Abstract
Ulcerative colitis (UC) is a chronic and recurrent inflammatory disease of the colon that result in the destruction and inflammation of the colonic mucosa. Current research has established a strong correlation between pyroptosis of colonic epithelial cells and the onset and progression of UC. In addition, miRNAs have been implicated in the development and progression of UC and pyroptosis. This aimed of this study was to identify specific miRNAs that could inhibit pyroptosis in colon epithelial cells and alleviate UC. Lipopolysaccharide (LPS) was used to induce inflammation in FHC normal colonic epithelial cells to construct an enteritis cell model and downregulated expression levels of miRNAs were detected in inflammatory bowel disease mucosal tissue model. Pyroptosis indicators were detected using Cell Counting Kit-8, flow cytometry, ELISA, qPCR, western blot, and immunofluorescence, and miRNA target genes were predicted by miRDB, TargetScan, pyroptosis pathway from KEGG, and double luciferase assay was used for verification. The effect of miR-141-3p on colitis was observed in the mouse DSS colitis model. The results showed that miR-141-3p was the most significantly downregulated miRNA in LPS-induced FHC cells, and promoted the proliferation of LPS-induced FHC cells and suppressed their apoptosis. In addition, miR-141-3p decreased the expression of pyroptosis-related proteins such as NLRP3, caspase-1, N-GSDMD, and the other proteins, as well as the release of IL-18 and IL-1β inflammatory factors. Conversely, the miR-141-3p inhibitor promoted LPS-induced FHC pyroptosis. Dual luciferase experiments confirmed that miR-141-3p could target the HSP90 molecular chaperone SUGT1. Further experiments demonstrated that SUGT1 overexpression could restore the inhibitory effect of miR-141-3p on pyroptosis, while SUGT1 knockdown could alleviate the promotion of pyroptosis induced by miR-141-3p inhibitor. Furthermore, miR-141-3p alleviated the inflammatory phenotype of mouse colonic mucosa in the mouse DSS colitis model. Therefore, miR-141-3p inhibits LPS-induced pyroptosis of colonic epithelial cells by targeting SUGT1. miR-141-3p could also alleviate DSS-induced colitis in mice, suggesting that miR-141-3p may become a nucleic acid drug for the treatment of UC.
Introduction
Ulcerative colitis (UC) is a chronic inflammatory disease that affects the colonic mucosa and is most prevalent in North America and northern Europe. The incidence of UC is increasing in developing countries due to the worldwide prevalence of Western diets [Citation1,Citation2]. It is characterised by the inflammation and ulcers that develop in the inner lining of the colon and rectum, resulting in various gastrointestinal symptoms, including diarrhoea, abdominal pain, and rectal bleeding [Citation3,Citation4].
The pathogenesis of UC is complex and involves multiple genetic, environmental, and immunological factors [Citation5,Citation6]. Recent studies have shown that the activation of the NLR family pyrin domain containing 3 (NLRP3) inflammasome plays a crucial role in the development and progression of UC [Citation7,Citation8]. The NLRP3 inflammasome is a cytoplasmic protein complex that is activated in response to various danger signals, including bacterial and viral infections, tissue damage, and oxidative stress [Citation9–11]. Activation of the NLRP3 inflammasome leads to the cleavage of pro-caspase-1 into active caspase-1 [Citation12], which further activates downstream effector proteins, including pro-IL-1β, pro-IL-18, and Gasdermin D (GSDMD) [Citation13,Citation14]. IL-1β and IL-18 are pro-inflammatory cytokines that are involved in the pathogenesis of UC [Citation15]. GSDMD is a pore-forming protein that mediates pyroptosis, a form of programmed cell death that is characterised by the release of pro-inflammatory cytokines and damage-associated molecular patterns (DAMPs) [Citation16]. N-(terminal) GSDMD is transferred to the cell membrane and changes its permeability, resulting in cell swelling and rupture [Citation17]. Thus, the NLRP3 inflammasome, IL-1β, IL-18, and GSDMD are potential targets for the inhibition of pyroptosis and inflammatory responses in UC.
MicroRNAs (miRNAs) are a class of small non-coding RNAs with a length of 19-23 bp, which mainly function by negatively regulating mRNA expression [Citation18]. miRNAs are fairly conserved among vertebrate species, with approximately 77% being highly conserved [Citation19]. miRNAs have been confirmed to be related to cancer, cardiovascular disease, inflammatory diseases, and other diseases [Citation20]. They can be used as therapeutic agents or antagonists for the treatment of diseases. For example, In a subset of patients with refractory advanced solid tumours, treatment with MRX34 (a liposome miR-34a mimic) and dexamethasone premedication exhibited evidence of antitumor activity and acceptable safety [Citation21]. RG-101 (an anti-miR-122) is an oligonucleotide that targets hepatocytes and is conjugated with N-acetylgalactosamine. It works by antagonising miR-122 in patients with chronic HCV infection [Citation22]. Therefore, miR-141-3p may be a promising nucleic acid drug for UC treatment.
In conclusion, understanding the molecular mechanisms underlying UC pathogenesis is essential for the development of effective therapeutic strategies. In this study, we investigated the role of miRNAs in the regulation of colonic epithelial cell pyroptosis and identified miR-141-3p as a potential drug for UC treatment. Our findings may provide new insights into the development of novel miRNA-based therapeutics for UC and other inflammatory bowel diseases.
