Abstract
There is a considerable body of information regarding bacterially enhanced corrosion, however, this review focuses on diatoms (unicellular algae) whose contribution to biocorrosion is less well studied. The reasons why diatoms have been neglected in studies of biocorrosion in natural waters are discussed and the question whether diatoms should be considered as inert with respect of electrochemical processes is considered. A particular focus is given to the case of stainless steels (SS), which are widely used in variety of applications in natural waters. Basic information on the cell biology of diatoms is included in the review, particularly with respect to their ability to ‘sense’ and adhere to surfaces. Investigations at the nanoscale are reviewed as these studies provide information about the behavior of cells at interfaces. Recent advances include the use of atomic force microscopy (AFM), although only a few studies have been applied to diatoms. Regarding the electrochemical behavior of SS, the mechanisms by which diatoms influence the potential ennoblement process is discussed. Such studies reveal the association of diatoms, in addition to bacteria, with biocorrosion processes.
Introduction
Owing to their outstanding resistance to corrosion, stainless steels (SS) are extensively used in many applications involving contact with biological compounds/solutions. They are used in the food industry (Jullien et al. Citation2003; Whitehead et al. Citation2011) and in the manufacture of vascular stents, guide wires, or other orthopedic implants (Hanawa Citation2002; Ratner et al. 2004). In addition, SS are frequently utilized in many structures located in marine and freshwater environments, including port installations, cooling water circuits, and ships and related equipment. When exposed to humid and non-sterile media, SS are usually colonized by a variety of microorganisms, which adhere and grow to form biofilms. This fouling process strongly affects the performance of the material and may cause its deterioration.
Over the two past decades, considerable progress has been made towards understanding the nature and mechanisms relative to (i) the adhesion of microorganisms and (ii) microbiologically influenced corrosion (MIC). Many experiments in natural media, or employing strains isolated from natural sources, have demonstrated the role of bacteria in biocorrosion (for reviews see Beech Citation2004; Beech and Sunner Citation2004; Mansfeld Citation2007; Little et al. Citation2008). By contrast, diatoms have attracted little interest, either in terms of biofouling, but particularly with respect to biocorrosion, in spite of the fact that they make up the dominant biomass on all wetted and illuminated surfaces (Wetherbee et al. Citation1998).
Understanding the behavior of diatoms on SS surfaces requires an understanding of the complexity of the interface. In this review, a description of how diatoms interact with SS surfaces in a range of aqueous media is presented, including natural waters (seawater, estuaries, lakes and freshwater) and other waters associated with human activities (dam-water, wastewater, domestic water). Details are presented to illustrate key points: (i) physico-chemical features of SS surfaces, (ii) biochemical properties of the diatom cell surface, including composition, structure and recognition, and (iii) metabolic activities that influence the electrochemical response of SS.
Regarding the adhesion of diatoms, relevant features involved in cell–surface and cell–cell interactions have been gained through the application of atomic force microscopy (AFM) to probe live cells at the nanoscale (Hinterdorfer and Dufrene Citation2006; Dufrêne Citation2008; Muller and Dufrene Citation2008; Dupres et al. Citation2010). Some studies have reported promising results implicating diatoms in the electrochemical behavior of SS upon immersion in aqueous media. However, there is a lack of more basic knowledge of the mechanism by which diatoms, by themselves or via their metabolites, influence the free corrosion potential of SS. The following reasons may be relevant: (1) microbiologists are more familiar with the biology of bacteria and tend to favor investigations on this class of microorganisms; (2) bacterial biofilms are a serious cause of persistent infections (eg Costerton et al. Citation1999), thus research focuses on bacteria of biomedical interest compared to other microorganisms; (3) many bacteria responsible for an electrochemical effect are already well-known (Ismail et al. Citation1999; Shi et al. Citation2002; Dumas et al. Citation2008a; Mansfeld Citation2007; Parot et al. Citation2011) while the involvement of diatoms, which may have a key role in these processes, is still not fully understood.
This review aims to point out particular aspects, either experimental or conceptual, which are of primary importance to understanding the behavior of diatoms on SS surfaces, and more generally, at interfaces between materials and aqueous media. An analysis of the different hypotheses reported in the literature indicate a connection between diatoms and the electrochemical response of SS. Considering these aspects is essential in order to make progress in deciphering interfacial mechanisms involved in fouling and biocorrosion processes.
Exposure of SS in natural waters
SS passive film
The surface properties of SS depend strongly on the presence of an oxide passive layer that forms during exposure of the bare alloy to an oxidising medium. Passivity results from the thermodynamic instability of the metal which tends to become covered by a film that insulates the material from the medium (Pourbaix Citation1963). Passivity occurs by anodic dissolution followed by the formation of a thin layer, typically with a thickness of a few nanometers (Olsson and Landolt Citation2003). The driving force of passive film growth and stability is the potential gap between the metal and the solution, inducing a high electrical field (up to 106 V cm−1) (Baroux et al. Citation1990). Passive film growth may be controlled by electrochemical polarization, or may occur spontaneously in the presence of an oxidising agent (electron acceptor). Theoretical aspects of the passivation process were reported in detail by Sato (Citation1990). Passive film formation slows down ionic transport and thus metal dissolution, leading to a substantial resistance to corrosion in conditions to which the bare metal would react significantly. Details regarding the properties of passive films (composition, structure, electronic properties and stability have been reviewed elsewhere (see Olsson and Landolt Citation2003, and references therein). It is now well established that the high corrosion resistance of SS in a wide range of aqueous media is due to the ability of the passive film to adapt to changes induced by physico-chemical parameters (eg ionic strength, pH, potential) or microbiological activities.
Regarding physico-chemical properties, in common with other metals and oxides, SS surfaces exhibit high surface energy, which can be reduced by the adsorption of organic species (Kinloch Citation1990; Mantel et al. Citation1995; Caillou et al. Citation2008). The distribution of surface charge of the passive film is associated with the presence of the electrical double layer that implies the dependence of surface charge on pH (Bockris and Reddy Citation1970). Accurate measurement of the surface charge of the passive film remains difficult due to experimental considerations (Lefèvre et al. Citation2006). Values approaching the point of zero charge (PZC) were reported for many oxides using zeta-potential measurements. The PZC value obtained on a standard SS was reported to be around 3–4 (Boulangé-Petermann et al. Citation1995). Accordingly, a SS surface is negatively charged in natural waters (pH ∼6–8).
Surface conditioning and biofilm formation
In the first seconds to minutes that follow the immersion of SS or other metal and alloys in natural waters, the surface becomes covered with inert material present in the liquid phase, namely ions, macromolecules (proteins, polysaccharides, lipids), and inorganic materials. This leads to the formation of a film, commonly called the primary or conditioning film (Loeb and Neihof 1975), which strongly modifies the physico-chemical properties of the SS surface (Characklis and Cooksey Citation1983; Little and Jacobus Citation1984; Callow and Fletcher Citation1994; Taylor et al. Citation1997; Jain and Boshle 2009). Details of the ways in which the surface physico-chemistry of SS are changed by the adsorbed film have been discussed elsewhere (Schneider Citation1996; Schneider et al. Citation1997). In the marine environment, the accumulation of proteins and carbohydrates was observed on SS surfaces (Compère et al. Citation2001).
Microorganisms interact with the surface and firmly adhere, owing to the secretion of extracellular polymeric substances (EPS). This step, usually considered as irreversible, leads through cell division and further recruitment, to the formation of biofilm, which is a highly hydrated polymeric matrix. The formation of biofilms is detailed in numerous reports (eg Characklis and Marshall Citation1990; Flemming and Geesay 1991; Geesey et al. Citation1994; Flemming et al. Citation2009). The influence of the major biochemical compounds which constitute the conditioning film, ie proteins and carbohydrates, on the adhesion of microorganisms was investigated by Jain and Bhosle (Citation2009). Although bacteria are considered to be the initial colonizers, followed by diatoms, other algae and invertebrate larvae, this trend should be considered carefully as the relationship may not always be sequential or causally related. For example, diatoms can attach to clean surfaces in the absence of bacteria (Cooksey Citation1981). The morphology of biofilms has evolved from the uniform representation of Hamilton (Citation1985) to the 3-D ‘mushroom-shaped’ model described by Costerton et al. (Citation1994). The characterization of biofilms in terms of composition and three-dimensional structure was made possible by the development of 3-D mapping techniques, microanalytical devices, new fluorochrome markers and fiber-optic sensors, which allowed analyses of the liquid phase within the biofilm to be performed with minimal disturbance (Stoodley et al. Citation1994; Strathmann et al. Citation2002; Grossmann et al. Citation2007; Hu et al. Citation2007; Ganesh and Radhakrishnan Citation2007). Even so, making generalizations about biofilm structure and physiological activities are difficult, although it is well established that biofilms permit the permeation of nutrients, extracellular enzymes and metabolites that are necessary for the survival of microorganisms and their growth (Lappin-Scott and Costerton Citation1995; Jenkinson and Lappin-Scott Citation2001; Sutherland Citation2001).
The development of biofilms on a SS surface creates a complex SS/biofilm interface where multiple and diverse processes take place, including: (1) modification of the SS passive film in terms of composition, morphology and physico-chemical properties as a function of the medium, in particular in terms of the range of microorganisms and related biomacromolecules (Ismail et al. Citation1999; Yuan and Pehkonen Citation2007; Landoulsi et al. Citation2008b); (2) biofilms may be considered as a multi-compartment system involving numerous chemical reactions and mass transport processes and include: (i) a semi-continuous liquid phase, containing ions, other chemical compounds, and macromolecules, (ii) microorganisms that may be aggregated, (iii) solid particles, including cellular debris with a variable level of dispersion and reactivity, (iv) a macromolecular gel, composed largely of sugar polymers (eg polymers of glucose, galactose and mannose) (Christensen and Characklis Citation1990; Bhosle et al. Citation1995) and (v) one or several interfaces in contact with the metal surface where adsorbed substances and compounds, originating from metal dissolution, accumulate. Studying the SS/biofilm interface is thus a challenge. The most promising method adopted consists of monitoring the electrochemical behavior of SS during immersion in natural waters. This approach allows information to be acquired in situ without noticeable disturbance of the interface. Recent progress regarding the electrochemical behavior of biofouled SS is detailed below.
Potential ennoblement
The free corrosion potential (Ecorr), also called open circuit potential (OCP), has been recognized as a relevant parameter to characterize the electrochemical behavior of SS in natural waters in situ. Mollica and Travis (personal communication) were the first to report that Ecorr shifted towards anodic values upon immersion of SS in natural waters. This potential shift considerably exceeds the one related to SS surface passivation and reaches values higher than +200 mV/SCE in most cases. The term ‘ennoblement’ was used to describe this phenomenon, but it does not mean that the surface becomes more resistant against corrosion. When the potential increases towards anodic values it could come close to the pitting potential (Ep) and affects the stability of the passive film.
