted and in this study patients also retained the capacity to produce antibodies against an ongoing infection during a phase of maximal IgG reduction induced by efgartigimod.
Immunosuppressive therapy is often utilised in both pemphigus and gMG [Citation3, Citation33] contributing to secondary immune deficiency and therefore an increased risk of infections, including COVID-19 [Citation11, Citation34–38]. The influence of disease pathology and immunosuppressive therapy are inseparable factors that collectively contribute to patients’ vulnerability to infections. In ADAPT, efgartigimod was well tolerated, and the majority of infections (reported by 46% of efgartigimod-treated patients and 37% of those receiving placebo) were mild to moderate in severity [Citation16]. Similarly, no increased infection pattern was observed in an open-label trial of efgartigimod in pemphigus [Citation15]. Upon efgartigimod treatment discontinuation, levels of total serum IgG quickly return to baseline levels. Low rates of infections documented in studies of antibody elimination by plasma exchange or immunoadsorption support the hypothesis that transient and selective reductions in IgG levels would be associated with a lower risk of infection than agents with broader immune actions [Citation35, Citation39–41].
Our analysis demonstrated that patients with gMG retained the ability to generate protective titres to vaccine challenge while receiving efgartigimod, regardless of the timing of vaccinations relative to efgartigimod cycles or the type of vaccine. Previous studies have shown that immune responses to influenza vaccination in patients with gMG were similar to those in healthy subjects, regardless of the use of some immunosuppressive therapies [Citation28]. In our study, an IgG response to T-cell dependent vaccines was observed in the majority (73.3%) of efgartigimod-treated patients, and in one patient to a T-cell independent vaccine, suggesting that FcRn antagonism did not interfere with immune responses to the vaccines studied. Similar numbers of unvaccinated patients in the efgartigimod and placebo groups of the ADAPT study were infected with influenza, suggesting efgartigimod did not necessarily increase susceptibility to influenza infection.
Broad immunosuppressants that affect T lymphocytes, B lymphocytes, or other components of the immune system, such as B-cell depleting agents, corticosteroids, abatacept, methotrexate, and mycophenolate mofetil have been reported to impair immune responses to SARS-CoV-2 vaccines and other vaccines [Citation26, Citation42–48]; indeed, patients in this study who were treated with high doses of mycophenolate mofetil had a reduced IgG response to COVID-19 vaccination. In contrast, patients who received more selective agents such as TNF-inhibitors exhibited a robust serological response after mRNA COVID-19 vaccination [Citation44, Citation49–51]. Efgartigimod also more selectively targets the immune system, reducing IgG levels while leaving other immune system components stated above that are necessary to mount significant immune responses largely untouched [Citation52].
Animal data support the observation that effective immune responses can be mounted in the presence of FcRn antagonism and reduced IgG levels [Citation17, Citation37,Citation38]. Furthermore, subjects with genetic defects resulting in lack of functional FcRn maintained the ability to mount immune responses upon vaccination [Citation53,Citation54]. While neither of these studies fully compare with transient pharmacologic FcRn antagonism, they provide insight on how the lack of functional FcRn can affect immune responses.
The data in our study are observational, and conclusions are limited by several factors. The analysis of ADAPT participants included only a small number of vaccinated patients, and the analysis of COVID-19 immunogenicity relied on limited availability of leftover serum samples. Dedicated sampling was not defined in the ADAPT or ADAPT + protocols, vaccination timing was not standardised, and several data points are missing due to limited sample volume. It was not obligatory to provide all the details on the vaccines such as brand name in the electronic data capture forms (eCRF). Furthermore, the immunogenicity elicited by vaccination varies widely based on age, gender, pre-existing immunity, immunosuppressive therapies, genetic polymorphisms, and the presence of chronic underlying conditions [Citation28, Citation44, Citation47, Citation55,Citation56]. Future studies including a placebo-controlled phase I pneumovax study (NCT05163834), will systematically monitor vaccination responses in efgartigimod-treated patients and will aim to not only assess the antigen-specific IgG responses to both T-cell dependent and T-cell independent antigens, but also evaluate other Ig classes and cellular responses.
In conclusion, reduction of IgG titres via FcRn antagonism with efgartigimod did not impair the ability of the patients analysed to generate new specific IgG responses, regardless of the timing of vaccinations. Immune titres typically increased whether vaccinations were given during or between efgartigimod cycles or when IgG levels were at nadir. Although vaccine-specific IgG levels were reduced proportionally to total IgG levels upon efgartigimod treatment, in general, they remained above the pre-vaccination levels and/or protective thresholds. Upon treatment discontinuation, total IgG levels returned to baseline, and protective titres were increased. These findings from this limited observational dataset suggest that FcRn antagonism does not prevent IgG production, with no impact on LLPCs, and protective antibody levels from vaccination can be maintained, even when total IgG levels are maximally reduced by efgartigimod.
