Abstract
A tropical marine bacterium isolated from the hard coral, Symphyllia sp. was identified as Serratia marcescens on the basis of morphological, biochemical and 16S rDNA analysis. The bacterium showed antimicrobial activity towards the pathogens Candida albicans and Pseudomonas aeruginosa and the marine biofouling bacterium Bacillus pumilus. S. marcescens displayed biosurfactant activity as evidenced by drop collapse, blood hemolysis and surface tension reduction (52.0–27 mN m−1). The active compound was purified by solvent extraction and silicic acid chromatography. Characterization was by thin layer chromatography, gas chromatography mass spectroscopy (GC-MS), Fourier transform infrared (FTIR) spectroscopy and 1H as well as 13C nuclear magnetic resonance (NMR) analysis. The surfactant was found to be a glycolipid composed of glucose and palmitic acid. The glycolipid prevented adhesion of C. albicans BH, P. aeruginosa PAO1 and B. pumilus TiO1. The glycolipid also disrupted preformed biofilms of these cultures in microtitre plates. Confocal laser scanning microscopy and electron microscopy confirmed the effective removal of biofilms from glass surfaces. The glycolipid derived from S. marcescens could thus serve as a potential anti-biofilm agent.
Introduction
Microbial communities termed biofilms are abundant in nature. They are prevalent in medical, dental, industrial and environmental settings where they are undesirable due to their pathogenicity, and resistance towards antimicrobial agents or biofouling technologies (Mah and O'Toole Citation2001). Although a variety of strategies have been developed to control biofilms, the search for novel, natural and effective anti-biofilm technologies continues (Fusetani Citation2004; Qian et al. Citation2010; Tahmourespour et al. Citation2011).
In recent decades, biosurfactants have emerged as efficient alternatives in controlling a variety of biofilms (Dusane et al. Citation2008, Citation2010; Rivardo et al. Citation2009). Because of their low toxicity, biodegradability, biocompatibility and effectiveness under different environmental conditions, they are more favored than their chemical counterparts and antimicrobial agents (Kosaric Citation2001; Singh and Cameotra Citation2004). Biosurfactants are important in the treatment of infections as most of them also display antimicrobial activity (Kosaric Citation2001). Biosurfactants reduce surface tension and are thus effective in modifying the surface properties of bacterial cells, which depend on the hydrophobicity of the cells as well as the concentration and type of biosurfactant (Ahimou et al. Citation2000). Polystyrene coated with biosurfactant markedly reduced bacterial and fungal colonization (Dusane et al. Citation2008). In the biomedical context, such coatings can also reduce the incidence of infections (Velraeds et al. Citation1996; Rivardo et al. Citation2009).
Marine microorganisms often produce biosurfactants that effectively control bacterial cell adhesion and biofilm formation (Das et al. Citation2009; Kiran et al. Citation2010). In particular, epibiotic bacteria associated with the surfaces of higher organisms produce biosurfactants or antimicrobial compounds that interfere with biofilm formation and cell to cell communication (Irie et al. Citation2005; Rasmussen and Givskov Citation2006; Valle et al. Citation2006). Several bacterial species produce a variety of extracellular products that inhibit the settlement of potential competitors, thereby preventing biofouling of surfaces (Burgess et al. Citation1999). A literature survey shows that marine environments are rich and diverse sources of novel anti-biofilm agents (Armstrong et al. Citation2000; Dworjanyn et al. Citation2006; Dobretsov Citation2008; Dobretsov et al. Citation2009). In this paper, the ability of a hitherto unreported tropical marine strain of Serratia marcescens is described that produces a biosurfactant that displays (i) antimicrobial, (ii) anti-adhesive and (iii) biofilm disrupting activities with respect to selected pathogenic and marine biofouling microorganisms.
Material and methods
Isolation and identification of the biosurfactant producing marine bacterial strain
A sample of the hard marine coral Symphyllia sp. was obtained from seawater near Mandapam, Tamil Nadu, India (9.28°N, 79.12°E). A reddish-pink pigment producing bacterium was isolated on Zobell agar (Dusane et al. Citation2011). The culture was maintained on Zobell agar (HiMedia, India) slants at 4°C. The isolate was stained by the Gram staining technique and subjected to catalase and oxidase tests. Molecular identification and phylogenetic analysis were carried out as follows. An isolated colony was suspended in 50 μl of colony lysis buffer (10 mM, Tris-HCl, pH 7.5, 10 mM EDTA and 50 μg ml−1 of proteinase K) and incubated at 55°C for 15 min. Proteinase K was inactivated at 80°C for 10 min and the mixture was centrifuged at 8000 × g at 4°C for 15 min. The supernatant containing genomic DNA was directly used as a template in the PCR reaction. PCR amplification of 16S rRNA gene was carried out with the eubacteria specific primers 16F27N: 5′CCAGAG TTTGATCMTGGCTCAG-3′ and 6R1525XP: 5′T TCTGCAGTCTAGAAGGAGGTGWTCCAGGC-3′ (Pidiyar et al. Citation2003). A PCR reaction (25 μl) was performed by using 10 ng of genomic DNA, 1X reaction buffer (10 mM Tris-HCl, pH 8.8 at 25°C, 1.5 mM MgCl2, 50 mM KCl and 0.1% Triton X-100), 0.4 mM (each) deoxynucleoside triphosphates (Invitrogen) and 0.5 U of DNA polymerase (New England Labs, UK) in an automated Gene Amp PCR system 9700 thermal cycler (Applied Biosystems, Foster City, USA). The amplification conditions were as follows 94°C for 1 min (denaturation), 55°C for 1 min (annealing), 72°C for 1.30 min (elongation) and 72°C for 10 min (final elongation). The expected PCR product of around 1.5 Kb was checked by electrophoresis on 1% agarose gel in 1X TBE buffer and stained with 0.5 μg ml−1 of ethidium bromide. The PCR product was precipitated by PEG-NaCl (20% PEG in 2.5 M NaCl) at 37°C for 30 min and centrifuged at 8000 × g for 30 min at room temperature. The pellet was washed twice with 70% ethanol, dried and resuspended in 4 μl of sterile nuclease free water. One microliter (50 ng) of purified PCR product was sequenced as described earlier (Pidiyar et al. Citation2003). The analysis of sequences was done by using the NCBI server (http://www.ncbi.nlm.nih.gov/BLAST). The analyzed sequence has been deposited in GenBank and an accession number (GQ214001) has been obtained.