Ennoblement has been observed in seawater, independent of parameters related to the composition (eg geographic location, season, immersion depth, hydrodynamic factors) or to the SS material (SS composition and microstructure, surface roughness, geometrical sample form) (Scotto et al. Citation1985; Bardal et al. Citation1993; Scotto and Lai Citation1998; Feron et al. personal communication; Fischer et al. personal communication). It appears that the various parameters only influence the time which precedes ennoblement and/or the rate of increase in potential. In contrast to seawater, a generalization appears to be more difficult to make for natural freshwaters, including estuaries, rivers and lakes. This is due to a high variability related to the composition of the water and microbial activity as a function of location. Nevertheless, ennoblement has been reported to occur systematically in natural rivers (Dickinson and Lewandowski Citation1996; Dickinson et al. Citation1996a; Marconnet et al. Citation2008; Landoulsi et al. unpublished data). Ennoblement was also observed in other low chloride media such as domestic waters (Percival et al. Citation1998a, Citation1998b) and dam-waters (Liao et al. Citation2010).
Diatoms: the predominant biofouling community
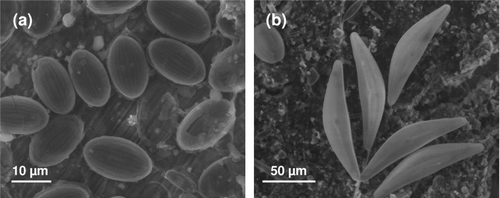
Although the literature on biofouling and resulting issues related to biocorrosion is dominated by studies on bacteria, biofilms formed on SS surfaces and other metal and alloys are typically dominated by diatoms, especially when SS surfaces are illuminated. Many authors have recorded diatoms on a SS surface when studying ennoblement in a wide range of media. The main results, summarized in , show the diversity of diatoms, independent of immersion conditions and SS type. In other reports, some authors have mentioned the presence of diatoms on SS surfaces without identification of the species (Scotto et al. Citation1986; Motoda et al. Citation1990; Mansfeld et al. Citation1994; Videla Citation1994; Mattila et al. Citation1997). Cooksey et al. (Citation1980) showed that the initial colonization of SS coupons by diatoms exposed in Biscayne Bay (Florida) was light dependent, but after the first cells attached, it was not possible to distinguish between further colonization and the division of attached cells that had adhered to the substratum first. In any event, the number of cells on the surfaces increased logarithmically during each light period over 1 week. There was no increase in cell density at night. In short-term laboratory-based experiments, adhesion of diatoms in the dark was far less than in the light. shows SS samples after immersion in a natural river using environmental scanning electron microscope (ESEM). The dominating presence of diatoms either in close contact with the SS surface () or when the surface is well covered with biofilm () is apparent. In these cases, Ecorr was observed to reach values ranging from +200 to +400 mV/SCE (Landoulsi et al. unpublished data). The presence of bacteria is not obvious from the images, but cannot be ruled out as samples were immersed in natural river water. A heterotrophic bacterial film requires a source of organic carbon for growth and since the level of free organic material is relatively low in natural waters, the initial bacterial film is probably carbon-limited. Diatoms, however are autotrophic and thus require only carbon dioxide and nutrients for growth and these are usually not limiting. Once the diatom film is established, a mutualistic relationship between diatoms and bacteria will be developed. Although the primary film is generally dominated by bacteria, especially after immersion for ∼1 day, the first major accumulation of biomass is attributed to diatoms (Cooksey et al. Citation1980, Citation1981).
Table 1. The main diatom species identified on stainless steel surface after immersion in natural waters at different locations.
General biology of diatoms
Diatoms are eukaryotic microalgae that form brown coloured ‘slimes’ on wet illuminated surfaces. They vary in size from about 2 μm to several hundred μm, but are most commonly in the range 10–100 μm. The cell wall (frustule), composed of silicon dioxide, consists of a top and bottom (hypotheca and epitheca) valve, the two valves being held together by girdle bands. Some diatoms have one or two slits (raphes) in the cell wall. Traditional taxonomists use, among other criteria, the shape, size and ‘decoration’ of the silica frustules (eg number of ridges and pores) and presence/absence of a terminal pore(s) to speciate diatoms (). Since diatom cells respond to stress by altering their shape, and even in unstressed situations they change their size when they divide, distinguishing between diatoms at the species level is challenging. When compared to similar technology used for bacteria, molecular taxonomy using 18s-RNA is not well developed. Although RNA extraction of cells can be made, the database available for comparisons is still scarce. Two main groups of diatoms can be designated viz. centric and the pinnate diatoms. The former shows radial symmetry of the frustules and the latter exhibits bilateral symmetry. Diatoms are found in all aquatic environments either in the water column (planktonic) or attached to surfaces (episammic or more generally, benthic). It is the attached organisms that cause biofouling. Attachment and motility are achieved via EPS secreted through the raphe slit(s), thus only raphid diatoms have these attributes (see review by Molino and Wetherbee Citation2008). Diatoms are most often obligately autotrophic, but some are facultative heterotrophs, many are mixotrophic and a few, having no chloroplast, are obligately heterotrophic (Chansang and Cooksey Citation1977; Werner Citation1977).
Diatoms at the nanoscale
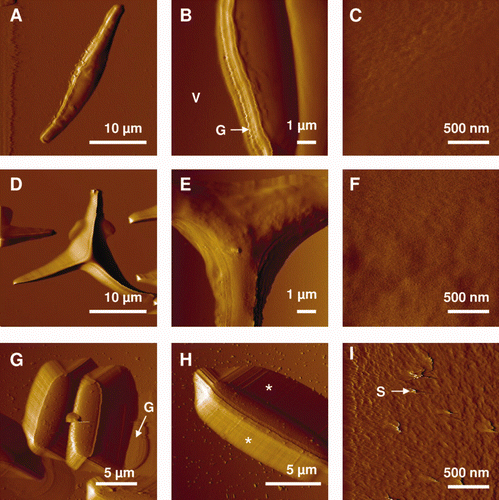
Diatoms exhibit unusual cell surfaces, compared to other common fouling microorganisms, which differentiates them when adhered in biofilm. Investigating diatoms at the nanoscale may help to decipher how diatoms interact and adhere to SS surfaces. Different techniques have been successfully used for the characterization of diatoms including scanning electron microscopy (SEM) and transmission electron microscopy (TEM) (De Stefano et al. Citation2003; Hildebrand et al. Citation2008), X-ray photoelectron spectroscopy (XPS) (Tesson et al. Citation2009), small angle X-ray scattering (SAXS) (Vrieling et al. Citation1999), confocal microscopy (Groger et al. Citation2008), Fourier transform infrared spectroscopy (FTIR) (Kiefer et al. Citation1997) and Raman mapping (Kammer et al. Citation2010). Traditional SEM and TEM are the most frequently used and high resolution images have provided information about the ultrastructure of diatom surfaces. However, such techniques are performed on dried samples and only provide limited information regarding adhesion. Recently, a quartz crystal microbalance with dissipation monitoring (QCM-D) has been used to this end (Molino et al. Citation2006, Citation2008). QCM-D allows the adhesion of diatoms to solid surfaces to be investigated, but spatial heterogeneity of the secreted adhesives is difficult to take into account. The use of atomic force microscopy (AFM) overcomes the limitation of the aforementioned methods by allowing a single living cell to be imaged (Muller and Dufrêne Citation2008; Dupres et al. 2010). Although the pioneering AFM experiments on diatoms were carried out on dried samples (Linder et al. Citation1992), imaging of cells in the native and hydrated state was quickly exploited (Crawford et al. Citation2001; Higgins et al. Citation2002, Citation2003a; Gebeshuber et al. Citation2003). In addition to not requiring a conductive layer that is required for SEM, AFM enables experiments with minimal preliminary sample preparation. Losic et al. (Citation2007a) used AFM to reveal details of frustule structure of Thalassiosira eccentric at the nanoscale, showing that the frustules are built from silica nanoparticles, with sizes varying from 20 to 70 nm. In another study, AFM was used to image the surface structure of Phaeodactylum (Francius et al. Citation2008a), a pennate diatom possessing three different morphotypes (ovoid, fusiform and triradiate). Fusiform cells were of an elongated shape in which the girdle region resulting from the valve overlapping was resolved (). Examination of the triradiate forms confirmed previous SEM images and revealed cells with three arms emerging from a central core and forming a star (, ). The ovoid morphotype was two to three times smaller than the two other morphotypes (). High resolution images revealed a rougher surface and ‘streaks’ following the scanning direction (). The authors suggested the presence of secreted polymers involved in adhesion and gliding motility, as reported elsewhere (Chiovitti et al. Citation2003; Dugdale et al. Citation2006a). Gebeshuber et al. (Citation2003) determined the thickness of the layer of EPS covering the siliceous frustules to be about 10 nm for benthic species, while more accurate measurements showed a thickness between 9–24 nm, depending on the species (Hildebrand et al. Citation2009).
Figure 3. AFM deflection images recorded in aqueous solution for the fusiform (A–C), triradiate (D–F) and ovoid (G–I) morphotypes of Phaeodactylum tricornutum. Labels V, G and S correspond to the following features: valve, girdle region and streaks. The features highlighted by the asterisks in (H) reflect tip convolution artifacts. Reproduced with permission from Francius et al. (2008a).
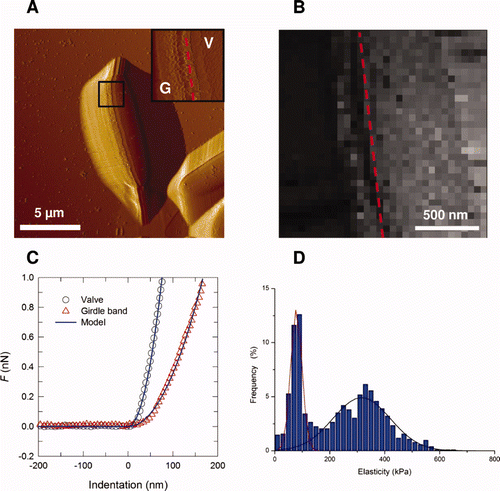
The elastic properties of the cell surface can also be obtained from AFM nanoindentation measurements performed on the siliceous cell walls. For example, Navicula pelliculosa has an elastic modulus varying from 7 to hundreds of GPa, depending on the location on the frustules (Almqvist et al. Citation2001). These values are similar to those found for silica. Other results showed that the elastic modulus varied at different parts of the frustules of Coscinodiscus sp. ranging from ∼2 GPa for the cribrum to ∼15 GPa for the internal plate (Losic et al. Citation2007b). By comparison, the elastic modulus of EPS secreted from the cell was found to be much lower, varying from 250 to 750 kPa (Higgins et al. Citation2003a, Citation2003b). Francius et al. (Citation2008a) investigated the cell wall elastic properties of different morphotypes of P. tricornutum. Elastic modulus values for the three morphotypes were lower than the GPa values reported for the walls of siliceous diatoms (Almqvist et al. Citation2001; Losic et al. Citation2007b) and differed from one morphotype to another. Indeed, the cell wall of the silicified ovoid form was found to be around five-fold stiffer (elastic modulus of ∼500 kPa) than that of the two non-silicified forms (∼100 kPa). In some situations, elasticity maps revealed heterogeneous contrast, as observed in the fusiform cell in . The girdle region appeared softer (∼80 kPa) than the valve (∼320 kPa), suggesting that the girdle has a lower silica content and is enriched in organic material.