Methods
Cell lines and culture
The normal colon epithelial cell line FHC was purchased from ATCC. 293 T cells were maintained in our laboratory. FHC cells were cultured in RPMI-1640 (Gibco, USA) and the cells were treated with 10 ng/mL lipopolysaccharide (LPS) for 6h to construct a cellular colitis model based on previous research [Citation23,Citation24]. 293 T cells were cultured in DMEM (Gibco) at 37 °C in 5% CO2. The medium was supplemented with 10% foetal bovine serum (FBS; HyClone), 100 U/ml penicillin, and 0.1 mg/mL streptomycin (Gibco).
Plasmid construction and cell transfection
To construct the SUGT1 overexpression (ov) vector, full-length human SUGT1 was subcloned into the pcDNA3.1 vector (Genechem, China). To knockdown SUGT1, siRNAs targeting SUGT1 (siRNA1, siRNA2, and siRNA3) and siRNA-negative control (NC; GenePharma, Shanghai, China) were synthesised. miR-141-3p mimics, mimics nc, miR-141-3p inhibitor, and inhibitor NC were purchased from GenePharma. All transfections were performed using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s instructions. The sequences of siRNAs, miR-139a-3p mimics, mimics NC, miR-141-3p inhibitor, and inhibitor NC are listed in .
Table 1. The sequences of siRNAs targeting SUGT1 and miR-141-3p related RNA.
Polymerase chain reaction (PCR) and real-time quantitative polymerase chain reaction (RT-qPCR)
Total RNA from the FHC cells treated with or without 10 ng/mL LPS for 6h was extracted using TRIzol reagent (Thermo Fisher, USA). Subsequently, the RNA was reverse transcribed into cDNA using RevertAid First Strand cDNA Synthesis Kit (Thermo Fisher), and cDNA was used as a template to amplify the target sequence in a 20 μL reaction using GoTaq® qPCR Master Mix (A6002, Promega). The relative gene expression levels were quantified using the 2−ΔΔCq method. The primer sequences are listed in .
Table 2. The RT-qPCR forward and reverse primer sequences.
Western blot (WB)
Total protein was extracted from the cell lysates (Beyotime, China). Equal amounts of denatured proteins (30 μg) were subjected to 10% sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE). The resolved proteins were transferred to polyvinylidene fluoride membranes (Millipore, USA) and probed with SUGT1 (1:300; sc-398625, Santa Cruz), NLRP3 (1:1,000; 13158, Cell Signalling Technology), pro-caspase-1 (1:1,000; 83383, Cell Signalling Technology), cleaved Caspase-1 (1:1,000; 4199, Cell Signalling Technology), and cleaved N-terminal GSDMD (1:1,000, ab215203, Abcam) antibodies at 4 °C overnight. The membranes were then incubated with goat anti-rabbit antibody (1:5,000; SA00001-2, Proteintech) or goat anti-mouse antibody (1:5,000; SA00001-1, Proteintech) for 1 h. The blots were visualised after chemical development using electrochemiluminescence reagent (Thermo Scientific). GAPDH was used as an internal reference antibody (1:5,000; 10494-1-AP, Proteintech).
Fluorescence in situ hybridisation (FISH)
FISH assay was performed using the Fluorescent in Situ Hybridisation Kit (GenePharma), according to the manufacturer’s instructions. The cell nuclei were stained with 4,6-diamidino-2-phenylindole (DAPI; Beyotime). Images were obtained using a fluorescence microscope (Leica, Germany).
Cell Counting Kit-8 (CCK8) assay
Briefly, single-cell suspensions were prepared using trypsin and inoculated into 96-well plates at a density of 3 × 103 cells/well. Next, 10 μL of the CCK8 reagent (Solarbio) was added and the cells were incubated for 60 min. Optical density at 450 nm was measured using a microplate reader (51119670DP, Thermo Scientific). All experiments were performed in triplicate.
Cell apoptosis assay
The cells (106 cells/ml) were incubated with 5 μL of fluorescein isothiocyante (FITC) annexin V, and then 5 μL of propidium iodide was added. The cells were incubated in the dark for 15 min at 23 ± 2 °C according to the manufacturer’s instructions of the Annexin V-FITC/PI Apoptosis Detection Kit. Apoptosis was assessed using flow cytometry (FCM; BD Biosciences). The experiments were performed in triplicates.
ELISA assay
The standard protein and supernatant were added to the coated ELISA plates, which were then incubated at 37 °C for 2 h, washed twice, and horseradish peroxidase-labeled secondary antibody was added before incubation at 37 °C for 1 h, then washed twice. The chromogenic substrate was added and incubated in the dark at room temperature for 10–15 min, the stop solution was added, and the absorbance at 450 nm was recorded using an enzyme-linked immunoassay. The detection indicators were human TNF-α (Beyotime, PT518), human-IL-1β (Abcam, ab214025), human-IL-18 (Sino Biological, KIT10119), mouse-IL-1β (Beyotime, PI301), and mouse-IL-18(Beyotime, PI553). The experiments were performed in triplicates.