Antimicrobial activity of the tropical marine S. marcescens culture
The bacterial isolate identified as S. marcescens was tested for its ability to inhibit growth of selected test microorganisms by using the standard disc assay. Pathogenic biofilm forming strains of the fungus Candida albicans and the bacterium Pseudomonas aeruginosa PAO1 (Dusane et al. Citation2008) were used, as well as the marine biofouling bacterium Bacillus pumilus TiO1 (Dusane et al. Citation2010). The bacterial and fungal strains were grown in LB (Luria Bertani) and YEPD (yeast extract 3.0; peptone 5.0; dextrose 10.0; malt extract 3.0 g l−1 of distilled water) broth, respectively. The pathogens (C. albicans and P. aeruginosa) were grown at 37°C and the biofouling isolate B. pumilus at 30°C for 24 h in LB (for bacterial cultures) and YEPD (for the fungal culture). The cultures were spread onto respective agar surfaces and the cell-free supernatant (CFS) of S. marcescens was assayed for antimicrobial activity. To obtain the CFS, S. marcescens was grown in Zobell marine broth for 24 h at 30°C, centrifuged at 6000 × g for 10 min and the resulting supernatant was passed through a 0.22 μ filter (Millipore, USA).
Determination of surfactant activity
The biosurfactant activity in the CFS was checked by three techniques, viz. (i) the drop collapse assay, (ii) hemolysis on blood agar and (iii) surface tension reduction. The drop collapse assay was performed on microtiter plate lids coated with DPX mount as described earlier (Dusane et al. Citation2011). Ten μl each of the cell-free supernatant of S. marcescens, 1% SDS and sterile broth (negative control) were individually placed onto the surface coated microtiter plate lids and drop collapse was noted after addition of crystal violet stain. Surfactant activity in the culture supernatant of S. marcescens was also determined by checking hemolysis on blood agar (Carillo et al. Citation1996). Wells were prepared in blood agar and culture supernatant (50 μl) was added. The plates were incubated at 30°C for 24 h. Surface tension reduction by S. marcescens grown in Zobell marine broth over a period of time was determined by using a tensiometer-DCAT 11 (Dataphysics, GmBH). The values were compared with the uninoculated growth medium.
Production, extraction and purification of the biosurfactant
S. marcescens was grown in 1 l of Zobell marine broth for 48 h at 30°C on a shaker at 120 rpm. The CFS obtained as described above was subjected to activity guided purification (Pruthi and Cameotra Citation2000) with some modifications to the procedure. Briefly, the CFS was extracted with equal volumes of chloroform: methanol (2:1), the organic fraction was collected and concentrated in a rotary evaporator. This crude biosurfactant preparation was loaded onto a pre-activated silicic acid (Sigma, USA) column and eluted with chloroform: methanol (2:1) with a flow rate of 0.25 ml min−1 (Brundish et al. Citation1966; Findlay and White Citation1987). The fractions were collected and those fractions displaying antimicrobial activity were characterized further.
Identification of the biosurfactants by TLC, GCMS, FTIR and NMR analysis
The bioactive fraction was loaded onto the thin-layer chromatography silica plates. The plates were developed in chloroform–methanol–water (65:25:4) and subjected to orcinol-sulfuric staining specific for glycolipids (Tuleva et al. Citation2002). The purified biosurfactant was hydrolyzed with 6 mol l−1 HCl at 100°C for 24 h as described earlier (Zinjarde et al. Citation1997). The hydrolysate was extracted twice with diethyl ether to obtain the fatty acid fraction. Fatty acid composition was determined by gas chromatography mass spectroscopy (GC-MS) analysis. Fatty acids were esterified with 2 mol l−1 HCl in methanol at 100°C for 40 min. These fatty acid methyl esters were extracted in n-hexane, concentrated and analyzed on a GC-MS (Shimadzu, model QP2010) equipped with a RTX-5MS (30 m × 0.2 mm) capillary column. Helium (1.5 ml min−1) was used as the carrier gas. The temperature was programmed from 60°C to 260°C at the rate of 5°C min−1 and held at 260°C for 10 min. The injector was maintained at 260°C and the electron impact ion source was maintained at 200°C. Electron impact spectra were recorded at 70 keV. Fatty acid methyl esters were identified and analyzed from the NIST database. The aqueous phase (after ether extraction) was neutralized and used to analyze the sugar components by TLC (Pruthi and Cameotra Citation2000; Kiran et al. Citation2010).
In order to determine the functional groups in the purified surfactant, FTIR analysis was carried out. The biosurfactant (1 mg dry weight) was ground with KBr to obtain a pellet. The infrared spectra were recorded with a spectral resolution and wave number accuracy of 4.0 and 0.01 cm−1, respectively, using a Shimadzu FTIR spectrophotometer (FTIR 8400). All experiments were carried out in triplicate and representative data are presented.