Figure 4. Mechanical properties of the ovoid girdle (G)/valve (V) interface of Phaeodactylum tricornutum diatom. (A) Deflection image (dashed line indicates the interface); (B) elasticity maps (z-range = 1000 kPa) corresponding to the inset image in (A); (C) typical force-indentation curve; (D) distribution of elasticity values (n = 1024 force curves). Reproduced with permission from Francius et al. (2008a).
Diatoms in biofilms
Comprehensive reviews of the involvement of diatoms in marine microfouling have been published elsewhere (eg Cooksey and Wigglesworth-Cooksey Citation2001; Cooksey et al. Citation2009). Diatoms form the bulk of the initial colonizing biomass on surfaces immersed in the marine environment (Cooksey Citation1981; Callow Citation1986; Wetherbee et al. Citation1998) and diatom biofilms generate hydrodynamic drag on vessels (Bohlander Citation1991; Schultz et al. Citation2011). A detailed description of the diatom community which adheres to a range of ship hull coatings can be found in a recent report by Zargiel et al. (Citation2011). Although there is more information appertaining to marine than freshwater biofilms, the biology of cell adhesion is likely to be similar. Attachment of all cells to surfaces (Berridge et al. Citation1998) is controlled by intracellular calcium levels (Cooksey et al. Citation1980; Cooksey Citation1981; Wetherbee et al. Citation1998). The intracellular calcium concentrations that invoke metabolic responses in all cells are changes in concentration in the range 10−7–10−6 M. A tenfold difference in environmental calcium levels between freshwater (ca 1 mM) and marine water (ca 10 mM) is not likely to be significant since intracellular calcium levels are 1000 times smaller. Thus, conclusions based upon studies of marine organisms are likely to be generally applicable.
Early work on the design of antifouling (AF) surfaces can be found in the publications of Baier (Citation1980), Characklis and Cooksey (Citation1983) and Wigglesworth-Cooksey et al. (Citation1999). Basic conclusions were that surfaces of intermediate surface energy (ca 25 dynes cm−1) were the least hospitable to cells in general and diatoms in particular, although cells attached to all substrata whatever their surface properties. Since this early work, there have been major advances stimulated by the need for fouling protection of marine structures without the release of toxic materials into the environment. Thus, AF coatings have become more complex. Recent studies using different strategies to control slimes dominated by diatoms can be found in the literature (eg Molino et al. Citation2009; Dobretsov et al. Citation2011; Patil and Jagadeesan Citation2011; Zargiel et al. Citation2011). Whereas earlier efforts used simple chemistries to generate differences in wettability of surfaces, more recent efforts have focused on mixed polymers. For example, Sommer et al. (Citation2010) used siloxane-polyurethane coatings based on aminopropyl terminated polydimethylsiloxane (PDMS). Urethane polymers alone have little fouling resistance, but provide mechanical strength whereas siloxanes, which are not mechanically strong, have fouling-release (FR) properties. The layering of these two components provided a coating with the positive properties of each component resulting in lower adhesion of bacteria, diatoms and macroalgae. The AF properties of polysiloxane polymers were also improved by the inclusion of tethered biocides (quaternary ammonium compounds (quats)), which were not released from the coating (Majumbar et al. Citation2008). Whilst coatings with 18 carbon length quats were effective in inhibiting bacterial biofilm formation, 14 carbon quats were most effective in inhibiting growth of the diatom Navicula sp. This technology demonstrates a two pronged attack on fouling control; the quat has AF properties, while the low surface energy surface reduces adhesion strength ie enhances FR. It has been shown that more hydraulic force is required to remove diatoms from a hydrophobic siloxane FR surface than to remove young plants of the macroalga Ulva (Cassé et al. Citation2007); the same relationship has been shown for other coating systems (see Bennett et al. Citation2010; Finlay et al. Citation2010). Since the extracellular adhesives of potential fouling organisms are diverse, it may not be possible to design a universal FR coating (Cooksey et al. Citation2009).
There is little information about AF coatings for application to SS that are specifically designed to resist diatoms. However studies performed on other surfaces, as described above, are expected to be generally applicable to chemically-modified SS surfaces. A widespread procedure to modify SS surfaces consists of grafting silane coupling agents onto the passive oxide film to form an anchoring layer (Landoulsi et al. 2011), and to use the amino-end groups to attach various molecules of AF interest, especially polymers. Other procedures of surface functionalistion have been also applied on SS, including the self-assembly of long chain aliphatic molecules with different headgroups, such as carboxylic acids, phosphonic acids and thiols (Shustak et al. Citation2004; Mahapatro et al. Citation2006; Raman and Gawalt Citation2007; Raman et al. 2010; Kruszewski and Gawalt Citation2011).
Since it appears that modifications to the surface energy of substrata, aimed at reducing adhesion do not prevent the adhesion of all fouling organisms, the question arises what properties of the surface could be altered to discourage/reduce cellular adhesion, ie what would be an ideal AF surface? One possible approach would be to alter the surface chemistry of a coating in order to induce a specific response by the potential fouling cell or larva. A number of recent papers have reported the benefits of using amphiphilic coating systems that present both hydrophobic and hydrophilic domains on the surface; such coatings show excellent AF and FR properties for both diatoms and macroalgae (eg Dobretsov and Thomason Citation2011; Martinelli et al. 2011; Sundaram et al. Citation2011).
There are also a number of concepts that moderate diatom adhesion. It has been shown that diatoms can sense sugars when presented as a concentration gradient (Wigglesworth-Cooksey and Cooksey Citation1992) and that an intracellular calcium concentration flux may be involved. The ability to sense the presence of a sugar was investigated using Amphora coffeaeformis and positive and negative taxis was found, depending on the sugar used. The ‘conditions’ for sensing involved orientation of a hydroxyl group at position 2 of the pyranose ring as well as the diatom being able to move towards a sugar gradient, suggesting the diatom cell has a sophisticated array of cell surface receptors. Support for the idea of sensing is found in the work of Wetherbee et al. (Citation1998), who showed that cells of Stauroneis decipiens were able to re-orientate so that the raphe slit in the cell wall is on the ventral side of the cell, instead of being uppermost. They postulated that surface recognition allowed the cell to ‘search’ for the substratum by strands of polymer secreted through the raphe slit. The strands then contract allowing the cell to turn so that motility is possible. The involvement of calcium transients in sensing has been shown in Phaeodactylum tricornutum and A. coffeaeformis (Falciatore et al. Citation2000; Cooksey et al. Citation2009). Further information was provided by Thompson et al. (Citation2008) who investigated the ability of diatom cells to detect and respond to the surface energy of the substratum. Cells adhere more strongly to hydrophobic surfaces and it would be reasonable to assume that the adhered state is preferable for survival. Thompson et al (Citation2008) measured the cellular level of nitric oxide, a general stress indicator found across the biological kingdom, in diatom cells on hydrophobic and hydrophilic surfaces. The level of nitric oxide was four-fold higher in cells on a hydrophilic surface (glass) than those on a hydrophobic surface (silicone) indicating that hydrophobic surfaces were less stressful. Molecules that induce stress in fouling organisms are candidates for inclusion in AF coatings, especially if they can be incorporated covalently into the coating. One such molecule is trans-trans-2,4- decadienal (DD) which has been implicated as a chemical defense molecule in that it inhibits invertebrate grazing of phytoplankton (Ianora et al. Citation2004, Citation2006). DD generates nitric oxide bursts which produce apoptosis, ie programmed cell death. As DD is produced by diatoms, it could be the trigger that causes clumps of diatoms to disperse (Wigglesworth-Cooksey et al. Citation1999). DD caused a rapid loss in motility and cells became permeable to Sytox Green 1 (a vital dye) soon afterwards (Cooksey et al. Citation2009). Based on the finding of Thompson et al. (Citation2008), the inhibition of cellular sensing may be a promising strategy. Since such molecular control mechanisms are often similar across biology, sensory inhibition may be a general AF strategy for organisms from both the plant and animal kingdoms. Such an approach would be applicable to metal and alloys, including SS and could provide a new way to design an efficient AF surface to prevent the adhesion of diatoms in natural waters. In a review on diatom adhesion, Molino and Wetherbee (Citation2008) concluded that ‘many questions remain unanswered’. Research is especially needed regarding the interaction between biofilm bacteria and diatom adhesion. It has been suggested that photorespiration in diatoms caused by a reduced oxygen diffusion in the biofilm matrix can be controlled by its utilization by heterotrophic bacteria (Wigglesworth-Cooksey et al. Citation2001). Diatom-bacterial interactions have been investigated by (Murray et al. Citation1986; Wigglesworth-Cooksey et al. Citation2001, Citation2005), but more work is needed in this area.
Diatom adhesion: guidelines for the future
Upon contact with a surface, adhesion forces are mediated by the physico-chemical properties of the substratum and those of the microorganism, eg hydrophobicity and surface charge. Although substratum properties are easily measured using traditional techniques of surface characterization, knowledge of cell surface properties at the single cell level remains challenging.
It is now established that the siliceous cell wall of diatoms is covered by an organic envelope composed of polysaccharides, proteins, and glycoproteins (Hecky et al. Citation1973; Staats et al. Citation1999; Chiovitti et al. Citation2003) and that adhesion of diatoms on surfaces is associated with the secretion of mucilaginous material (EPS) (Hoagland et al. Citation1993). Diatom EPS have some common attributes; most are carbohydrate-based polymers with some protein content, which provides the ability to bind to both hydrophilic and hydrophobic substrata. However, analyses of extracted polymers, eg by time of flight mass spectroscopy, provides only limited information compared to in situ sampling because extraction techniques may introduce artifacts (de Brouwer et al. Citation2006).
AFM has been used to determine the adhesive and mechanical properties of individual proteins secreted by live diatoms cells (Dugdale et al. Citation2005, Citation2006a, Citation2006b). Force curves recorded for the benthic diatom Toxarium undulatum revealed a regular sawtooth-like pattern, which is a reliable signature of modular protein unfolding. Dugdale et al. (Citation2005) hypothesized that single adhesive nanofibers were each made of a specific number of modular proteins aligned in parallel, forming a cohesive unit. The modular and flexible nature of these proteins conveys both strength and toughness, making it ideally suited for adhesion to the substratum. However, one question remains: what is the contribution of each of these macromolecules in the attachment of diatoms to a surface? The use of force spectroscopy with modified tips will provide insight into the distribution of specific sugar moieties on live diatoms, while AFM tips functionalized with specific antibodies should resolve protein mapping. Such studies will aid understanding of the physical properties of diatom EPS.