Dual luciferase assay
The sequences of the SUGT1 3′-UTR containing the wild-type or mutant binding site of hsa-miR-141-3p were designed and synthesised by GenePharma. These sequences were subcloned into the psiCHECK2 vector (Promega). 293 T cells and FHC cells were co-transfected with the psiCHECK2 plasmid containing the insertion sequence and hsa-miR-141-3p mimics/mimics-NC or hsa-miR-141-3p inhibitors/inhibitor-NC, using Lipofectamine 2000 (Invitrogen). After 48 h of incubation, the activities of firefly and Renilla luciferase were measured using the Dual Luciferase Reporter Assay Kit (Promega) with firefly fluorescence as an internal reference.
Animals and haematoxylin–eosin (HE) staining
Animal research was approved by the local ethics committee of Guangzhou Medical University, Guangzhou, China. Male C57BL/6 mice (age 7–8 weeks, weight 20–22 g) were regularly treated with 2.5% dextran sulphate sodium (DSS, MW 40-50 kDa; MP Biomedicals, USA) in drinking water for 1 month. To evaluate the function of miR-141-3p, the mice after establishment of experimental colitis were inject agomiR-141-3p or agomiR-141-3p nc (Sangon Biotech, Shanghai, China) via tail vein, once every 3 days, 5 μ mol/kg each time, and inhale 5 times. Intestinal mucosa was harvested for HE staining, the samples were fixed with 4% paraformaldehyde, and sliced into 5 μm sections. Deparaffinization was then performed using xylene, and the samples were rehydrated using gradient solution. Subsequently, the sections were stained with haematoxylin for 10 min and with eosin for 1 min. After dehydration, the sections were vitrified and examined under a microscope (Olympus, Japan).
Statistical analysis
Data are expressed as the mean ± standard deviation. Multiple comparisons were performed using one-way analysis of variance (ANOVA), followed by Dunnett’s post-hoc test. All statistical analyses were performed using SPSS (version 19.0; IBM Corp.). Statistical significance was set at p < 0.05.
Results
LPS induced pyroptosis of the FHC normal colon epithelial cells
To mimic the inflammatory response observed in UC, the FHC normal colon epithelial cells were treated with 10 ng/mL LPS. The results showed that LPS inhibited the proliferation of the normal col onepithelial cells and promoted cell death (). In addition, LPS induced the upregulation of expression of inflammatory factors in colonic epithelial cells (). Pyroptosis is the main cell death mechanism that causes tissue inflammation, and the pyroptosis markers IL-1β and IL-18 were upregulated in the LPS-treated colon cells. Following the LPS treatment, the FHC colon epithelial cells were swollen and raptured. Severe blistering was observed (). Therefore, we used WB to detect key effector proteins in pyroptotic pathways, such as NLRP3, caspase-1, and N-GSDMD, and FISH to detect the expression of the pyroptotic membrane signal N-GSDMD. The results showed that NLRP3, caspase-1, and N-GSDMD were highly expressed in the LPS-induced FHC cells. Therefore, LPS induced pyroptosis in the FHC cells ().
Figure 1. Lipopolysaccharide (LPS)-induced pyroptosis of FHC normal colon epithelial cells. A: Cell Counting Kit-8 was used to examine the effect of LPS on the proliferation of FHC cells. B: FCM was used to determine the effect of LPS on FHC cell apoptosis. C-E: ELISA was used to investigate the effect of LPS on the secretion of TNF-α, IL-1β, and IL-18 cytokines. F: The effect of LPS on cell morphology was observed under a light microscope. G: In the LPS-treated and untreated FHC cells, the expression of NLRP3, caspase-1, N-GSDMD, and key factors of pyroptosis was examined using WB. H: The expression of N-GSDMD was detected using FISH in the LPS-treated and untreated FHC cells. * p < 0.05, **p < 0.01, ***p < 0.01.
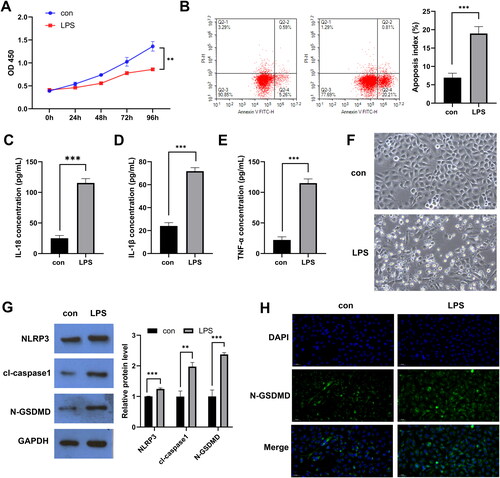
miR-141-3p inhibits LPS-induced pyroptosis in the FHC cells
We screened for unproven miRNAs (such as miR-141-3p, miR-188-5p, miR-192-5p, miR-215-5p, miR-320a-3p, miR-346, miR-375-3p, and miR-378a-3p) in UC that have been suggested in previous studies to be closely related to UC processes or inflammatory responses [Citation25–32]. In the LPS-stimulated epithelial cell model, the expression of miR-141-3p, miR-215-5p, miR-320a-3p, miR-346, and miR-375-3p were all dysregulated, with miR-141-3p showing the greatest difference in expression and being downregulated (). Since we urgently searched for miRNAs that suppressed intestinal inflammation, miR-141-3p might be a promising therapeutic target for UC. We then transfected the synthetic miR-141-3p mimics/inhibitor into FHC cells and found that miR-141-3p expression was upregulated in the mimics group and downregulated in the inhibitor group (), suggesting that the synthetic miR-141-3p mimics/inhibitor was effective. Subsequently, we found that miR-141-3p mimics promoted proliferation (), and inhibited apoptosis () and secretion of pyroptotic indicators IL-1β and IL-18 and inflammatory factor TNF-α () of LPS-induced epithelial cell models. miR-141-3p inhibitor had the exact opposite effect of mimics. WB and FISH results showed that miR-141-3p mimic inhibited the expression of the key pyroptotic effector proteins NLRP3, caspase-1, and N-GSDMD and the membrane expression of N-GSDMD in the LPS-stimulated epithelial cell model ().