To determine the structure of the purified surfactant, 1H-NMR and 13C-NMR were performed. The spectra were acquired at 500 MHz in CDCl3 solution on a Varian Mercury YH-300 spectrometer. The biosurfactant was dissolved in the solvent (CDCl3) and subjected to NMR and based on the spectra the probable structure of the biosurfactant was drawn using the ChemDraw Ultra 12.0 software (Cambridge Soft, USA).
Determination of minimum inhibitory concentration (MIC) and half effective concentration (EC50)
During the initial studies on the determination of the antimicrobial activity of the glycolipid against the test cultures (C. albicans, P. aeruginosa and B. pumilus), the standard broth microdilution method was employed (Dusane et al. Citation2010). The MIC was determined as the lowest concentration of the antimicrobial agent that inhibited the growth of microorganisms after 24 h and the half maximal effective concentration (EC50) was determined as the concentration that induced a response halfway between the baseline and maximum inhibition after exposure to the antimicrobial agent. The overnight grown test cultures were diluted to 0.5 McFarland with the growth medium and the microtitre plate wells were inoculated. Glycolipid concentrations (0.0007–25 mg ml−1) were prepared in the respective media added to the microtitre plate wells. The plates were incubated at respective temperatures overnight. After the incubation period, growth in the presence of glycolipid was estimated by measuring absorbance at 600 nm. Wells without glycolipid and those lacking cells were used as controls. All experiments were done in triplicates with two biological replicates.
Anti-adhesive activity of the glycolipid
The anti-adhesive activity of glycolipid towards the three test cultures was determined in 96 well microtiter plates (Dusane et al. Citation2010). The cells were allowed to adhere for 4 h in the microtiter plate wells. After the incubation period, different concentrations of glycolipid (0.0125–25 mg ml−1) were added and the plates were further incubated at 30 and 37°C (for biofouling and pathogenic cultures, respectively) for 1 h. Wells without glycolipids and only medium served as controls during the experiments. After the incubation period, the medium was aspirated, the wells were rinsed with PBS, air dried and stained with 0.2% crystal violet for 5 min and the absorbance at 600 nm was measured by following standard techniques (Dusane et al. Citation2008). These experiments were carried out in triplicate with two biological replicates and average values indicating standard deviation (SD) are presented here.
Glycolipid mediated disruption of preformed biofilms
C. albicans, P. aeruginosa and B. pumilus biofilms were allowed to form in 96 well microtitre plates (Dusane et al. Citation2008). After incubation for 24 h, the planktonic cells and the medium were discarded and fresh growth medium containing different concentrations of glycolipid (0.0125–25 mg ml−1) were added. These plates were further incubated for 24 h, planktonic cells were discarded and the biofilms were quantified using the standard crystal violet assay (Dusane et al. Citation2008). The wells untreated with glycolipids served as controls. The values are expressed in terms of the percentage of biofilm formed in comparison to the untreated control biofilms. All experiments were performed in triplicate with two biological replicates and mean values indicating the SD are presented here.
Visualization of biofilms by confocal laser scanning microscopy (CLSM)
Biofilms were formed on pre-sterilized microscopic glass slides. Aliquots (200 μl) of overnight cultures of C. albicans, P. aerguinosa and B. pumilus were inoculated in sterile Petri plates containing 20 ml of appropriate growth medium. Pre-sterilized microscopic glass slides were immersed in the medium. The Petri plates were incubated at respective temperatures for 24 h on a rocker. After incubation, the slides were removed and placed in fresh medium containing glycolipid (50 and 100 μg ml−1) and incubated further for 24 h. After the incubation period, the slides were removed, rinsed twice with PBS (0.1 M, pH 7.0) and stained with Live/Dead BacLight viability stains (Molecular Probes, Eugene). BacLight stains differentiate live cells from the dead ones on the basis of loss of membrane integrity (Jin et al. Citation2005). The Live/Dead stain was prepared by mixing 3 μl each of Syto 9 and propidium iodide (PI) in 1 ml of PBS. The biofilms were stained by incubating with the dyes for 10 min at room temperature and the unbound stain was removed by washing in PBS for 15 min. Cell viability was assessed by using a confocal laser scanning microscope (CLSM). A CLSM (TCS SP2 AOBS) equipped with a DM IRE 2-inverted microscope (Leica Microsystems, Germany) and a 63 × 1.2 NA water immersion lens was used to image fungal and bacterial biofilms. The confocal microscope was equipped with the 488 nm and 543 nm Ar lasers for excitation and emission, collected in the bandwidth 515–540 nm and 560 nm, respectively. SYTO 9 was excited by a 488 nm laser and the emitting light was collected by the filter 515–540, while PI was excited by a 543 nm laser and the filter 560 nm were used to collect emission light. Twenty biofilm stacks measuring an area of 238.1 × 238.1 μm were scanned and the images were acquired at 2 μm z-intervals, from the base to the top of the biofilm. Line averaging was applied and the percentage biofilm disruption was determined by using the digital image analysis freeware ImageJ, downloadable from the site http://rsb.info.nih.govij of NIH, USA. The percentage coverage for control samples (untreated with glycolipid) was determined and compared with those after treatment with the glycolipid. The untreated values were considered as 100% and the percentage inhibition values were calculated as reported earlier (Dusane et al. Citation2008).
Scanning electron microscopy (SEM)
Biofilms of C. albicans, P. aeruginosa and B. pumilus were formed on glass surfaces for 24 h. These biofilms were then treated with glycolipid (50 and 100 μg ml−1) and incubated further for 24 h. These glass slides were removed, rinsed with PBS, air dried and treated with 4% glutaraldehyde (Sigma, USA). The treated slides were kept in darkness for 2 h and were successively dehydrated through grades of alcohol (33%–99.9%). The samples were air dried, coated with platinum vapor and observed under a scanning laser electron microscope (Jeol 6360A LV, Japan). Biofilms without glycolipid treatment served as controls.