Cell probe
Diatoms can be used as probes to investigate cellular adhesion. Bowen et al. (Citation1998) were the first to use a single, living, immobilised cell as a ‘cell probe’ for the study of cell-surface adhesion. Following this study, a large variety of cell probes from different microorganisms have been used, including fungal spores (Bowen et al. Citation2002; Wargenau and Kwade Citation2010), yeast cells (Bowen et al. Citation2001) and bacteria (Dague et al. Citation2010). In the biofouling and biocorrosion contexts, recent experiments have been performed to probe the interaction between bacteria immobilized on an AFM tip and different metal surfaces, including SS (Sheng et al. Citation2007, Citation2008). Despite the interest in this approach to probe interactions between cells and surfaces, very few studies have been reported using diatoms. Arce et al. (Citation2004) used AFM to compare the adhesion of Navicula sp. to surfaces of different physico-chemical properties. Live diatom cells were immobilized at the end of tipless cantilevers and both hydrophobic and hydrophilic surfaces were tested with the same diatom probe to avoid artefacts (). Force vs distance curves revealed comparable cell adhesion strengths on Intersleek® and mica, indicating that Navicula secretes EPS with both hydrophobic and hydrophilic properties ().
Figure 5. Cell probe experiments (A and B). (A) SEM micrograph of a single diatom cell attached with epoxy glue to an AFM tipless cantilever; (B) representative force vs distance curves obtained with bioprobe diatoms in the stationary phase on Intersleek (a–c) and mica (d–f) surfaces. The work of detachment, W, is given in fJ units (10−15 J) for each curve. The arrow represents the approach and retraction directions. Reproduced with permission from Arce et al. (Citation2004). Nanoscale structure and hydrophobicity of Aspergillus fumigatus. (C) Deflection image and (D) adhesion force map obtained with a hydrophobic tip on SDS-treated conidia, revealing highly correlated structural and hydrophobic heterogeneities. Reproduced with permission from Dague et al. (Citation2007). Detecting individual galactose-rich polysaccharides on LGG bacteria (E and F). (E) AFM deflection image of single LGG bacteria trapped into porous polymer membrane and adhesion force map (inset, gray scale: 200 pN) and (F) representative force curves recorded with PA–1 tip on LGG wild-type. Reproduced and adapted with permission from Francius et al. (Citation2008b).
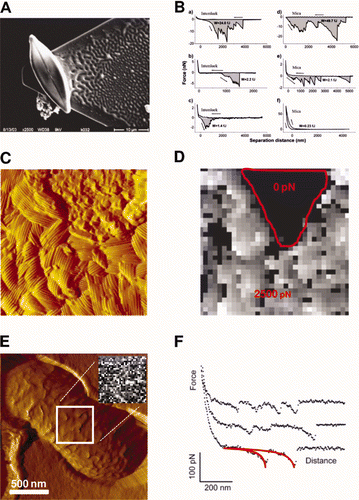
Chemical properties of the cell surface
Ahimou et al. (Citation2002) used AFM tips functionalized with ionizable carboxyl groups (COO−/COOH) to probe the surface charges of Saccharomyces cerevisiae at the nanometer level. Force–distance curves were strongly influenced by pH: no adhesion was measured at neutral/alkaline pH, while multiple adhesion forces were recorded at acidic pH. The change of adhesion force as a function of pH was interpreted as resulting from a change of cell surface electrostatic properties. Using a similar approach, it has been shown that hydrophilic (OH) and hydrophobic (CH3) tips can be used to map cell surface hydrophobicity (Dufrêne Citation2000). Moreover, this technique, called chemical force microscopy (CFM) now makes it possible to map the spatial arrangement of chemical groups on live cells (Alsteens et al. Citation2007; Dague et al. Citation2007; Hu et al. Citation2011). Using CFM with hydrophobic tips Dague et al. (Citation2007) demonstrated large adhesion forces on the surface of Aspergillus fumigatus conidia, reflecting strong hydrophobic properties, in agreement with the presence of hydrophobins in the outer rodlet layer. Variations in hydrophobicity on a single cell could also be resolved, revealing contrasted hydrophobicity between rodlet and polysaccharide regions (, ). These studies demonstrate that chemically functionalized tips enable quantitative measurement of surface properties at the subcellular level and could be of interest to probe the distribution of EPS on live diatoms.
Identifying cell surface proteins and polysaccharides
Force spectroscopy experiments using biospecific tips, ie tips in which specific biological molecules are immobilized, have been shown to be particularly useful in identifying individual polysaccharides and proteins on living cells, and to measure their adhesion (Dupres et al. Citation2005; Dufrêne Citation2008). Notably, force spectroscopy offers a means of probing the conformational properties of microbial polysaccharides (Camesano and Abu-Lail Citation2002; Abu-Lail and Camesano Citation2003; Camesano et al. Citation2007). For example, AFM tips modified with lectins were used to specifically detect, localize and analyse individual polysaccharides on live Lactobacillus rhamnosus GG (LGG) (Francius et al. Citation2008b, Citation2009). Two types of polysaccharides were identified using AFM tips functionalized with two polysaccharide-specific lectins (, ). Additionally, the properties of the polysaccharide (distribution, adhesion, extension) of LGG wild-type were markedly different from those of a derived mutant impaired in terms of adhesion, biofilm formation and exopolysaccharide production.
Implication of diatoms in electrochemical processes
Oxygen plays a pivotal role in processes associated with biocorrosion of SS as it is involved in both abiotic and biotic mechanisms, which influence the electrochemical behavior of these alloys (Landoulsi et al. Citation2008a). The involvement of diatoms in these processes may be mediated by photosynthetic activity, which produces O2 at the SS/biofilm interface. Though this has not been shown directly in biocorrosion studies, many reports in the literature suggest that diatoms are involved. The role of aerobic activities within biofilms on the electrochemical behavior of SS is detailed below.
Mechanism of ennoblement involving aerobic activities
Since early observations on the potential ennoblement of SS in natural seawater (Mollica and Trevis 1976), many hypotheses have been proposed to explain the interfacial processes involved in ennoblement. However, progress which has been gained regarding the structure and properties of biofilm changed the vision of researchers regarding its role. Taking into account the high level of biofilm heterogeneity and thus of the SS/biofilm interface, some hypotheses have been revised (for recent reviews see Beech et al. Citation2005; Mansfeld Citation2007; Landoulsi et al. Citation2008a).
Within the biofilm, oxygen is involved in the metabolic pathways of many microorganisms. It acts as a final electron acceptor in the oxidation process of organic molecules, eg lipids and sugars, or inorganic species such as manganese. Due to energetic considerations, the reduction reaction of oxygen leads to the formation of highly reactive free radicals or molecular species. Such intermediate products, commonly called reactive oxygen species (ROS), are involved in biocorrosion because their reactivity is higher than that of oxygen itself.
Biogenic formation of H2O2
Hydrogen peroxide (H2O2) is one of the main intermediates of the oxygen reduction reaction. The presence of H2O2 has been reported within biofilms formed on SS surfaces immersed in natural seawaters (Dickinson et al. 1996b; Xu et al. Citation1998; Washizu et al. Citation2004) and freshwaters (Marconnet et al. Citation2008; Liao et al. Citation2010; Landoulsi et al. unpublished data). In these studies, the concentration of H2O2 was detected in the range of several mM. The generation of H2O2 is governed by two antagonist processes: (i) production by enzymes using O2 as electron acceptors (oxidases) and (ii) degradation by enzymes involved in the defense of microorganisms against oxidative stress (catalases, peroxidases).
The generation of H2O2 in biofilms has attracted much interest in biocorrosion studies, owing to the ability of H2O2 to influence the electrochemical behavior of SS. In natural waters, cathodic processes on SS are mainly due to the oxygen reduction reaction. However, H2O2 exhibits a redox potential (E° = 1.776 V/SHE), significantly higher than that of oxygen (E° = 1.228 V/SHE), making it a good candidate to initiate ennoblement.
Enzymatic system
Previously, it has pointed out that the biogenic generation of H2O2 is at the crossroads of many enzymatic reactions and plays a key role in the ennoblement of SS. Hence, there is growing interest in using purified enzymes in electrochemical tests to study SS ennoblement (Landoulsi et al. Citation2008a). In particular, an enzymatic system mimicking the generation of H2O2 in biofilms has been used. To this end, glucose oxidase (EC. 1.1.3.4) was used, which catalyzes the formation of H2O2 by converting glucose into gluconolactone, then spontaneously decomposed in gluconic acid (Equation (1)):
In addition to practical experimental considerations, the choice of this enzyme was justified by the fact that glucose, the substrate of the enzyme, is the major sugar in polysaccharides present in natural waters and glucose has also been detected in biofilms formed on SS surfaces (Bhosle et al. Citation1990). Furthermore, the amount of H2O2 produced may be adjusted to be in the range of few mM, as observed in natural biofilms. Electrochemical tests using this enzyme have been performed in natural sterilized seawater (Amaya and Miyuki Citation1995, Citation1997, Citation1999; Dupont et al. Citation1998), in artificial seawater (Amaya and Miyuki Citation1995, Citation1997, Citation1999) and artificial freshwater (Landoulsi et al. Citation2008c; Marconnet et al. Citation2008). Experimental parameters relating to enzymatic activity, including pH and the ratio of enzyme and substrate, were optimized to be relevant to biocorrosion studies. Ennoblement occurred similar to that observed in natural waters reaching values ranging from +250 to +350 mV/SCE. In synthetic freshwater, simulating natural rivers, ennoblement was observed on SS type 316L whether H2O2 was generated in situ (ie produced by the enzymatic reaction) or added to the solution (). By combining electrochemical measurements and detailed surface characterization by XPS, it was shown that ennoblement was due to the electrochemical effect of H2O2. Furthermore, modification of the passive film during immersion was not sufficient to initiate such ennoblement (Landoulsi et al. Citation2008c), even if it influenced cathodic processes, especially the oxygen reduction reaction (Le Bozec et al. Citation2001). These findings were reinforced by further electrochemical measurements, which showed an increase in cathodic current density in the vicinity of Ecorr, when H2O2 was present in the solution ().
Figure 6. Panel (A) and (B). Electrochemical measurements in laboratory controlled model (Ecorr evolution and cathodic polarization curves, respectively). H2O2-induced ennoblement obtained in synthetic freshwater, simulating natural rivers, (a) before and after the addition of (b) H2O2 (2 mM, pH∼8), (c) free or (d) immobilized enzymes. Panels (C) and (D). Schematic representation of the enzymatic system used to generate H2O2. When enzymes (designated ‘E’) are free (C), the formation of H2O2 occurs randomly in the solution, while immobilized enzymes (D) catalyze the reaction near the SS surface, leading to an enrichment of H2O2 and depletion of O2.