Figure 2. miR-141-3p inhibits lipopolysaccharide (LPS)-induced pyroptosis in FHC cells. A: RT-qPCR was used for the identification of miRNAs with down-regulated expression in mucosal tissues of patients with UC. B: qPCR analysis of miR-141-3p mimics/inhibitor effectiveness. C: Cell Counting Kit-8 was used to examine the effect of miR-141-3p increase or inhibition on LPS-induced FHC cell proliferation. D: FCM was used to investigate effect of miR-141-3p increase or inhibition on LPS-induced FHC cell apoptosis. E: ELISA was used to examine whether miR-141-3p increases or inhibits the secretion of IL-1β, IL-18 and TNF-α in LPS-induced FHC cells F: WB was used to examine whether miR-141-3p increases or inhibits the expression of the key apoptosis factors NLRP3, caspase-1, and N-GSDMD. G: FISH analysis of the effect of miR-141-3p on the expression of N-GSDMD. *p < 0.05, **p < 0.01, ***p < 0.01.
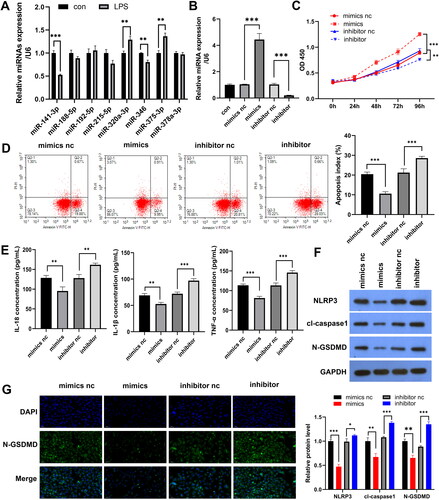
miR-141-3p targets SUGT1
To determine the molecular mechanism by which miR-141-3p regulates pyroptosis, we used miRDB and TargetScan to predict the binding targets of miR-141-3p and combined the prediction results with the analysis of the Kyoto Encyclopaedia of Genes and Genomes signalling pathway (hsa04621) related to pyroptosis. The results showed that miR-141-3p targets four pathway proteins: TNFAIP3, SUGT1, IFNAR1, and TAB (). Subsequently, qPCR results showed that the miR-141-3p mimics only downregulated the expression of SUGT1 (). qPCR and WB results showed that miR-141-3p mimic decreased SUGT1 expression and that miR-141-3p inhibitor increased SUGT1 expression (). Dual-luciferase experiments confirmed that miR-141-3p binds to the SUGT1 3′-UTR region in the 293 T and FHC cells (). WB indicated that the treatment of FHC cells with LPS resulted in increased expression of SUGT1 compared to that of the untreated FHC cells. This indicated that SUGT1 expression was inversely correlated with the expression of miR-141-3p ().
Figure 3. miR-141-3p targets SUGT1. A: Venn diagram showing the process of screening the target genes of miR-141-3p through prediction software and signalling pathways. B: RT-qPCR results showing the targeting efficiency of miR-141-3p against candidate target genes. C,D: qPCR and WB analysis of the targeting efficiency of miR-141-3p mimic and inhibitor against SUGT1. E: Schematic diagram of the 3’ UTR of miR-141-3p target SUGT1. F: Dual-luciferase experiments were performed to examine the binding efficiency of miR-141-3p to SUGT1 3′-UTR in 293 T cells. G: Dual-luciferase experiments were performed in FHC cells. H: WB analysis of the expression of SUGT1 in the lipopolysaccharide-induced and uninduced FHC cells. *p < 0.05, **p < 0.01, ***p < 0.01.
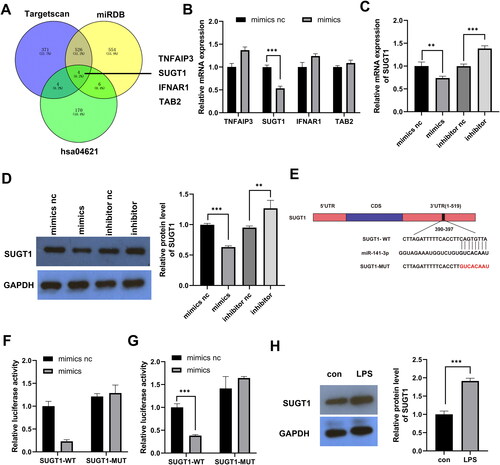
SUGT1 knockdown reverses the promoting effect of miR-141-3p inhibitor on LPS-induced FHC cell pyroptosis
The results showed that siRNA1 and siRNA3 had significant knockdown effects on SUGT1, with siRNA1 having the most significant knockdown effect (). CCK8 and FCM results showed that SUGT1 knockdown prevented the inhibitory effect of miR-141-3p inhibitor on LPS-induced FHC cell proliferation and the effect of miR-141-3p inhibitor on the promotion of epithelial cell model apoptosis (). ELISA results showed that SUGT1 knockdown restored the promoting effect of the miR-141-3p inhibitor on IL-1β, IL-18 and TNF-α secretion in the LPS-treated FHC cells (). WB results showed that knockdown of SUGT1 also inhibited the expression of key effector proteins of pyroptosis, NLRP3, caspase-1, and N-GSDMD, which compensated for the promoting effect of the miR-141-3p inhibitor on the expression of these proteins (). Similar results were obtained using FISH, in which SUGT1 knockdown inhibited the expression of N-GSDMD on the cell surface ().