Statistical analysis
The effect of glycolipid on biofilms was analyzed statistically by the Students t-test and treatments were considered significantly different if P ≤ 0.05.
Results
Identification of the marine isolate
The tropical marine bacterium was a Gram-negative, motile, rod shaped organism with catalase positive and oxidase negative characteristics. It produced reddish-pink pigment in both liquid and solid media ( ). Based on the morphological, biochemical and genetic characteristics, the strain was identified as Serratia marcescens. The 16S rRNA gene sequence of this marine S. marcescens strain has been submitted to GenBank with an accession number (GQ214001). The sequence showed 99.22% similarity with S. marcescens strains DAP36 (EU302861) as well as with SRM (EF596776) and 99.24% with Serratia sp. 48P (EU100391).
Table 1. Morphological, biochemical and genetic characteristics of the marine isolate.
Antimicrobial and surfactant activity of S. marcescens
During the preliminary studies, the antimicrobial property of the tropical marine bacterium was determined by the standard disc assay method. The CFS displayed zones of inhibition against all the three test cultures (C. albicans, P. aeruginosa and B. pumilus). The zone diameters were found to be 16, 15 and 19 mm, respectively, when 10 μl aliquots of the CFS were used. No zones were obtained with the sterile medium (negative control). Fluconazole (50 μg) displayed a zone of inhibition of 10 mm against C. albicans and streptomycin (10 μg) showed a zone of inhibition of 10 and 12 mm against P. aeruginosa and B. pumilus, respectively.
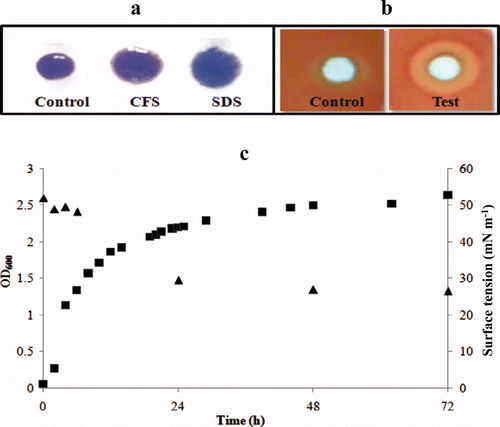
To determine the biosurfactant activity of the S. marcescens CFS, three different standard assays (drop collapse, hemolysis on blood agar and surface tension measurements) were performed. The zone diameter of the drop collapse after addition of S. marcescens supernatant onto the surface coated microtiter plate surface was 5.5 mm and that obtained with SDS (1%) was 6.0 mm (a). These values were significantly higher (P < 0.05) than those observed for control experiments (sterile broth). Distinct clearance zones on blood agar plates were observed after incubation for 24 h. These suggested β-hemolysis and were a possible indication of the presence of surfactant activity. A representative zone is shown in b (test). Negative control samples (sterile broth) did not show such zones (b, control). On the other hand, a rhamnolipid sample (used as a positive control) also displayed the hemolysis zones as reported earlier (Tuleva et al. Citation2002). The third criterion tested to determine the surfactant activity was a reduction in the surface tension of the CFS over a period of time (c). The surface tension of the growth medium was reduced from 52 mN m−1 to 29.6 and 27.0 mN m−1 after incubation for 24 and 48 h, respectively.
S. marcescens biosurfactant extraction, purification and characterization
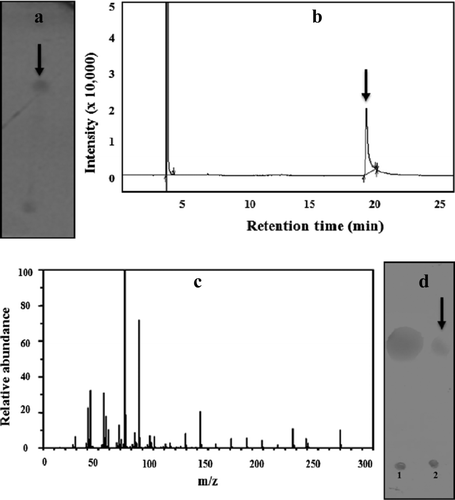
After solvent extraction and silicic acid chromatography, the bioactive fractions were concentrated and the compound was analyzed to determine the structural details. The compound gave a positive reaction with orcinol reagent on TLC plates. A single spot with an R f of 0.72 was observed (a black arrow). The lipid and sugar components in the glycolipid were further determined by GCMS and TLC, respectively. The methyl esterified hydrolyzed sample when analyzed by GC displayed a single major peak (b black arrow). The mass spectrum analysis identified this major peak as palmitic acid methyl ester with m/z ratio of 270 (c). The sugar component in the hydrolyzed product was identified as glucose on the basis of the R f value on TLC when compared to standard glucose (d black arrow).
Figure 2. Structural characterization of the surfactant from S. marcescens. a = TLC profile stained with orcinol reagent showing a single spot marked with a black arrow; b = GC profile of the methyl esterified lipid fraction showing a single peak marked with a black arrow; c = mass spectrum of the peak in the GC profile identified as palmitic acid methyl ester from the NIST database; d = TLC profile of the hydrolyzed surfactant showing the presence of (1) standard glucose (2) test sample showing the glucose spot marked with a black arrow.