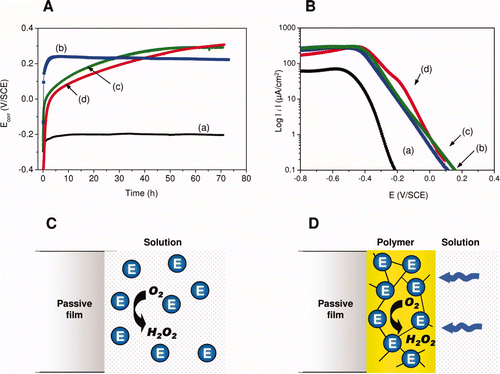
Landoulsi et al. (Citation2008d) elaborated a SS-modified electrode based on an enzyme immobilization method, to concentrate enzymatic activity near to the SS surface. This strategy was aimed at mimicking the physico-chemical conditions of the SS/biofilms interface (ie depletion of oxygen and production of oxidant species). Moreover, it allowed the activity of the immobilized enzymes to be preserved longer, since the polymer film confined the enzyme in a stable configuration and thus avoided its inactivation. When glucose oxidase was immobilized in a polymeric film coated onto a SS surface ( and ) H2O2 was mainly produced within the polymeric film according to Equation (1), leading to local accumulation. This was accompanied by a strong depletion of oxygen near the SS/film interface, owing to the fact that (i) the polymeric film partially hindered access of dissolved oxygen to the SS surface and (ii) the oxygen was consumed by the entrapped enzymes (). These experiments provided information about the cathodic processes and demonstrated the separate roles of O2 and H2O2 in ennoblement. The same approach was applied on SS, based on the use of a wire beam electrode to mimic the heterogeneity of the SS/biofilms interface (Wang et al. Citation2009). These authors showed a heterogeneous distribution of potential and current due to the generation of H2O2 catalyzed by glucose oxidase.
Consequences on corrosion behavior
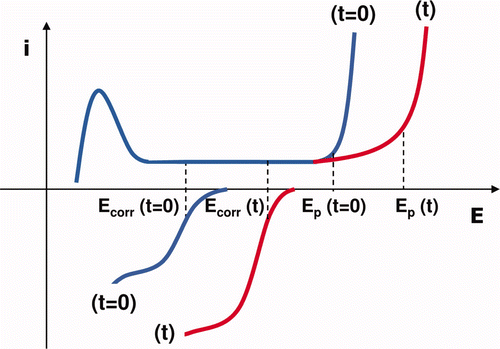
Although the mechanism of ennoblement involving H2O2 and related species is now known, one issue of primary importance in biocorrosion studies still remains poorly understood, viz. does ennoblement lead to localized corrosion of SS?
In natural waters, although the systematic feature of ennoblement is well established, pitting corrosion or other forms of localized corrosion, were not always observed. The correlation between ennoblement and corrosion is still a topic of debate. On the one hand, some authors have observed a beneficial effect of biofilms against corrosion and reported the notion of inhibition of MIC. This observation stems from the presence of EPS secreted by bacteria or other microorganisms (Mansfeld Citation2007; Videla and Herrera Citation2009), leading to protection against corrosion for several metal and alloys (Chongdar et al. Citation2005; Stadler et al. Citation2008). On the other hand, the pitting corrosion of SS has been investigated using the enzymatic generation of H2O2 to mimic aerobic activity of biofilms (Landoulsi et al. Citation2009). The results showed that the presence of H2O2 may limit pit propagation, leading to a noticeable shift of the pitting potential. From the electrochemical point of view, the involvement of H2O2 both in ennoblement and in the pitting corrosion behavior of SS may be explained on the basis of anodic and cathodic branches, as depicted in . All these findings enable reappraisal of the commonly acknowledged hypothesis that ennoblement increases the risk of localized attacks. Both EPS and dissolved compounds, such as H2O2 and related species, may play a beneficial role in protecting SS against localized corrosion. Hence, ennoblement does not necessarily increase the susceptibility of the passive film to pitting.
Figure 7. Hypothetical polarization curves of the cathodic and anodic processes on SS under MIC conditions: before (t = 0) and after (t) the formation of H2O2, causing a cathodic and an anodic response. These mechanisms result in the shift of both the corrosion potential (Ecorr) and the pitting potential (Ep) towards anodic values.
Light-dependent ennoblement
The effect of light on Ecorr evolution has been investigated by exposing SS to dark conditions (Dexter and Zhang Citation1991; Little et al. Citation1991). Little et al. (Citation1991) observed that immersion of SS in natural seawater lead to the formation of biofilms which were dominated by diatoms. However, the presence of the biofilms did not result in an ennoblement of Ecorr. On the basis of dissolved oxygen profiles through the biofilm and microprobe pH measurements, the authors suggested that diatoms modify the interfacial chemical properties by influencing the local oxygen concentration and the pH.
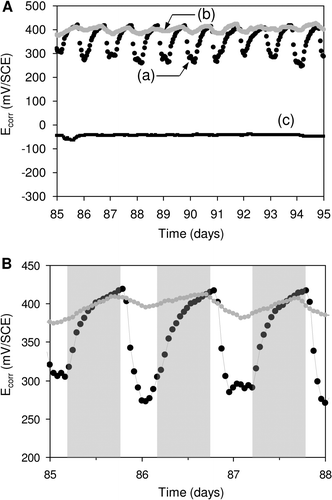
In a later report, periodic fluctuations of Ecorr were observed on SS immersed in natural waters (Maruthamuthu et al. Citation1993). Interestingly, these variations were concomitant with the day/night cycle, suggesting a light-dependence of Ecorr evolution. These findings implicate diatoms through their photosynthetic metabolism. The authors suggest that the ‘loss’ of ennoblement is due to a decrease in the pH induced by a significant proportion of acidophilic sessile bacteria (∼50% of the total aerobic bacteria), creating an unfavorable pH for enzymatic reactions. Ennoblement is restored because photosynthesis by diatoms produces alkalization within the biofilm (Maruthamuthu et al. Citation1993).
An alternative hypothesis to explain ennoblement through the photosynthetic activity of diatoms was reported by Eashwar and Maruthamuthu (Citation1995). The authors proposed a hypothetical model, based on the work of Little et al. (Citation1991) involving a change in pH and dissolved oxygen within the biofilm near the SS/biofilm interface. However, their interpretation is not straightforward because the heterogeneity of the biofilms was not taken into account. The authors used a homogenous layer to describe the microbial biofilm present on the SS surface, which is now accepted as too simplistic as the high heterogeneity of biofilms is now well known. For instance, the use of microelectrodes demonstrated that the concentration of oxygen decreased with increasing depth into the biofilm (Little et al. Citation1991; Xu et al. Citation1998). However, the spatial distribution of oxygen inside the biofilm is difficult to determine with accuracy. Recently, a three-dimensional map of oxygen concentration revealed the existence of some highly concentrated pockets of oxygen within the biofilm (De La Rosa and Yu Citation2005).
In more recent studies, the day/night cycle-dependance of Ecorr upon immersion of SS in natural river and in fresh-dam water has been reported (Marconnet et al. Citation2008; Liao et al. Citation2010). In both studies, the authors reported the dominating presence of diatoms on the SS surfaces. It was shown that Ecorr increased at night and decreased during daytime (). The potential values fluctuated as a function of the day/night cycle with an amplitude <+200 mV. In contrast, without light, the diurnal fluctuations were reduced and the Ecorr was kept at a value ∼+400 mV/SCE.
Figure 8. Light-dependent evolution of Ecorr on SS samples. Panel (A). Potential variation recorded in (a) natural exposure conditions (dam-water), (b) the same without light and (c) the same after addition of filter. Panel (B). Detailed Ecorr variation in a short period: the grey shaded regions indicate the night periods. Reproduced and adapted with permission from Liao et al. (Citation2010).
The correlation between light-dependent ennoblement and corrosion remains obscure. As mentioned above, the major issue relates to the complexity of the interface. Furthermore, it must be kept in mind that sunlight may influence the physical properties of the passive film, as it behaves as a semiconductor. In the marine environment, Eashwar et al. (Citation2011) have demonstrated the influence of exposure to sunlight on the susceptibility of SS to localized corrosion.
How may diatoms be involved in ennoblement?
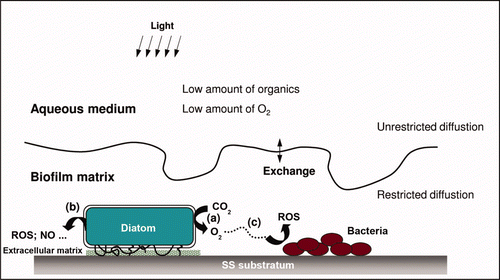
Because of the dominant presence of diatoms on SS surfaces, considerable care in interpreting the electrochemical behavior of SS, namely potential ennoblement, is necessary. The light-dependence of Ecorr evolution suggests the involvement of diatoms on the ennoblement process. The question of the mechanism by which diatoms, directly or via their metabolism, influence the potential of SS potential is difficult to answer because few studies based on electrochemical tests on SS using pure cultures of diatoms are documented. Furthermore, as mentioned above the biofilm ensemble is a heterogeneous complex. An analysis of data reported in the literature reveals the possibility of different mechanisms, as follows: (1) Direct action on Ecorr via photosynthetic metabolic activity in various aqueous media (Maruthamuthu et al. Citation1993; Ishihara and Tsujikawa Citation1998; Marconnet et al. Citation2008; Liao et al. Citation2010) although photosynthetic metabolism did not inhibit potential ennoblement (Liao et al. Citation2010). The latter was deduced from observations under reduced illumination () and may explain why ennoblement occurred in dark conditions. Furthermore, the electrochemical response time of the SS electrode indicated that the variation in day/light potential could be attributed to enrichment/depletion cycles of oxygen at the SS/biofilm interface (, process a). It is easily understandable if the Nernst equation is considered, which predicts that production of oxygen would increase the electrode potential and vice versa. A future challenge is to examine this mechanism by means of real-time measurements of dissolved oxygen close to the SS/biofilm interface. (2) Direct action mediated by diatom metabolites, in particular ROS, that react with the SS surface. Indeed, as observed in other microorganisms, the oxidative stress of diatoms may lead to the production of H2O2 or other ROS (, process b). These processes were observed in diatoms and other algae that were exposed to various forms of stress including mechanical stress, variation of light or temperature, addition of herbicides (Collén et al. Citation1994; Sundström et al. Citation1996; Abrahamsson et al. Citation2003). Although the mechanism remains poorly understood, it was shown that stress induced H2O2 may be related to the formation of volatile halocarbons involving haloperoxidase-catalyzed reactions (Wever et al. Citation1991). This process was recorded for the diatom Pleurosira laevis (Abrahamsson et al. Citation2003). The generation of ROS was also observed for Nitzschia in response to the toxic effect of redox-active compounds and their copper complexes. (Stauber and Florence Citation1985; Florence and Stauber Citation1986). The effect appears to be due to inhibition of the enzyme that breaks down H2O2 formed during oxidation of copper compounds. H2O2 may react with lipids to form hydroxyl radicals or diffuse into the extracellular space. OH• and superoxide radicals (O2 •) are also generated extracellularly (Florence and Stauber Citation1986), but they did not influence the growth of diatoms. The production of H2O2 and related species in biofilms was reinforced by recent studies, in which the presence of diatoms on ennobled SS samples was accompanied by the production of a significant amount of H2O2 (Marconnet et al. Citation2008; Liao et al. Citation2010; Landoulsi et al. unpublished data). (3) Indirect action by providing metabolic products, namely oxygen, to other heterotrophic microorganisms present in the biofilm. Ishihara and Tsujikawa (Citation1998, Citation1999) examined the potential for ennoblement by incubating SS samples in two stages: in ‘stage I’, SS was immersed in natural seawater for several days in a way that potential ennoblement did not exceed ∼ +100 mV/SCE. In ‘stage II’, SS samples were transferred to a diatom-enriched solution in which ennoblement reached ∼ +400 mV/SCE. The authors observed that ‘stage II’ alone could not lead to significant ennoblement and that ‘stage I’ was needed. These findings may imply two processes: (i) without ‘stage I’, diatoms are not able to adhere to the SS surface, possibly due to the physico-chemical properties of the interface, (ii) ennoblement is the result of the combined activities of diatoms and bacteria, based on the production of oxygen by diatoms and its consumption by heterotrophic bacteria as described above (, process c). That heterotrophic bacteria may influence photorespiration in diatoms supports this scenario (Wigglesworth-Cooksey et al. Citation2001). Other investigations on diatom-bacterial interactions have also been reported (Murray et al. Citation1986; Wigglesworth-Cooksey and Cooksey Citation2005).