Supplemental Material
Download MS Word (164.4 KB)Disclosure statement
J.T.G. consultant for Immunovant, Alexion, Apellis, Momenta, Ra Pharma, Grifols, Jacobus, Becton Dickinson, Cabaletta, Regeneron, argenx, Janssen, UCB, Toleranzia and Piedmont Pharmaceuticals. He receives industry grant support from UCB pharma for a fellowship training grant. Full disclosure statement available at: https://dcri.org/about-us/conflict-of-interest/. He is an MG trial site investigator for: Alexion, Janssen, UCB Pharma, argenx, Takeda; receives grant/research support from: NIH (NIAID, NINDS, NIMH), Centres for Disease Control and Prevention, and the Myasthenia Gravis Foundation of America. Is now an employee of argenx, but was not directly affiliated during the studies or post hoc analyses.
J.W.S. is a consultant for argenx and CSL Behring.
S.S., M.S., D.G., H.d.H. Are employees of argenx.
A.A. consultant or grants from Grifols, CSL, X4, argenx, Optinose
K. L. W. consultant to GSK, Novartis, Roche, BMS, Pfizer, UCB, Lilly, Galapagos, Abbvie, and Sanofi
Data availability statement
argenx is committed to responsible data sharing regarding the clinical trials they fund. Included in this commitment is access to anonymised, individual, and trial-level data (analysis datasets), and other information (e.g. protocols and clinical study reports), as long as the trials are not part of an ongoing or planned regulatory submission. This includes requests for clinical trial data for unlicensed products and indications. These clinical trial data can be requested by qualified researchers who engage in rigorous independent scientific research and will only be provided after review and approval of a research proposal and statistical analysis plan and execution of a data sharing agreement. Data requests can be submitted at any time and the data will be accessible for 12 months. Requests can be submitted to [email protected].
Additional information
Funding
References
- Ludwig RJ, Vanhoorelbeke K, Leypoldt F, et al. Mechanisms of autoantibody-induced pathology. Front Immunol. 2017;8:603.
- Kridin K, Schmidt E. Epidemiology of pemphigus. JID Innov. 2021;1(1):100004.
- Schmidt E, Kasperkiewicz M, Joly P. Pemphigus. Lancet. 2019;394(10201):882–894.
- Stanley JR, Amagai M. Pemphigus, bullous impetigo, and the staphylococcal scalded-skin syndrome. N Engl J Med. 2006;355(17):1800–1810.
- Gilhus NE, Verschuuren JJ. Myasthenia gravis: subgroup classification and therapeutic strategies. Lancet Neurol. 2015;14(10):1023–1036.
- Gilhus NE, Tzartos S, Evoli A, et al. Myasthenia gravis. Nat Rev Dis Primers. 2019;5(1):30.
- Zisimopoulou P, Evangelakou P, Tzartos J, et al. A comprehensive analysis of the epidemiology and clinical characteristics of anti-LRP4 in myasthenia gravis. J Autoimmun. 2014;52:139–145.
- Costamagna G, Abati E, Bresolin N, et al. Management of patients with neuromuscular disorders at the time of the SARS-CoV-2 pandemic. J Neurol. 2021;268(5):1580–1591.
- Muppidi S, Guptill JT, Jacob S, et al. COVID-19-associated risks and effects in myasthenia gravis (CARE-MG). Lancet Neurol. 2020;19(12):970–971.
- Jakubíková M, Týblová M, Tesař A, et al. Predictive factors for a severe course of COVID-19 infection in myasthenia gravis patients with an overall impact on myasthenic outcome status and survival. Eur J Neurol. 2021;28(10):3418–3425.
- Drenovska K, Vassileva S, Tanev I, Joly K. Impact of COVID-19 on autoimmune blistering diseases. Clin Dermatol. 2021;39(3):359–368.
- Ulrichts P, Guglietta A, Dreier T, et al. Neonatal Fc receptor antagonist efgartigimod safely and sustainably reduces IgGs in humans. J Clin Invest. 2018;128(10):4372–4386.
- Grevys A, Nilsen J, Sand KMK, et al. A human endothelial cell-based recycling assay for screening of FcRn targeted molecules. Nat Commun. 2018;9(1):621.
- Howard JF, Bril V, Burns TM, Jr., Efgartigimod MG Study Group, et al. Randomized phase 2 study of FcRn antagonist efgartigimod in generalized myasthenia gravis. Neurology. 2019;92(23):e2661–e2673.,
- Goebeler M, Bata-Csörgő Z, De Simone C, et al. Treatment of pemphigus vulgaris and foliaceus with efgartigimod, a neonatal Fc receptor inhibitor: a phase 2 multicentre, open-label feasibility trial. Br J Dermatol. 2022;186(3):429–439.