Figure 4. SUGT1 knockdown reverses the promoting effect of miR-141-3p inhibitor on lipopolysaccharide-induced FHC pyroptosis. A: RT-qPCR was used to determine the efficiency of siRNA-mediated knockdown of SUGT1. B: WB was used to determine the efficiency of siRNA-mediated knockdown of SUGT1. C: Cell Counting Kit-8 was used to examine whether SUGT1 knockdown can reverse the inhibitory effect of miR-141-3p inhibitor on LPS-stimulated epithelial cell model proliferation. D: FCM was used to examine whether SUGT1 knockdown reverses the effect of miR-141-3p inhibitor on the promotion of LPS-stimulated epithelial cell model apoptosis. E: Statistical Chart of Apoptosis. F: ELISA was used to examine the effect of SUGT1 knockdown on miR-141-3p inhibitor-promoted secretion of IL-1β, IL-18 and TNF-α in LPS-stimulated epithelial cell model. G: WB was used to investigate the effect of knockdown of SUGT1 on the expression of pyroptosis-related proteins in LPS-stimulated epithelial cell model, and the recovery effect of miR-141-3p inhibitor on the expression of these proteins. H: FISH was used to determine the effect of knockdown of SUGT1 on the expression of N-GSDMD protein in LPS-stimulated epithelial cell model, and the recovery effect of miR-141-3p inhibitor on the expression of N-GSDMD protein. *p < 0.05, **p < 0.01, ***p < 0.01.
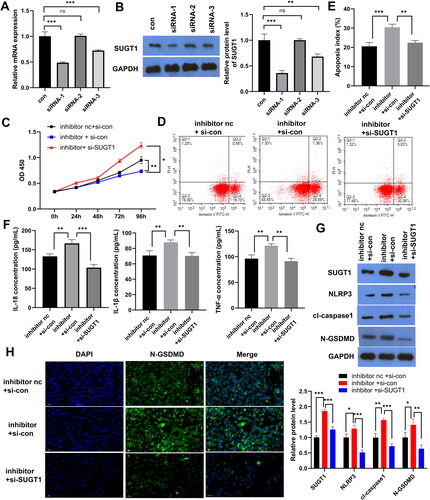
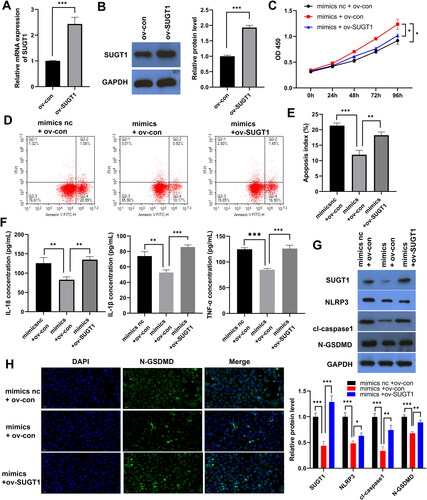
Ov-SUGT1 reverses the inhibitory effect of miR-141-3p mimics on LPS-induced FHC pyroptosis
qPCR and WB results showed that SUGT1 was successfully overexpressed in the LPS-treated FHC cells (). CCK8 results showed that ov-SUGT1 reversed the promoting effect of miR-141-3p mimics on LPS-induced FHC cell proliferation (). FCM results showed that SUGT1 upregulation inhibited the effect of miR-141-3p mimics on LPS-induced FHC cell apoptosis (). The ELISA results showed that ov-SUGT1 reversed the inhibitory effect of miR-141-3p mimics on IL-1β, IL-18 and TNF-α secretion in the LPS-induced FHC cells (). WB results showed that ov-SUGT1 also promoted the expression of key effector proteins of pyroptosis, NLRP3, caspase-1, and N-GSDMD, which compensated for the inhibitory effect of miR-141-3p mimics on the expression of these proteins (). Similar results were obtained using FISH, with the upregulation of SUGT1 expression promoting the expression of the N-GSDMD on the cell surface ().
Figure 5. SUGT1 overexpression restores the inhibitory effect of miR-141-3p mimics on lipopolysaccharide-induced FHC pyroptosis. A: RT-qPCR was used to confirm SUGT1 overexpression. B: The overexpression of SUGT1 was detected using WB. C: The recovery effect of SUGT1 overexpression on miR-141-3p mimic-promoted LPS-stimulated epithelial cell model proliferation was determined using Cell Counting Kit-8. D: FCMwas used to examine the effect of SUGT1 overexpression on miR-141-3p mimic-mediated inhibition of LPS-stimulated epithelial cell model apoptosis. E: Statistical Chart of Apoptosis. F: ELISA was used to examine the inhibitory effect of SUGT1 overexpression on the inhibition of IL-1β, IL-18 and TNF-α secretion by miR-141-3p mimic in LPS-stimulated epithelial cell model. G: WB was used to determine the effect of overexpression of SUGT1 on the expression of pyroptosis-related proteins in LPS-stimulated epithelial cell model, and the recovery effect of miR-141-3p mimic on the expression of pyroptosis-related proteins. H: FISH was used to examine the effect of overexpression of SUGT1 on the expression of N-GSDMD protein in LPS-stimulated epithelial cell model, and the recovery effect of miR-141-3p mimic on N-GSDMD protein expression. *p < 0.05, **p < 0.01, ***p < 0.01.