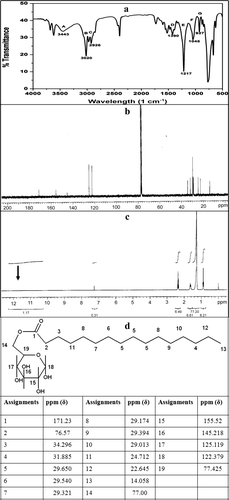
The FTIR spectrum of the pure compound is shown in a. The regions marked A and G (3500–2700 cm−1 and 950–900 cm−1) are known to indicate the presence of ester compounds (Tuleva et al. Citation2002). The peak at 3443 cm−1 (A) is characteristic of the ‒OH free stretching due to hydrogen bonding. Labels B and C (3025 and 2926 cm−1) indicate the aliphatic bond stretch of ‒CH3, ‒CH2 and ‒CH groups; label D (1400–1380 cm−1) indicates the bending of ‒OH bonds in the carboxylic acid group and labels E and F (1220–1040 cm−1) depict the C‒O‒C stretching (Tuleva et al. Citation2002; Rahman et al. 2010). The 13C and 1H-NMR analysis were performed to further elucidate the structure of the purified surfactant. The 13C-NMR spectrum (b) showed the presence of 22 peaks in agreement with the postulated structure (ester of palmitic acid and glucose). The –COOH group in palmitic acid is known to show a ppm (δ) of 183. However, in the observed 13C-NMR spectrum, this shifted to 171.23 ppm (δ) indicating a conjugation of this COOH group with the C6 of the glucose moiety. The remaining peaks were assigned as per the frequency and corresponding ppm (δ) values. The 1H-NMR profile for free palmitic acid would have displayed a peak at 12 ppm (δ). However, the profile obtained with the glycolipid did not show this peak (c black arrow) indicating a conjugation. The assignments of the 13C-NMR peaks to the postulated structure are shown in d. This figure also enlists δ values and their C assignments. On the basis of the FTIR and the NMR data, the biosurfactant produced by this tropical marine strain of S. marcescens was elucidated. The presence of the ester bond was indicated from the FTIR as well as the 13C-NMR data. d thus shows the deduced structure of the glycolipid composed of glucose and palmitic acid.
Anti-biofilm activity of the glycolipid
The MIC values of the glycolipid as determined by the standard broth microdilution method were > 25.0 μg ml−1 against the pathogens (C. albicans and P. aeruginosa) and > 12.5 μg ml−1 against B. pumilus. The EC50 values calculated on the basis of these results were found to be 12.5 μg ml−1 for C. albicans and P. aeruginosa and 6.25 μg ml−1 for B. pumilus. Based on the MIC values the anti-adhesive property of glycolipids at different concentrations (0.0125–25 μg ml−1) against C. albicans, P. aeruginosa and B. pumilus were also tested in microtiter plates. The attachment of B. pumilus cells to polystyrene microtiter plate surfaces were significantly inhibited (89% and 94%, P < 0.05) at concentrations of 50 and 100 μg ml−1 of the glycolipid, respectively. P. aeruginosa was inhibited up to 75% and 88% and C. albicans up to 76% and 82% at 50 and 100 μg ml−1, respectively (a). Cells of B. pumilus were more effectively removed (P = 0.01) from the surface when compared to C. albicans and P. aeruginosa PAO1. In the present study, preformed biofilms (24 h grown) of C. albicans, P. aeruginosa and B. pumilus in polystyrene microtitre plates were disrupted upto 55%, 62% and 55%, respectively, with the glycolipid at 50 μg ml−1 (b). The preformed biofilms were more effectively removed (70–80%) at a concentration of 100 μg ml−1. Statistical analysis using the Student's t-test showed significant difference (P < 0.05) in biofilm disruption by glycolipid at concentrations of 50 and 100 μg ml−1 when compared to untreated biofilms. Based on these results, the concentrations 50 and 100 μg ml−1 were used for the CLSM imaging studies on the disruption of preformed biofilms on glass surfaces (). Results of the image analysis depicted in clearly indicated that the biofilms on glass surfaces were disrupted efficiently. The biofilms were significantly disrupted (P < 0.01) as compared to the untreated biofilms at the tested concentrations of 50 and 100 μg ml−1. Preformed biofilms of C. albicans (A) were disrupted up to 60% and 88% at concentrations of 50 and 100 μg ml−1 of the biosurfactant, respectively (B and C). The disruption was significant (70.6 and 90.5%, P < 0.01) in the case of P. aeruginosa at concentrations of 50 and 100 μg ml−1 when compared to control experiments (D, E and F). With B. pumilus, the disruption was 69.5 and 89.5%, respectively ( G, H and I). The differences in biofilm disruption within the test cultures were found to be statistically insignificant in case of B. pumilus and P. aeruginosa (P = 0.15) and B. pumilus and C. albicans (P = 0.25) at 50 and 100 μg ml−1, suggesting a comparable effect of the glycolipid against these cultures. depicts representative SEM images of control and glycolipid treated biofilms of C. albicans, P. aeruginosa and B. pumilus. These images also confirm the anti-biofilm nature of the glycolipid and its effectiveness in removing biofilms from glass surfaces.
Figure 4. a = Anti-adhesive ability of the S. marcescens glycolipid towards C. albicans (▪), P. aeruginosa (▪) and B. pumilus (□). The bars represent average values of triplicate experiments with two biological replicates per experiment; error bars indicate the SD. b = the effect of S. marcescens glycolipid on preformed biofilms of C. albicans (▪), P. aeruginosa (▪) and B. pumilus (□). The bars represent average values of triplicate experiments with two replicates per experiment; error bars indicate the SD.