- Howard JF, Bril V, Vu T, Jr., et al. Safety, efficacy, and tolerability of efgartigimod in patients with generalised myasthenia gravis (ADAPT): a multicentre, randomised, placebo-controlled, phase 3 trial. Lancet Neurol. 2021;20(7):526–536.
- Nixon AE, Chen J, Sexton DJ, et al. Fully human monoclonal antibody inhibitors of the neonatal fc receptor reduce circulating IgG in non-human primates. Front Immunol. 2015;6:176.
- Galazaka AM. The immunological basis for immunization series, module 3: tetanus. In Global programme for vaccines and immunization, expanded programme on immunization; 1993. Geneva, Switzerland: World Health Organization.
- Chris Maple PA, Gunn A, Sellwood J, et al. Comparison of fifteen commercial assays for detecting varicella zoster virus IgG with reference to a time resolved fluorescence immunoassay (TRFIA) and the performance of two commercial assays for screening sera from immunocompromised individuals. J Virol Methods. 2009;155(2):143–149.
- Maple PAC, Rathod P, Smit E, et al. Comparison of the performance of the LIAISON VZV-IgG and VIDAS automated enzyme linked fluorescent immunoassays with reference to a VZV-IgG time-resolved fluorescence immunoassay and implications of choice of cut-off for LIAISON assay. J Clin Virol. 2009;44(1):9–14.
- Orange JS, Ballow M, Stiehm ER, et al. Use and interpretation of diagnostic vaccination in primary immunodeficiency: a working group report of the basic and clinical immunology interest section of the American Academy of Allergy, Asthma & Immunology. J Allergy Clin Immunol. 2012;130(3 Suppl):S1–S24.
- Kamchaisatian W, Wanwatsuntikul W, Sleasman J, et al. Validation of current joint American Academy of Allergy, Asthma & Immunology and American College of Allergy, Asthma and Immunology guidelines for antibody response to the 23-valent pneumococcal vaccine using a population of HIV-infected children. J Allergy Clin Immunol. 2006;118(6):1336–1341.
- Jódar L, Butler J, Carlone G, et al. Serological criteria for evaluation and licensure of new pneumococcal conjugate vaccine formulations for use in infants. Vaccine. 2003;21(23):3265–3272.[12804857
- World Health Organization. Recommendations to assure the quality, safety and efficacy of pneumococcal conjugate vaccines: replacement of WHO technical report series No. 927, Annex 2, 2013. [cited 2021 6 October]; Available from: https://www.who.int/biologicals/vaccines/TRS_977_Annex_3.pdf.
- World Health Organization. WHO Expert Committee on Biological Standardization. World Health Organ Tech Rep Ser, 2005;927:1–154.
- Boekel L, Steenhuis M, Hooijberg F, et al. Antibody development after COVID-19 vaccination in patients with autoimmune diseases in The Netherlands: a substudy of data from two prospective cohort studies. Lancet Rheumatol. 2021;3(11):E778–E788.
- Khoury DS, Cromer D, Reynaldi A, et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021;27(7):1205–1211.
- Strijbos E, Tannemaat MR, Alleman I, et al. A prospective, double-blind, randomized, placebo-controlled study on the efficacy and safety of influenza vaccination in myasthenia gravis. Vaccine. 2019;37(7):919–925.
- Zhao X, Fang VJ, Ohmit SE, et al. Quantifying protection against influenza virus infection measured by hemagglutination-inhibition assays in vaccine trials. Epidemiology. 2016;27(1):143–151.
- WHO. Recommended composition of influenza virus vaccines for use in the 2019-2020 Northern hemisphere influenza season. 2019. World Health Organization. [cited 2021 May 20].
- Maho-Vaillant M, Sips M, Golinski M-L, et al. FcRn antagonism leads to a decrease of desmoglein-specific B cells: Secondary analysis of a phase 2 study of efgartigimod in pemphigus vulgaris and pemphigus foliaceus. Front Immunol. 2022;13:863095.
- Newland AC, Sánchez-González B, Rejtő L, et al. Phase 2 study of efgartigimod, a novel FcRn antagonist, in adult patients with primary immune thrombocytopenia. Am J Hematol. 2020;95(2):178–187.
- Gilhus NE. Myasthenia gravis. N Engl J Med. 2016;375(26):2570–2581.
- Jolles S, Chapel H, Litzman J. When to initiate immunoglobulin replacement therapy (IGRT) in antibody deficiency: a practical approach. Clin Exp Immunol. 2017;188(3):333–341.
- Patel SY, Carbone J, Jolles S. The expanding field of secondary antibody deficiency: Causes, diagnosis, and management. Front Immunol. 2019;10:33.
- Ungaro RC, Agrawal M, Park S, et al. Autoimmune and chronic inflammatory disease patients with COVID-19. ACR Open Rheumatol. 2021;3(2):111–115.