Figure 9. Proposed metabolic pathways to explain the possible involvement of diatoms in influencing the electrochemical behavior of SS. (a) Direct action via photosynthetic metabolic activity, influencing physico-chemical conditions of the SS/biofilm interface; (b) direct action via the effect of other diatom metabolic substances: production of reactive oxygen species (ROS) due to oxidative stress; (c) Indirect action by providing metabolic products, namely oxygen, to other microorganisms: potential metabolic interactions within the biofilms between diatom (phototrophic) and bacteria (heterotrophic).
Prospects
It is obvious that diatoms are important in the biofouling community that develops on SS and other metals and alloys in natural waters. While the effect of bacteria in potential ennoblement has been widely discussed in the literature, diatoms are usually neglected in investigations of the electrochemical processes that may lead to biocorrosion. In the present review, it has been shown that the role of diatoms in such processes cannot be excluded. The ways in which diatoms may be involved in the potential ennoblement of SS have been identified, thereby opening new possibilities to gain an understanding of the ability of diatoms to initiate an electrochemical effect on SS electrodes. Investigating diatoms at the nanoscale will provide unique insights into how diatoms ‘sense’ surfaces and how they are involved in cell–surface and cell–cell interactions. Biomimetic systems, based on the use of either cultured axenic diatoms or in the presence of both mixed consortia of diatoms and bacteria, should be used in electrochemical tests. Furthermore, experiments based on the combination of AFM and electrochemical tests may pave the way for new comprehensive approaches to understanding features regarding diatom–SS and diatom–bacteria interactions. The ability of diatoms to induce an electrochemical response when they are in close contact to an SS electrode may be exploited in many areas of research, especially in the design of new microbial fuel cells. Indeed, studies have reported the construction of microbial fuel cell prototypes based on the use of marine biofilms and SS electrodes as the anode or cathode (Bergel et al. Citation2005; Dumas et al. Citation2007; Dumas et al. Citation2008b).
References
- Abrahamsson , K , Choo , K-S , Pedersén , M , Johansson , G and Snoeijs , P . 2003 . Effects of temperature on the production of hydrogen peroxide and volatile halocarbons by brackish-water algae . Phytochemistry , 64 : 725 – 734 .
- Abu-Lail , N I and Camesano , T A . 2003 . Polysaccharide properties probed with atomic force microscopy . J Microsc-Oxford , 2 : 217 – 238 .
- Ahimou , F , Denis , F , Touhami , A and Dufrene , Y F . 2002 . Probing microbial cell surface charges by atomic force microscopy . Langmuir , 18 : 9937 – 9941 .
- Almqvist , N , Delamo , Y , Smith , B L , Thomson , N H , Bartholdson , A , Lal , R , Brzezinski , M and Hansma , P K . 2001 . Micromechanical and structural properties of a pennate diatom investigated by atomic force microscopy . J Microsc-Oxford , 202 : 518 – 532 .
- Alsteens , D , Dague , E , Rouxhet , P G , Baulard , A R and Dufrêne , Y F . 2007 . Direct measurement of hydrophobic forces on cell surfaces using AFM . Langmuir , 23 : 11977 – 11979 .
- Amaya , H and Miyuki , H . 1995 . Development of accelerated evaluation method for microbially influenced corrosion resistance of stainless steels . Corros Eng , 44 : 123 – 133 .
- Amaya , H and Miyuki , H . 1997 . Mechanism of microbially influenced corrosion on stainless steels in natural seawater and the effect of surface treatment on corrosion resistance . Corros Eng , 46 : 567 – 581 .
- Amaya , H and Miyuki , H . Laboratory reproduction of potential ennoblement of stainless steels in natural seawater. COROSION 99 . Paper No 168 . Houston, TX : NACE International . Houston (TX): NACE International
- Andrewartha , J , Perkins , K , Sargison , J , Osborn , J , Walker , G , Henderson , A and Hallegraeff , G . 2010 . Drag force and surface roughness measurements on freshwater biofouled surfaces . Biofouling , 26 : 487 – 496 .
- Arce , F T , Avci , R , Beech , I B , Cooksey , K E and Wigglesworth-Cooksey , B . 2004 . A live bioprobe for studying diatom-surface interactions . Biophys J , 87 : 4284 – 4297 .
- Baier , R E . 1980 . “ Substrata influences on adhesion microorganisms and their resultant new surface properties ” . In Adsorption of microorganisms to surfaces , Edited by: Bitton , K CM . 59 – 104 . New York : John Wiley .
- Bardal , E , Drugli , J M and Gartland , P O . 1993 . The behaviour of corrosion-resistant steels in seawater: a review . Corros Sci , 35 : 257 – 267 .
- Baroux , B , Béranger , G and Lemaître , C . 1990 . “ Passivité et rupture de la passivité des aciers inoxydables ” . In Les aciers inoxydables , Edited by: Lacombe , P , Baroux , B and Béranger , G . 161 – 181 . Les Ulis : Editions de Physiques .
- Beech , I B . 2004 . Corrosion of technical materials in the presence of biofilms-current understanding and state-of-the art methods of study . Int Biodeterior Biodegr , 53 : 177 – 183 .
- Beech , I B and Sunner , J . 2004 . Biocorrosion: towards understanding interactions between biofilms and metals . Curr Opin Biotechnol , 15 : 181 – 186 .
- Beech , I B , Sunner , J A and Hiraoka , K . 2005 . Microbe-surface interactions in biofouling and biocorrosion processes . Int Microbiol , 8 : 157 – 168 .
- Bennett , S M , Finlay , J A , Gunari , N , Wells , D D , Meyer , A E , Walker , G C , Callow , M E , Callow , J A , Bright , F V and Detty , M R . 2010 . The role of surface energy and water wettability in aminoalkyl/fluorocarbon/hydrocarbon-modified xerogel surfaces in the control of marine biofouling . Biofouling , 26 : 235 – 246 .
- Bergel , A , Féron , D and Mollica , A . 2005 . Catalysis of oxygen reduction in PEM fuel cell by seawater biofilm . Electrochem Comm , 7 : 900 – 904 .
- Berridge , M J , Bootman , M D and Lipp , P . 1998 . Calcium-a life or death signal . Nature , 395 : 645 – 648 .
- Bhosle , N B , Sankaran , P B and Wagh , A B . 1990 . Carbohydrate sources of microfouling material developed on aluminium and stainless steel panels . Biofouling , 2 : 151 – 164 .
- Bhosle , N B , Sawant , S S , Garg , A and Wagh , A B . 1995 . Isolation and partial chemical analysis of exopolysaccharides from the marine fouling diatom Navicula subinflata . Bot Mar , 38 : 103 – 110 .
- Bockris , J OM and Reddy , A KN . 1970 . “ Modern electrochemistry ” . In Volume 1: Inonics , 2053 pp New York : Plenum Press .
- Bohlander , G H . 1991 . Biofilm effects on drag: measurements on ships . Trans Inst Mar Eng , 103 : 135 – 138 .
- Boulangé-Petermann , L , Doren , A , Baroux , B and Bellon-Fontaine , M-N . 1995 . Zeta potential measurements on passive metals . J Colloid Interface Sci , 171 : 179 – 186 .
- Bowen , W R , Lovitt , R W and Wright , C J . 2001 . Atomic force microscopy study of the adhesion of Saccharomyces cerevisiae . J Colloid Interface Sci , 237 : 54 – 61 .
- Bowen , W R , Hilal , N , Lovitt , R W and Wright , C J . 1998 . Direct measurement of the force of adhesion of a single biological cell using an atomic force microscope . Colloid Surface A , 136 : 231 – 234 .
- Bowen , W R , Fenton , A S , Lovitt , R W and Wright , C J . 2002 . The measurement of Bacillus mycoides spore adhesion using atomic force microscopy, simple counting methods, and a spinning disk technique . Biotechnol Bioeng , 79 : 170 – 179 .
- Caillou , S , Gerin , P A , Nonckreman , C J , Fleith , S , Dupont-Gillain , C C , Landoulsi , J , Pancera , S M , Genet , M J and Rouxhet , P G . 2008 . Enzymes at solid surfaces: nature of the interfaces and physico-chemical processes . Electrochim Acta , 54 : 116 – 122 .
- Callow , M E . 1986 . Fouling of “in service” ships . Bot Mar , 29 : 351 – 357 .
- Callow , M E and Fletcher , R L . 1994 . The influence of low surface energy materials on bioadhesion – a review . Int Biodeterior Biodegr , 34 : 333 – 348 .
- Camesano , T A and Abu-Lail , N I . 2002 . Heterogeneity in bacterial surface polysaccharides, probed on a single-molecule basis . Biomacromolecules , 3 : 661 – 667 .
- Camesano , T A , Liu , Y and Datta , M . 2007 . Measuring bacterial adhesion at environmental interfaces with single-cell and single-molecule techniques . Adv Water Resour , 30 : 1470 – 1491 .
- Cassé , F , Stafslien , S J , Bahr , J A , Daniels , J , Finlay , J A , Callow , J A and Callow , M E . 2007 . Combinatorial research applied to the development of new surface coatings V. Application of spinning water-jet for semi-high through put assessment of the attachment strength of marine of marine fouling algae . Biofouling , 23 : 121 – 130 .
- Chansang , H and Cooksey , K E . 1977 . The glucose transport system of A. coffeaeformis (Bacillariophyceae) . J Phycol , 13 : 51 – 57 .