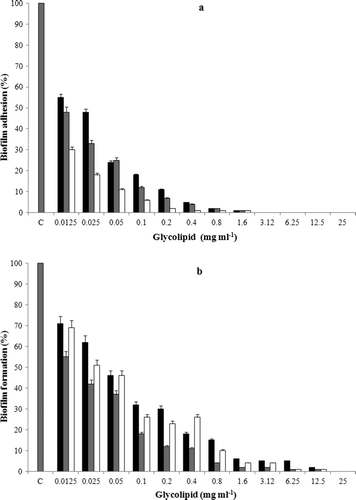
Figure 5. CLSM analysis of preformed biofilms of C. albicans (A, B, C); P. aeruginosa (D, E, F) and B. pumilus (G, H, I). Controls (A, D, G); treated with 50 μg ml−1 (B, E, H) and 100 μg ml−1 (C, F, I) of the glycolipid.
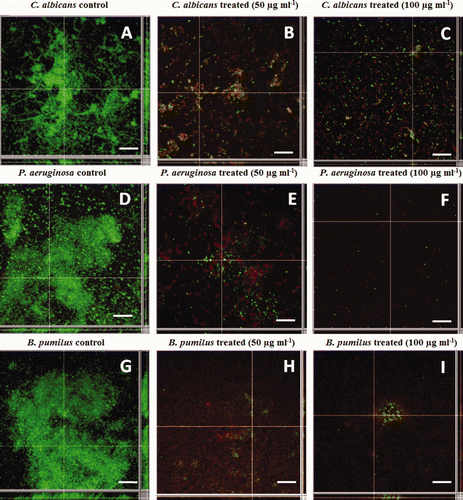
Figure 6. Representative SEM images of preformed biofilms of C. albicans, P. aeruginosa and B. pumilus. Control (a, c and e); treated with 100 μg ml−1 glycolipid biosurfactant after 24 h (b, d, f). The scale bar represents 10 μm for a and b and 5 μm for c to f.
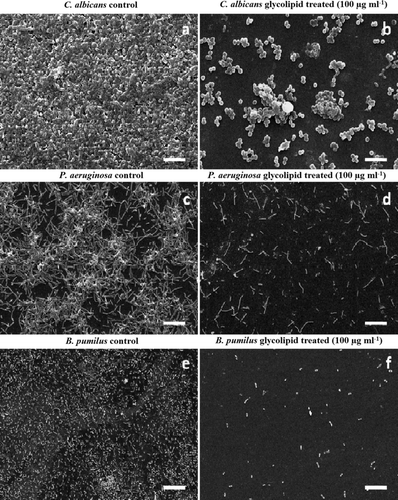
Table 2. Quantitative data from CLSM image analysis (N = 20) for disruption of preformed biofilm by glycolipid.
Discussion
Marine microorganisms are exposed to a variety of stress conditions with respect to pressure, temperature, salinity and micronutrient depletion. Their survival and proliferation often depends on their inherent ability to produce bioactive compounds. In particular, epibiotic bacteria associated with different living forms produce molecules that prevent the attachment, growth and/or survival of challenging microorganisms in competitive environments (de Carvalho and Fernandes Citation2010). As a result, these microorganisms are extensively exploited in screening programs related to the isolation of novel compounds with commercially interesting biological functions. In the present investigation, a tropical marine strain of S. marcescens produced a potential biosurfactant that displayed anti-biofilm activity towards the test pathogenic and biofouling microorganisms. Although some strains of this organism are known to produce surfactants and one such glycolipid (derived from MTCC 86) has been exploited in enhanced oil recovery (Pruthi and Cameotra Citation2000), to the best of the authors' knowledge, there are no reports on the application of S. marcescens biosurfactants as anti-biofilm agents.
The biosurfactant activity of this S. marcescens strain was evident from the drop collapse assay, hemolysis on blood agar and surface tension reduction compared to the controls. The surface tension reduction obtained with the tropical marine strain under investigation is similar to that observed with Serratia rubidaea and S. marcescens MTCC 86 (Matsuyama et al. Citation1990; Pruthi and Cameotra Citation2000). c also indicates that the surface tension reduction occurred in the late log phase of growth indicating that the biosurfactant is a secondary metabolite as also reported for another marine biosurfactant-producing tropical yeast (Zinjarde and Pant Citation2002).
The biosurfactant was identified as a glycolipid on the basis of the orcinol test as also reported earlier (Kiran et al. Citation2010) having palmitic acid as the major fatty acid esterified to glucose. The structure was deduced on the basis of FTIR and NMR results by making comparisons with earlier work on glycolipids (Park et al. Citation1998). There are a few reports on the production of glycolipid surfactants by Serratia spp. although a strain of S. marcescens (MTCC 86) is also reported to produce a glycolipid biosurfactant (Pruthi and Cameotra Citation2000). However, the composition of the MTCC 86 glycolipid [composed of sucrose and a mixture of 3-(3’-hydroxytetradecanoyloxy) decanoate and 3-(3’-hydroxyhexadecanoyloxy) decanoate] was different from that produced by this tropical marine strain.
The glycolipid biosurfactant derived from the tropical marine strain of S. marcescens showed antimicrobial and surfactant activity. The antimicrobial activity displayed by the biosurfactant against C. albicans, P. aeruginosa and B. pumilus was significant at MIC concentrations (12.5 and 25.0 μg ml−1). There is a recent report on a marine bacterium (Brevibacterium casei) producing a glycolipid biosurfactant that showed MIC values in the range of 68 to 72 μg ml−1 (Kiran et al. Citation2010). In comparison to the aforementioned and other glycolipids (Dusane et al. Citation2010), the surfactant used in the present study was found to be effective at even at lower concentrations. Other compounds such as alkenals containing C11 and C12 aliphatic groups also display low MIC values (12.5 and 6.25 μg ml−1, respectively), against a test culture (Salmonella choleraesuis) as has been reported earlier (Kubo et al. Citation2004).