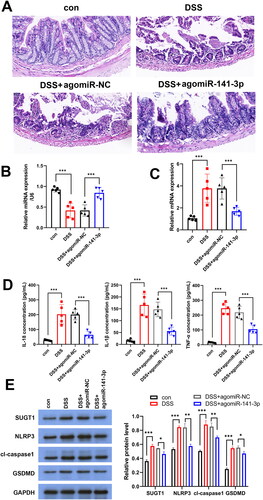
miR-141-3p alleviates DSS-induced UC in mice
To study the therapeutic effect of miR-141-3p on colitis in vivo, we established a DSS-induced mouse UC model and injected agomiR-miR-141-3p and agomiR-NC into the tail vein of DSS-induced mice. The results showed that agomiR-miR-141-3p attenuated the damage to colonic epithelial tissue in DSS mice (). After agomiR-141-3p injection, down-regulated expression of miR-141-3p and up-regulated expression of SUGT1 in DSS-induced mouse UC model were reversed (). miR-141-3p downregulated the expression of TNF-α, IL-1β, and IL-18 in mouse serum (). This indicated that miR-141-3p can alleviate DSS-induced UC pathology in mice and reduce the release of inflammatory factors, such as TNF-α, IL-1β, and IL-18. In addition, WB results appeared that miR-141-3p inhibited the expression of pyroptosis factors in enterocytes by suppressing SUGT1(Figure6E). Therefore, miR-141-3p may be a nucleic acid drug for the treatment of UC.
Figure 6. miR-141-3p alleviates DSS-induced ulcerative colitis in mice. A: HE staining was used to detect the protective effect of agomiR-141-3p on DSS-induced mouse intestinal epithelial tissue. B, C: RT-qPCR analysis the expression of miR-141-3p (B) and SUGT1 (C) in vivo. D: ELISA was used to determine the expression of IL-1β, IL-18 and TNF-α in serum of UC mice inject with agomiR-141-3p. *p < 0.05, **p < 0.01, ***p < 0.01.
Discussion
During the active exacerbation of the disease in patients with inflammatory bowel disease (including UC and Crohn’s disease), intestinal epithelial cell death through various mechanisms, especially cell pyroptosis and cell necrosis, is observed, leading to damage to the intestinal tissue[Citation33]. In this study, LPS-treated colonic epithelial cells were used to establish an intestinal inflammation model. The expression of the pyroptosis-related proteins IL-1β, IL-18, NLRP3, caspase-1, and N-GSDMD was upregulated, that is, LPS successfully induced pyroptosis in intestinal epithelial cells. We also examined the expression levels of miRNAs [Citation34–36] that have been reported to be downregulated in the intestinal mucosa of patients with UC in LPS-induced colonic epithelial cells and found that the expression of miR-141-3p was the most significantly downregulated. Furthermore, we also transfected miR-141-3p mimic, inhibitor and the corresponding controls into LPS-induced upper colon cells to examine the effect of miR-141-3p on epithelial cell model. miR-141-3p mimic inhibited LPS-induced pyroptosis of colonic epithelial cells, while the inhibitor further aggravated pyroptosis of colonic epithelial cells. A variety of miRNAs have been confirmed to be involved in the occurrence and development of UC. miR-21-5p inhibitor can inhibit the inflammatory development of UC in RAW264.7 cells [Citation37], and miR-21-5p is abnormally expressed in patients with inflammatory bowel disease and is considered to be a marker of inflammatory[Citation38]. In this study, miR-141-3p was found to play a role in relieving or treating colitis.
To study the mechanism by which miR-141-3p inhibits epithelial cell pyroptosis and alleviates colitis, the miRNA target prediction software TargetScan and miRDB predicted the target genes of miR-141-3p. The NOD-like receptor signalling pathway (hsa04621) was combined to screen the target genes of miR-141-3p, and the target efficiency of miR-141-3p on the screened genes was detected using qPCR. The results showed that miR-141-3p had the best effect on targeting SUGT1. SUGT1 is a molecular chaperone of HSP90. Before NLRP3 is activated, a complex including NLRP3, SUGT1 and HSP90 inhibits the ubiquitination and degradation of NLRP3 [Citation39]. Previous studies have shown that inhibition of HSP90 or its molecular chaperone SUGT1 inhibits the activation of the NLRP3 inflammasome [Citation40,Citation41]. When NLRP3 is knocked out or antagonised by inhibitors in mice, pyroptosis is significantly reduced and NLRP3 cross-talk with other innate immune pathways is also reduced [Citation42,Citation43], suggesting that NLRP3 inhibition may have broad inhibitory effects on inflammatory responses. Therefore, NLRP3 is an attractive drug target for patients with inflammatory diseases [Citation44]. This study found that miR-141-3p mimic inhibited the activation of NLRP3 by targeting SUGT1, thereby inhibiting the expression of caspase-1, N-GSDMD, IL-1β, and IL-18, which are downstream effector molecules of pyroptosis. In particular, the expression of N-GSDMD is increased and the protein is transferred to the membrane, which disrupts the permeability of the cell membrane and ruptures the cell, triggering an inflammatory response [Citation45].