- Characklis , W G and Cooksey , K E . 1983 . Biofilms and microbial fouling . Adv Appl Microbiol , 29 : 93 – 138 .
- Characklis , W G and Marshall , K C . 1990 . Biofilms , 816 pp New York : Wiley-Interscience Publications .
- Chiovitti , A , Bacic , A , Burke , J and Wetherbee , R . 2003 . Heterogeneous xylose-rich glycans are associated with extracellular glycoproteins from the biofouling diatom Craspedostauros australis (Bacillariophyceae) . Eur J Phycol , 38 : 351 – 360 .
- Chongdar , S , Gunasekaran , G and Kumar , P . 2005 . Corrosion inhibition of mild steel by aerobic biofilm . Electrochim Acta , 50 : 4655 – 4665 .
- Christensen , B E and Characklis , W G . 1990 . “ Physical and chemical properties of biofilms ” . In Biofilms , Edited by: Characklis , W G and Marshall , K C . 93 – 130 . New York : Wiley-Interscience Publications .
- Collén , J , Ekdahl , A , Abrahamsson , K and Pedersén , M . 1994 . The involvement of hydrogen peroxide in the production of volatile halogenated compounds by Meristiella gelidium . Phytochemistry , 36 : 1197 – 1202 .
- Compère , C , Bellon-Fontaine , M-N , Bertrand , P , Costa , D , Marcus , P , Poleunis , C , Pradier , C-M , Rondot , B and Walls , W G . 2001 . Kinetics of conditioning layer formation on stainless steel immersed in seawater . Biofouling , 17 : 129 – 145 .
- Cooksey , K E . 1981 . Requirement for calcium in adhesion of a fouling diatom to glass . Appl Environ Microbiol , 4 : 1378 – 1382 .
- Cooksey , K E and Wigglesworth-Cooksey , B . 1980 . Calcium is necessary for motility in the diatom Amphora coffeaeformis . Plant Physiol , 65 : 129 – 131 .
- Cooksey , K E and Wigglesworth-Cooksey , B . 2001 . “ Diatoms in biofilms ” . In Encyclopedia of environmental microbiology , Edited by: Flemming , H-C . 1052 – 1063 . New York : Wiley .
- Cooksey , K E , Wiggleworth-Cooksey , B and Long , R . 2009 . “ A strategy to pursue in selecting a natural antifoulant: a perspective ” . In Marine and industrial biofouling , Edited by: Flemming , H-C , Murthy , P S , Venkatesan , R and Cooksey , K E . 165 – 177 . Berlin, Germany : Springer .
- Cooksey , K , Cooksey , B , Miller , C A and Paul , J H . 1980 . “ Attachment of diatoms to surfaces: field and laboratory studies ” . In Microbial adhesion to surfaces , Edited by: Lynch , J M , Melling , J , Rutter , P R and Vincent , B . 526 – 528 . Chichester, UK : Ellis Horwood .
- Costerton , J W , Stewart , P S and Greenberg , E P . 1999 . Bacterial biofilms: a common cause of persistent infections . Science , 284 : 1318 – 1322 .
- Costerton , J W , Lewandowski , Z , Debeer , D , Caldwell , D E , Korber , D R and James , G . 1994 . Biofilms, the customised microniche . J Bacteriol , 176 : 2137 – 2142 .
- Crawford , S A , Higgins , M J , Mulvaney , P and Wetherbee , R . 2001 . Nanostructure of the diatom frustule as revealed by atomic force and scanning electron microscopy . J Phycol , 37 : 543 – 554 .
- Dague , E , Doan , T L , Zanna , S , Marcus , P , Loubiere , P and Mercier-Bonin , M . 2010 . Probing in vitro interactions between Lactococcus lactis and mucins using AFM . Langmuir , 26 : 11010 – 11017 .
- Dague , E , Alsteens , D , Latge , J P , Verbelen , C , Raze , D , Baulard , A R and Dufrêne , Y F . 2007 . Chemical force microscopy of single live cells . Nano Lett , 7 : 3026 – 3030 .
- De Brouwer , J , Stal , L , Avci , R , Staal , M and Cooksey , K E . 2006 . Time of flight – secondary ion mass spectrometry on isolated extracellular fractions and intact biofilms of three species of benthic diatoms . J Microbiol Methods , 65 : 562 – 572 .
- De La Rosa , C and Yu , T . 2005 . Three-dimensional mapping of oxygen distribution in wastewater biofilms using an automation system and microelectrodes . Environ Sci Technol , 39 : 5196 – 5202 .
- De Messano , L VR , Sathler , L , Reznik , L Y and Coutinho , R . 2009 . The effect of biofouling on localized corrosion of the stainless steels N08904 and UNS S32760 . Int Biodeterior Biodegr , 63 : 607 – 614 .
- De Stefano , M , Kooistra , W and Marino , D . 2003 . Morphology of the diatom genus Campyloneis(Bacillariophyceae, Bacillariophyta), with a description of Campyloneis juliae sp. nov. and an evaluation of the function of the valvocopulae . J Phycol , 39 : 735 – 753 .
- Dexter , S C and Zhang , H J . 1991 . Effect of biofilms, sunlight and salinities on corrosion potential and corrosion initiation of stainless alloys , Electric Power Research Institute : Palo Alto (CA) . EPRI NP-7275
- Dickinson , W H and Lewandowski , Z . 1996 . Manganese biofouling and the corrosion behavior of stainless steel . Biofouling , 10 : 79 – 93 .
- Dickinson , W H , Caccavo , F and Lewandowski , Z . 1996a . The ennoblement of stainless steel by manganic oxide biofouling . Corros Sci , 38 : 1407 – 1422 .
- Dickinson , W H , Lewandowski , Z and Geer , R D . 1996b . Evidence for surface changes during ennoblement of type 316L stainless steel: dissolved oxidant and capacitance measurements . Corrosion , 52 : 910 – 920 .
- Dobretsov , S and Thomason , J C . 2011 . The development of marine biofilms on two commercial non-biocidal coatings: a comparison between silicone and fluoropolymer technologies . Biofouling , 27 : 869 – 880 .
- Dobretsov , S , Teplitski , M , Bayer , M , Gunasekera , S , Proksch , P and Paul , V J . 2011 . Inhibition of marine biofouling by bacterial quorum sensing inhibitors . Biofouling , 27 : 893 – 905 .
- Dufrêne , Y F . 2000 . Direct characterization of the physicochemical properties of fungal spores using functionalized AFM probes . Biophys J , 78 : 3286 – 3291 .
- Dufrêne , Y F . 2008 . Towards nanomicrobiology using atomic force microscopy . Nature Rev Microbiol , 6 : 674 – 680 .
- Dugdale , T M , Willis , A and Wetherbee , R . 2006a . Adhesive modular proteins occur in the extracellular mucilage of the motile, pennate diatom Phaeodactylum tricornutum . Biophys J , 90 : L58 – L60 .
- Dugdale , T M , Dagastine , R , Chiovitti , A and Wetherbee , R . 2006b . Diatom adhesive mucilage contains distinct supramolecular assemblies of a single modular protein . Biophys J , 90 : 2987 – 2993 .
- Dugdale , T M , Dagastine , R , Chiovitti , A , Mulvaney , P and Wetherbee , R . 2005 . Single adhesive nanofibers from a live diatom have the signature fingerprint of modular proteins . Biophys J , 89 : 4252 – 4260 .
- Dumas , C , Basseguy , R and Bergel , A . 2008a . Electrochemical activity of Geobacter sulfurreducens biofilms on stainless steel anodes . Electrochim Acta , 53 : 5235 – 5241 .
- Dumas , C , Mollica , A , Féron , D , Basséguy , R , Etcheverry , L and Bergel , A . 2007 . Marine microbial fuel cell: use of stainless steel electrodes as anode and cathode materials . Electrochim Acta , 53 : 468 – 473 .
- Dumas , C , Mollica , A , Féron , D , Basseguy , R , Etcheverry , L and Bergel , A . 2008b . Checking graphite and stainless anodes with an experimental model of marine microbial fuel cell . Bioresource Technol , 99 : 8887 – 8894 .
- Dupont , I , Féron , D and Novel , G . 1998 . Effect of glucose oxidase activity on corrosion potential of stainless steels in seawater . Int Biodeterior Biodegr , 41 : 13 – 18 .
- Dupres , V , Alsteens , D , Andre , G and Dufrêne , Y F . 2010 . Microbial nanoscopy: a closer look at microbial cell surfaces . Trends Microbiol , 18 : 397 – 405 .
- Dupres , V , Menozzi , F D , Locht , C , Clare , B H , Abbott , N L , Cuenot , S , Bompard , C , Raze , D and Dufrêne , Y F . 2005 . Nanoscale mapping and functional analysis of individual adhesins on living bacteria . Nature Meth , 2 : 515 – 520 .
- Eashwar , M and Maruthamuthu , S . 1995 . Mechanism of biologically produced ennoblement: ecological perspectives and a hypothetical model . Biofouling , 8 : 203 – 213 .
- Eashwar , M , Subramanian , G , Palanichamy , S and Rajagopal , G . 2011 . The influence of sunlight on the localized corrosion of UNS S31600 in natural seawater . Biofouling , 27 : 837 – 849 .
- Eashwar , M , Subramanian , G , Palanichamy , S , Rajagopal , G , Madhu , S and Kamaraj , P . 2009 . Cathodic behaviour of stainless steel in coastal Indian seawater: calcareous deposits overwhelm biofilms . Biofouling , 25 : 191 – 201 .
- Falciatore , A , D'alcala , M R , Groot , P and Bowler , C . 2000 . Perception of environmental signals by a marine diatom . Science , 288 : 2363 – 2366 .
- Finlay , J A , Bennett , S M , Brewer , L H , Sokolova , A , Clay , G , Gunari , N , Meyer , A E , Walker , G C , Wendt , D E Callow , M E . 2010 . Barnacle settlement and adhesion of protein and diatom microfouling to xerogel films with varying wettability . Biofouling , 26 : 657 – 666 .
- Flemming , H-C and Geesey , G G . 1991 . Biofouling and biocorrosion in industrial water systems , 220 pp Heidelberg : Springer .
- Flemming , H-C , Murthy , P S , Venkatesan , R and Cooksey , K E . 2009 . Marine and industrial biofouling , Vol. 4 , Springer Series on Biofilms Vol. 4. Berlin (Germany): Springer. 334 pp .
- Florence , T M and Stauber , J L . 1986 . Toxicity of copper complexes to the marine diatom Nitzschia closterium . Aquat Toxicol , 8 : 11 – 26 .
- Francius , G , Tesson , B , Dague , E , Martin-Jézéquel , V and Dufrêne , Y F . 2008a . Nanostructure and nanomechanics of live Phaeodactylum tricornutum morphotypes . Environ Microbiol , 10 : 1344 – 1356 .