Biosurfactants have potential applications in the treatment of medical and industrial biofilms (Rodrigues et al. Citation2006; Dusane et al. Citation2010). Their anti-adhesive and disruptive properties make them significant molecules in controlling various kinds of biofilms. The authors' laboratory has recently demonstrated the effective use of rhamnolipids as anti-adhesion and biofilm disrupting agents against the marine biofouling bacterium B. pumilus (Dusane et al. Citation2010). The S. marcescens glycolipid was also effective as an anti-adhesive agent (displaying > 60% anti-adhesive effect) against the test cultures at MIC concentrations. An increase in biosurfactant concentration resulted in a better reduction in fungal and bacterial cell adhesion to the microtiter plate surfaces. Rodrigues et al. (Citation2006) demonstrated that rhamnolipids inhibit bacterial adhesion over a range varying from 21% to 81%. The glycolipid derived from the tropical marine strain of S. marcescens was more effective and exhibited higher anti-adhesive activity (95 to 99%). The results observed with this glycolipid are comparable with those of a biosurfactant derived from Lactobacillus that inhibited the adhesion of Enterococcus faecalis (Velraeds et al. Citation1996, Citation2000). Compounds that cause a decrease in bacterial adhesion and a reduction of biofilm are potentially useful for both clinical and environmental applications.
Preformed biofilms of C. albicans, P. aeruginosa PAO1 and B. pumilus formed in microtiter plate wells and on glass surfaces were effectively disrupted by the glycolipid surfactant. CLSM and SEM images confirmed the removal of cells from glass surfaces, while the former revealed that a large population of cells was killed after the treatment with the glycolipid. The inhibition of E. faecalis biofilms on glass surfaces using surfactants produced by Lactobacillus sp. has been reported (Velraeds et al. Citation1996). In addition, rhamnolipids are also known to disperse biofilms of Bordetella bronchiseptica and B. pumilus (Irie et al. Citation2005; Dusane et al. Citation2010). Treatment of B. pumilus biofilms with rhamnolipids resulted in the removal of the exopolymeric substances (EPS). This was postulated to be the mechanism by which biofilms were removed from glass surfaces (Dusane et al. Citation2010). The increased anti-biofilm ability of the glycolipid currently being investigated may be due to the two important properties that it displays, namely, antimicrobial and surfactant activity.
Conclusion
The marine strain of S. marcescens produced a glycolipid biosurfactant consisting of glucose and palmitic acid. The glycolipid inhibited growth and was found to be a potent anti-adhesive and anti-biofilm agent for the pathogenic and biofouling microbial strains tested. This study highlights the significance of hitherto unreported tropical marine microflora in producing novel bioactive compounds that may find a variety of applications.
Acknowledgements
DHD is a recipient of Senior Research Fellowship (SRF) under the BARC-UoP collaborative research programme. The authors would like to thank Professor Rosario Oliveira, Portugal for the C. albicans culture and Dr Ayesha Khan, IBB for NMR data analysis.
References
- Ahimou , F , Jacques , P and Deleu , M . 2000 . Surfactin and iturin A effects on Bacillus subtilis surface hydrophobicity . Enzyme Microbial Technol , 27 : 749 – 754 .
- Armstrong , E , Boyd , K G and Burgess , G J . 2000 . Prevention of marine biofouling . Biotechnol Ann Rev , 6 : 221 – 241 .
- Brundish , D E , Shaw , N and Baddiley , J . 1966 . Bacterial glycolipids . Biochem J , 99 : 546 – 549 .
- Burgess , J G , Jordan , E M , Bregu , M , Mearns-Spragg , A and Boyd , K G . 1999 . Microbial antagonism: a neglected avenue of natural products research . J Biotechnol , 70 : 27 – 32 .
- Carillo , P , Mardarz , C and Pitta-Alvarez , S . 1996 . Isolation and selection of biosurfactant producing bacteria . World J Microbiol Biotechnol , 12 : 82 – 84 .
- Das , P , Mukherjee , S and Sen , R . 2009 . Antiadhesive action of a marine microbial surfactant . Colloids Surf B: Biointerfaces , 71 : 183 – 186 .
- De Carvalho , C CR and Fernandes , P . 2010 . Production of metabolites as bacterial responses to the marine environment . Mar Drugs , 8 : 705 – 727 .
- Dobretsov , S . 2008 . “ Inhibition and induction of marine biofouling by biofilms ” . In Marine and industrial biofouling , Edited by: Venkatesan , R , Murthy , P S , Cooksey , K and Flemming , H C . Vol. 4 , 293 – 314 . Vol. 4. Berlin (Germany) : Springer .
- Dobretsov , S , Teplitski , M and Paul , V . 2009 . Mini-review: quorum sensing in the marine environment and its relationship to biofouling . Biofouling , 25 : 413 – 427 .
- Dusane , D H , Nancharaiah , Y V , Zinjarde , S S and Venugopalan , V P . 2010 . Rhamnolipid mediated disruption of marine Bacillus pumilus biofilms . Colloids Surf B: Biointerfaces , 81 : 242 – 248 .
- Dusane , D H , Matkar , P , Venugopalan , V P , Kumar , A R and Zinjarde , S S . 2011 . Cross species induction of antimicrobial compounds, biosurfactants and quorum sensing inhibitors in tropical marine epibiotic bacteria by pathogens and biofouling microorganisms . Curr Microbiol , 62 : 974 – 978 .
- Dusane , D H , Rajput , J K , Kumar , A R , Nancharaiah , Y V , Venugopalan , V P and Zinjarde , S S . 2008 . Disruption of fungal and bacterial biofilms by lauroyl glucose . Lett Appl Microbiol , 47 : 374 – 379 .
- Dworjanyn , S A , de Nys , R and Steinberg , P D . 2006 . Chemically mediated antifouling in the red algae Delisea pulchra . Mar Ecol Prog Ser , 318 : 153 – 163 .