In addition, we found that the expression of IL-1β and IL-18 in the serum was upregulated in the DSS-induced mouse UC model, and the results of immunohistochemistry also revealed abnormal necrosis of colonic epithelial cells. However, necrosis of colonic epithelial cells was significantly reduced in UC mice treated with miR-141-3p mimic. Therefore, miR-141-3p may improve colitis in mice, and can be a therapeutic agent for alleviating colitis in the future.
Authors’ contribution
RY designed the research and planned the experimental procedures. RY provided the financial support for this study. RY was a major contributor to writing the manuscript. XL participated in data collection and literature analysis. JH performed the data analysis. All authors have read and approved the final manuscript.
| Abbreviations | ||
| LPS | = | lipopolysaccharide |
| UC | = | ulcerative colitis |
| NLRP3 | = | NLR Family Pyrin Domain Containing 3 |
| HSP90 | = | Heat Shock Protein 90 Alpha Family Class A Member 1 |
| SUGT1 | = | SGT1 Homolog, MIS12 Kinetochore Complex Assembly cochaperone |
| KEGG | = | Kyoto Encyclopaedia of Genes and Genomes |
| IL | = | Interleukin |
| GSDMD | = | Gasdermin D |
Acknowledgments
Not applicable.
Availability of data and materials
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
Disclosure statement
The authors declare that no competing interests exist.
Additional information
Funding
References
- Eisenstein M. Ulcerative colitis: towards remission. Nature. 2018;563(7730):1.
- Ordas I, Eckmann L, Talamini M, et al. Ulcerative colitis. Lancet. 2012;380(9853):1606–12.
- Lasa JS, Olivera PA, Danese S, et al. Efficacy and safety of biologics and small molecule drugs for patients with moderate-to-severe ulcerative colitis: a systematic review and network meta-analysis. Lancet Gastroenterol Hepatol. 2022;7(2):161–170.
- Kobayashi T, Siegmund B, Le Berre C, et al. Ulcerative colitis. Nat Rev Dis Primers. 2020;6(1):74.
- Ahluwalia B, Moraes L, Magnusson MK, et al. Immunopathogenesis of inflammatory bowel disease and mechanisms of biological therapies. Scand J Gastroenterol. 2018;53(4):379–389.
- Ananthakrishnan AN. Epidemiology and risk factors for IBD. Nat Rev Gastroenterol Hepatol. 2015;12(4):205–217.
- Lv Q, Xing Y, Liu J, et al. Lonicerin targets EZH2 to alleviate ulcerative colitis by autophagy-mediated NLRP3 inflammasome inactivation. Acta Pharm Sin B. 2021;11(9):2880–2899.
- Tong L, Hao H, Zhang Z, et al. Milk-derived extracellular vesicles alleviate ulcerative colitis by regulating the gut immunity and reshaping the gut microbiota. Theranostics. 2021;11(17):8570–8586.
- Zhao N, Di B, Xu LL. The NLRP3 inflammasome and COVID-19: activation, pathogenesis and therapeutic strategies. Cytokine Growth Factor Rev. 2021;61:2–15.
- Hu R, Wang MQ, Ni SH, et al. Salidroside ameliorates endothelial inflammation and oxidative stress by regulating the AMPK/NF-kappaB/NLRP3 signaling pathway in AGEs-induced HUVECs. Eur J Pharmacol. 2020;867:172797.
- Munoz-Planillo R, Kuffa P, Martinez-Colon G, et al. K(+) efflux is the common trigger of NLRP3 inflammasome activation by bacterial toxins and particulate matter. Immunity. 2013;38(6):1142–1153.
- Sun L, Ma W, Gao W, et al. Propofol directly induces caspase-1-dependent macrophage pyroptosis through the NLRP3-ASC inflammasome. Cell Death Dis. 2019;10(8):542.
- Tan Y, Zhang M, Pan Y, et al. Suppression of the caspase-1/GSDMD-mediated pyroptotic signaling pathway through dexamethasone alleviates corneal alkali injuries. Exp Eye Res. 2022;214:108858.
- Wang Y, Liu X, Shi H, et al. NLRP3 inflammasome, an immune-inflammatory target in pathogenesis and treatment of cardiovascular diseases. Clin Transl Med. 2020;10(1):91–106.
- Steiner A, Reygaerts T, Pontillo A, et al. Recessive NLRC4-autoinflammatory disease reveals an ulcerative colitis locus. J Clin Immunol. 2022;42(2):325–335.
- Frank D, Vince JE. Pyroptosis versus necroptosis: similarities, differences, and crosstalk. Cell Death Differ. 2019;26(1):99–114.
- Karmakar M, Minns M, Greenberg EN, et al. N-GSDMD trafficking to neutrophil organelles facilitates IL-1beta release independently of plasma membrane pores and pyroptosis. Nat Commun. 2020;11(1):2212.
- Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116(2):281–297.
- Bartel DP. Metazoan MicroRNAs. Cell. 2018;173(1):20–51.
- Paul P, Chakraborty A, Sarkar D, et al. Interplay between miRNAs and human diseases. J Cell Physiol. 2018;233(3):2007–2018.