- Stabler S, Giovannelli J, Launay D, et al. Serious infectious events and immunoglobulin replacement therapy in patients with autoimmune disease receiving rituximab: a retrospective cohort study. Clin Infect Dis. 2021;72(5):727–737.
- Kovvuru S, Nalleballe K, Onteddu SR, et al. Immunosuppression in chronic autoimmune neurological disorders during the COVID-19 pandemic. J Neurol Sci. 2021;420:117230.
- Guptill JT, Juel VC, Massey JM, et al. Effect of therapeutic plasma exchange on immunoglobulins in myasthenia gravis. Autoimmunity. 2016;49(7):472–479.
- Schmaldienst S, Müllner M, Goldammer A, et al. Intravenous immunoglobulin application following immunoadsorption: benefit or risk in patients with autoimmune diseases? Rheumatology (Oxford). 2001;40(5):513–521.
- Stummvoll GH, Aringer M, Jansen M, et al. Immunoadsorption (IAS) as a rescue therapy in SLE: considerations on safety and efficacy. Wien Klin Wochenschr. 2004;116(21–22):716–724.
- Boyarsky BJ, Werbel WA, Avery RK, et al. Antibody response to 2-Dose SARS-CoV-2 mRNA vaccine series in solid organ transplant recipients. Jama. 2021;325(21):2204–2206.
- Deepak P, Kim W, Paley MA, et al. Glucocorticoids and B cell depleting agents substantially impair immunogenicity of mRNA vaccines to SARS-CoV-2. medRxiv. 2021;2021.04.05.21254656.
- Furer V, Eviatar T, Zisman D, et al. Immunogenicity and safety of the BNT162b2 mRNA COVID-19 vaccine in adult patients with autoimmune inflammatory rheumatic diseases and in the general population: a multicentre study. Ann Rheum Dis. 2021;80(10):1330–1338.
- Geisen UM, Berner DK, Tran F, et al. Immunogenicity and safety of anti-SARS-CoV-2 mRNA vaccines in patients with chronic inflammatory conditions and immunosuppressive therapy in a monocentric cohort. Ann Rheum Dis. 2021;80(10):1306–1311.
- Haberman RH, Herati R, Simon D, et al. Methotrexate hampers immunogenicity to BNT162b2 mRNA COVID-19 vaccine in immune-mediated inflammatory disease. Ann Rheum Dis. 2021;80(10):1339–1344.
- Picchianti-Diamanti A, Aiello A, Laganà B, et al. Immunosuppressive therapies differently modulate humoral- and T-cell-specific responses to COVID-19 mRNA vaccine in rheumatoid arthritis patients. Front Immunol. 2021;12:740249.
- Spiera R, Jinich S, Jannat-Khah D. Rituximab, but not other antirheumatic therapies, is associated with impaired serological response to SARS- CoV-2 vaccination in patients with rheumatic diseases. Ann Rheum Dis. 2021;80(10):1357–1359.
- Avouac J, Miceli-Richard C, Combier A, et al. Risk factors of impaired humoral response to COVID-19 vaccination in rituximab treated patients. Rheumatology (Oxford). 2022;61(SI2):SI163–SI168.
- Bugatti S, De Stefano L, Balduzzi S, et al. Methotrexate and glucocorticoids, but not anticytokine therapy, impair the immunogenicity of a single dose of the BNT162b2 mRNA COVID-19 vaccine in patients with chronic inflammatory arthritis. Ann Rheum Dis. 2021;80(12):1635–1638.
- Wong SY, Dixon R, Martinez Pazos V, et al. Serologic response to messenger RNA coronavirus disease 2019 vaccines in inflammatory bowel disease patients receiving biologic therapies. Gastroenterology. 2021;161(2):715–718.e4.
- Madelon N, Lauper K, Breville G, et al. Robust T cell responses in anti-CD20 treated patients following COVID-19 vaccination: a prospective cohort study. Clin Infect Dis. 2021:ciab954.
- Waldmann TA, Terry WD. Familial hypercatabolic hypoproteinemia. A disorder of endogenous catabolism of albumin and immunoglobulin. J Clin Invest. 1990;86(6):2093–2098.
- Ardeniz Ö, Unger S, Onay H, et al. β2-Microglobulin deficiency causes a complex immunodeficiency of the innate and adaptive immune system. J Allergy Clin Immunol. 2015;136(2):392–401.
- Castrucci MR. Factors affecting immune responses to the influenza vaccine. Hum Vaccin Immunother. 2018;14(3):637–646.
- Pellini R, Venuti A, Pimpinelli F, et al. Initial observations on age, gender, BMI and hypertension in antibody responses to SARS-CoV-2 BNT162b2 vaccine. EClinicalMed. 2021;36:100928.