- Findlay , R H and White , D C . 1987 . A simplified method for bacterial nutritional status based on the simultaneous determination of phospholipid and endogenous storage lipid poly-β-hydroxyalkanoate . J Microbiol Meth , 6 : 113 – 120 .
- Fusetani , N . 2004 . Biofouling and antifouling . Nat Prod Rep , 21 : 94 – 104 .
- Irie , Y , O'Toole , G and Yuk , M H . 2005 . Pseudomonas aeruginosa rhamnolipids disperse Bordetella bronchiseptica biofilms . FEMS Microbiol Lett , 250 : 237 – 243 .
- Jin , Y , Zhang , T , Samaranayake , Y H , Fang , H P , Yip , H K and Samaranayake , L P . 2005 . The use of new probes and stains for improved assessment of cell viability and extracellular polymeric substances in Candida albicansbiofilms . Mycopatholgy , 159 : 353 – 360 .
- Kiran , G S , Sabarathnam , B and Selvin , J . 2010 . Biofilm disruption potential of a glycolipid biosurfactant from marine Brevibacterium casei . FEMS Immunol Med Microbiol , 59 : 432 – 438 .
- Kosaric , N . 2001 . Biosurfactants and their application for soil bioremediation . Food Technol Biotechnol , 39 : 295 – 301 .
- Kubo , I , Fujita , K and Kubo , A . 2004 . Anti-Salmonella activity of (2E)-alkenals . J Appl Microbiol , 96 : 693 – 699 .
- Mah , T FC and O'Toole , G A . 2001 . Mechanisms of biofilm resistance to antimicrobial agents . Trends Microbiol , 9 : 34 – 39 .
- Matsuyama , T , Kaneda , K , Ishizuka , I , Toida , T and Yano , I . 1990 . Surface-active novel glycolipid and linked 3-hydroxy fatty acids produced by Serratia rubidaea . J Bacteriol , 172 : 3015 – 3022 .
- Park , O J , Lee , Y E , Cho , J H , Shin , H J , Yoon , B D and Yang , J . 1998 . Purification and structural characterization of glycolipid biosurfactants from Pseudomonas aeruginosa YPJ-80 . Biotechnol Bioprocess Eng , 3 : 61 – 66 .
- Pidiyar , V J , Jangid , K , Dayanand , M , Patole , M S , Gonzales , J M , Kaznowski , A and Shouche , Y S . 2003 . Phylogenetic affiliation of Aeromonas culicicola MTCC 3249 based on gyrB gene sequence and PCR amplicon sequence analysis of cytolytic enterotoxin gene . Syst Appl Microbiol , 26 : 197 – 202 .
- Pruthi , V and Cameotra , S S . 2000 . Novel sucrose lipid produced by Serratia marcescens and its application in enhanced oil recovery . J Surf Deter , 3 : 533 – 537 .
- Qian , P Y , Xu , Y and Fusetani , N . 2010 . Natural products as antifouling compounds: recent progress and future perspectives . Biofouling , 26 : 223 – 234 .
- Rasmussen , T B and Givskov , M . 2006 . Quorum-sensing inhibitors as anti-pathogenic drugs . Int J Med Microbiol , 296 : 149 – 161 .
- Rivardo , F , Turner , R J , Allegrone , G , Ceri , H and Martinotti , M G . 2009 . Anti-adhesion activity of two biosurfactants produced by Bacillus spp. prevents biofilm formation of human bacterial pathogens . Appl Microbiol Biotechnol , 83 : 541 – 553 .
- Rodrigues , L R , Banat , I M , Teixeira , J A and Oliviera , R . 2006 . Biosurfactants: potential applications in medicine . J Antimicrob Chemother , 57 : 609 – 618 .
- Singh , P and Cameotra , S S . 2004 . Potential applications of microbial surfactants in biomedical sciences . Trends Biotechnol , 22 : 142 – 146 .
- Tahmourespour , A , Salehi , R , Kermanshahi , R K and Eslami , G . 2011 . The anti- biofouling effect of Lactobacillus fermentum-derived biosurfactant against Streptococcus mutans . Biofouling , 27 : 385 – 392 .
- Tuleva , B K , Ivanov , G R and Christova , N E . 2002 . Biosurfactant production by a new Pseudomonas putida strain . Z Naturforsch , 57 : 356 – 360 .
- Valle , J , da Re , S , Henry , N , Fontaine , T , Balestrino , D , Latour-Lambert , P and Ghigo , J M . 2006 . Broad-spectrum biofilm inhibition by a secreted bacterial polysaccharide . Proc Natl Acad Sci USA , 103 : 12558 – 12563 .
- Velraeds , M M , van der Mei , H C , Reid , G and Busscher , H J . 1996 . Inhibition of initial adhesion of uropathogenic Enterococcus faecalis by biosurfactants from Lactobacillus isolates . Appl Environ Microbiol , 62 : 1958 – 1963 .
- Velraeds , M M , van de Belt-Gritter , B , Busscher , H J , Reid , G and van der Mei , H C . 2000 . Inhibition of uropathogenic biofilm growth on silicone rubber in human urine by lactobacilli – a teleologic approach . World J Urol , 18 : 422 – 426 .
- Zinjarde , S S and Pant , A . 2002 . Emulsifier from a tropical marine yeast Yarrowia lipolytica NCIM 3589 . J Basic Microbiol , 42 : 67 – 73 .
- Zinjarde , S S , Sativel , C , Lachke , A H and Pant , A . 1997 . Isolation of an emulsifier from Yarrowia lipolytica NCIM 3589 using a modified mini-isoelectric focusing unit . Lett Appl Microbiol , 24 : 117 – 121 .