- Beg MS, Brenner AJ, Sachdev J, et al. Phase I study of MRX34, a liposomal miR-34a mimic, administered twice weekly in patients with advanced solid tumors. Invest New Drugs. 2017;35(2):180–188.
- van der Ree MH, de Vree JM, Stelma F, et al. Safety, tolerability, and antiviral effect of RG-101 in patients with chronic hepatitis C: a phase 1B, double-blind, randomised controlled trial. Lancet. 2017;389(10070):709–717.
- Liu C, Yan X, Zhang Y, et al. Oral administration of turmeric-derived exosome-like nanovesicles with anti-inflammatory and pro-resolving bioactions for murine colitis therapy. J Nanobiotechnology. 2022;20(1):206.
- Wei YY, Fan YM, Ga Y, et al. Shaoyao decoction attenuates DSS-induced ulcerative colitis, macrophage and NLRP3 inflammasome activation through the MKP1/NF-kappaB pathway. Phytomedicine. 2021;92:153743.
- Adamowicz M, Milkiewicz P. Kempinska-Podhorodecka A. 5-aminosalicylic acid inhibits the expression of oncomiRs and pro-inflammatory microRNAs: an in vitro study. J Physiol Pharmacol. 2021;72(4):529–535.
- Li H, Xuan J, Zhang W, et al. Long non-coding RNA SNHG5 regulates ulcerative colitis via microRNA-375/janus kinase-2 axis. Bioengineered. 2021;12(1):4150–4158.
- Lu JW, Rouzigu A, Teng LH, et al. The construction and comprehensive analysis of inflammation-related ceRNA networks and tissue-infiltrating immune cells in ulcerative progression. Biomed Res Int. 2021;2021:6633442.
- Cordes F, Demmig C, Bokemeyer A, et al. MicroRNA-320a monitors intestinal disease activity in patients with inflammatory bowel disease. Clin Transl Gastroenterol. 2020;11(3):e00134.
- Dubois-Camacho K, Diaz-Jimenez D, De la Fuente M, et al. Inhibition of miR-378a-3p by inflammation enhances IL-33 levels: a novel mechanism of alarmin modulation in ulcerative colitis. Front Immunol. 2019;10:2449.
- Yan S, Yue Y, Wang J, et al. LINC00668 promotes tumorigenesis and progression through sponging miR-188-5p and regulating USP47 in colorectal cancer. Eur J Pharmacol. 2019;858:172464.
- Pekow J, Meckel K, Dougherty U, et al. Increased mucosal expression of miR-215 precedes the development of neoplasia in patients with long-standing ulcerative colitis. Oncotarget. 2018;9(29):20709–20720.
- Feng X, Wang H, Ye S, et al. Up-regulation of microRNA-126 may contribute to pathogenesis of ulcerative colitis via regulating NF-kappaB inhibitor IkappaBalpha. PLoS One. 2012;7(12):e52782.
- Patankar JV, Becker C. Cell death in the gut epithelium and implications for chronic inflammation. Nat Rev Gastroenterol Hepatol. 2020;17(9):543–556.
- Cai M, Chen S, Hu W. MicroRNA-141 is involved in ulcerative colitis pathogenesis via aiming at CXCL5. J Interferon Cytokine Res. 2017;37(9):415–420.
- Fasseu M, Treton X, Guichard C, et al. Identification of restricted subsets of mature microRNA abnormally expressed in inactive colonic mucosa of patients with inflammatory bowel disease. PLoS One. 2010;5(10):e13160.
- Wu F, Zikusoka M, Trindade A, et al. MicroRNAs are differentially expressed in ulcerative colitis and alter expression of macrophage inflammatory peptide-2 alpha. Gastroenterology. 2008;135(5):1624–1635 e1624.
- Lu X, Yu Y, Tan S. The role of the miR-21-5p-mediated inflammatory pathway in ulcerative colitis. Exp Ther Med. 2020;19(2):981–989.
- Yan H, Zhang X, Xu Y. Aberrant expression of miR-21 in patients with inflammatory bowel disease: a protocol for systematic review and meta analysis. Medicine (Baltimore). 2020;99(17):e19693.
- Sutterwala FS, Haasken S, Cassel SL. Mechanism of NLRP3 inflammasome activation. Ann N Y Acad Sci. 2014;1319(1):82–95.
- Nizami S, Arunasalam K, Green J, et al. Inhibition of the NLRP3 inflammasome by HSP90 inhibitors. Immunology. 2021;162(1):84–91.
- Piippo N, Korhonen E, Hytti M, et al. Hsp90 inhibition as a means to inhibit activation of the NLRP3 inflammasome. Sci Rep. 2018;8(1):6720.
- Venegas C, Heneka MT. Inflammasome-mediated innate immunity in Alzheimer’s disease. Faseb J. 2019;33(12):13075–13084.
- Rathinam VA, Vanaja SK, Waggoner L, et al. TRIF licenses caspase-11-dependent NLRP3 inflammasome activation by gram-negative bacteria. Cell. 2012;150(3):606–619.
- Yang Y, Wang H, Kouadir M, et al. Recent advances in the mechanisms of NLRP3 inflammasome activation and its inhibitors. Cell Death Dis. 2019;10(2):128.
- Burdette BE, Esparza AN, Zhu H, et al. Gasdermin D in pyroptosis. Acta Pharm Sin B. 2021;11(9):2768–2782.