- Francius , G , Alsteens , D , Dupres , V , Lebeer , S , De Keersmaecker , S , Vanderleyden , J , Gruber , H J and Dufrene , Y F . 2009 . Stretching polysaccharides on live cells using single molecule force spectroscopy . Nature Prot , 4 : 939 – 946 .
- Francius , G , Lebeer , S , Alsteens , D , Wildling , L , Gruber , H J , Hols , P , De Keersmaecker , S , Vanderleyden , J and Dufrêne , Y F . 2008b . Detection, localization and conformational analysis of single polysaccharide molecules on live bacteria . ACS Nano , 2 : 1921 – 1929 .
- Ganesh , A B and Radhakrishnan , T K . 2007 . Fiber-optic sensors for the estimation of pH within natural biofilms on metals . Sensor Actuat B-Chem , 123 : 1107 – 1112 .
- Gebeshuber , I C , Kindt , J H , Thompson , J B , Del Amo , Y , Stachelberger , H , Brzezinski , M A , Stucky , G D , Morse , D E and Hansma , P K . 2003 . Atomic force microscopy study of living diatoms in ambient conditions . J Microsc-Oxford , 212 : 292 – 299 .
- Geesey , G G , Lewandowski , Z and Flemming , H-C . 1994 . Biofouling and biocorrosion in industrial water systems , 297 pp Chelsea, Michigan : Lewis Publishers .
- Groger , C , Sumper , M and Brunner , E . 2008 . Silicon uptake and metabolism of the marine diatom Thalassiosira pseudonana: solid-state Si–29 NMR and fluorescence microscopic studies . J Struct Biol , 161 : 55 – 63 .
- Grossmann , K , Arnold , T , Krawczyk-Bärsch , E , Diessner , S , Wobus , A , Bernhard , G and Krawietz , R . 2007 . Identification of fluorescent U(V) and U(VI) microparticles in a multispecies biofilm by confocal laser scanning microscopy and fluorescence spectroscopy . Environ Sci Technol , 41 : 6498 – 6504 .
- Hamilton , W A . 1985 . Sulphate-reducing bacteria and anaerobic corrosion . Annu Rev Microbiol , 39 : 195 – 217 .
- Hanawa , T . 2002 . “ Metallic biomaterials ” . In Recent research and developments in biomaterials , Edited by: Ikada , Y . 11 – 31 . Kerala, India : Research Signpost .
- Hecky , R E , Mopper , K , Kilham , P and Degens , E T . 1973 . The amino acid and sugar composition of diatom cell-walls . Mar Biol , 19 : 323 – 331 .
- Higgins , M J , Crawford , S A , Mulvaney , P and Wetherbee , R . 2002 . Characterization of the adhesive mucilages secreted by live diatom cells using atomic force microscopy . Protist , 153 : 25 – 38 .
- Higgins , M J , Molino , P , Mulvaney , P and Wetherbee , R . 2003a . The structure and nanomechanical properties of the adhesive mucilage that mediates diatom-substratum adhesion and motility . J Phycol , 39 : 1181 – 1193 .
- Higgins , M J , Sader , J E , Mulvaney , P and Wetherbee , R . 2003b . Probing the surface of living diatoms with atomic force microscopy: the nanostructure and nanomechanical properties of the mucilage layer . J Phycol , 39 : 722 – 734 .
- Hildebrand , M , Doktycz , M J and Allison , D P . 2008 . Application of AFM in understanding biomineral formation in diatoms . Pflug Arch Eur J Physiol , 456 : 127 – 137 .
- Hildebrand , M , Holton , G , Joy , D C , Doktycz , M J and Allison , D P . 2009 . Diverse and conserved nano- and mesoscale structures of diatom silica revealed by atomic force microscopy . J Microsc-Oxford , 235 : 172 – 187 .
- Hinterdorfer , P and Dufrene , Y F . 2006 . Detection and localization of single molecular recognition events using atomic force microscopy . Nature Meth , 3 : 347 – 355 .
- Hoagland , K D , Rosowski , J R , Gretz , M R and Roemer , S C . 1993 . Diatom extracellular polymeric substances – function, fine structure, chemistry and physiology . J Phycol , 29 : 537 – 566 .
- Hu , Y , Ulstrup , J , Zhang , J , Molin , S and Dupres , V . 2011 . Adhesive properties of Staphylococcus epidermidis probed by atomic force microscopy . Phys Chem Chem Phys , 13 : 9995 – 10003 .
- Hu , Z , Jin , J , Abruna , H D , Houston , P L , Hay , A G , Ghiorse , W C , Shuler , M L , Hidalgo , G and Lion , L W . 2007 . Spatial distributions of copper in microbial biofilms by scanning electrochemical microscopy . Environ Sci Technol , 41 : 936 – 941 .
- Ianora , A , Miralto , A , Poulet , S , Carotenuto , Y and Buttino , I . 2004 . Aldehyde suppression of copepod recruitment in blooms of a ubiquitous planktonic diatom . Nature , 429 : 403 – 407 .
- Ianora , A , Boersma , M , Casotti , R , Fontana , A , Harder , J , Hoofman , F , Pavia , H , Potin , P , Poulet , S A and Toth , G . 2006 . New trends in marine chemical ecology . Estuar Coast , 29 : 531 – 551 .
- Ishihara , Y and Tsujikawa , S . 1998 . Effect of diatoms on ennoblement of electrode potential for stainless steels in natural sea water . Zairyo-to-Kankyo , 47 : 260 – 266 .
- Ishihara , Y and Tsujikawa , S . 1999 . Effect of bacteria combined with diatom on ennoblement of electrode potential for stainless steels in natural sea water . Zairyo-to-Kankyo , 48 : 520 – 527 .
- Ismail , K M , Jayaraman , A , Wood , T K and Earthman , J C . 1999 . The influence of bacteria on the passive film stability of 304 stainless steel . Electrochim Acta , 44 : 4685 – 4692 .
- Jain , A and Bhosle , N B . 2009 . Biochemical composition of the marine conditioning film: implications for bacterial adhesion . Biofouling , 25 : 13 – 19 .
- Jenkinson , H F and Lappin-Scott , H M . 2001 . Biofilms adhere to stay . Trends Microbiol , 9 : 9 – 10 .
- Jullien , C , Bénézech , T , Carpentier , B , Lebret , V and Faille , C . 2003 . Identification of surface characteristics relevant to the hygienic status of stainless steel for the food industry . J Food Eng , 56 : 77 – 87 .
- Kammer , M , Hedrich , R , Ehrlich , H , Popp , J , Brunner , E and Krafft , C . 2010 . Spatially resolved determination of the structure and composition of diatom cell walls by Raman and FTIR imaging . Anal Bioanal Chem , 398 : 509 – 517 .
- Kiefer , E , Sigg , L and Schosseler , P . 1997 . Chemical and spectroscopic characterization of algae surfaces . Environ Sci Technol , 31 : 759 – 764 .
- Kinloch , A J . 1990 . Adhesion and adhesive: science and technology , 460 pp London, UK : Chapman & Hall .
- Kruszewski , K M and Gawalt , E S . 2011 . Perfluorocarbon thin films and polymer brushes on stainless steel 316 L for the control of interfacial properties . Langmuir , 27 : 8120 – 8125 .
- Landoulsi , J , El Kirat , K , Richard , C , Féron , D and Pulvin , S . 2008a . Enzymatic approach in microbial-influenced corrosion: a review based on stainless steels in natural waters . Environ Sci Technol , 42 : 2233 – 2242 .
- Landoulsi , J , Genet , M J , Richard , C , El Kirat , K , Pulvin , S and Rouxhet , P G . 2008b . Evolution of the passive film and organic constituents at the surface of stainless steel immersed in fresh water . J Colloid Interface Sci , 318 : 278 – 289 .
- Landoulsi , J , Genet , M J , Richard , C , El Kirat , K , Rouxhet , P G and Pulvin , S . 2008c . Ennoblement of stainless steel in the presence of glucose oxidase: nature and role of interfacial processes . J Colloid Interface Sci , 320 : 508 – 519 .
- Landoulsi , J , El Kirat , K , Richard , C , Sabot , R , Jeannin , M and Pulvin , S . 2008d . Glucose oxidase immobilization on stainless steel to mimic the aerobic activities of natural biofilms . Electrochim Acta , 54 : 133 – 139 .
- Landoulsi , J , Genet , M J , El Kirat , K , Richard , C , Pulvin , S and Rouxhet , P G . 2011 . “ Silanization with APTES for controlling the interactions between stainless steel and biocomponents: reality vs expectation ” . In Biomaterials – physics and chemistry , Edited by: Pignatello , R . 978-953-307-418-4 Croatia : In Tech .
- Landoulsi , J , Dagbert , C , Richard , C , Sabot , R , Jeannin , M , El Kirat , K and Pulvin , S . 2009 . Enzyme-induced ennoblement of AISI 316L stainless steel: focus on pitting corrosion behavior . Electrochim Acta , 54 : 7401 – 7406 .
- Lappin-Scott , H M and Costerton , J W . 1995 . “ Introduction to microbial biofilms ” . In Microbial biofilms , Edited by: Lappin-Scott , H M and Costerton , J W . 1–11 Cambridge, UK : Cambridge University Press .
- Le Bozec , N , Compère , C , L'her , M , Laouenan , A , Costa , D and Marcus , P . 2001 . Influence of stainless steel surface treatment on the oxygen reduction reaction in seawater . Corros Sci , 43 : 765 – 786 .
- Lefèvre , G DM , Fédoroff , M and Johannes , L . 2006 . “ Accuracy in the determination of acid-base properties of metal oxides surfaces ” . In Surface complexation modelling , Edited by: Lützenkirchen , J . 35 – 66 . Amsterdam (The Netherlands) : Academic Press .
- Liao , J , Fukui , H , Urakami , T and Morisaki , H . 2010 . Effect of biofilm on ennoblement and localized corrosion of stainless steel in fresh dam-water . Corros Sci , 52 : 1393 – 1403 .
- Linder , A , Colchero , J , Apell , H J , Marti , O and Mlynek , J . 1992 . Scanning force microscopy of diatom shells . Ultramicroscopy , 42 : 329 – 332 .
- Little , B and Jacobus , J . 1984 . A comparison of two techniques for the isolation of adsorbed dissolved organic material from seawater . Org Geochem , 5 : 1 – 6 .
- Little , B , Lee , J S and Ray , R I . 2008 . The influence of marine biofilms on corrosion: a concise review . Electrochim Acta , 54 : 1 – 7 .
- Little , B , Ray , R , Wagner , P , Lewandowski , Z , Lee , W C , Characklis , W G and Mansfeld , F . 1991 . Impact of biofouling on the electrochemical behavior of 304 stainless steel in natural seawater . Biofouling , 3 : 45 – 59 .
- Loeb , G I and Neihof , R A . 1975 . “ Marine conditioning films ” . In Applied chemistry at protein interfaces , Edited by: Baier , R E . Vol. 145 , 319 – 335 . Washington (DC) : American Chemical Society .
- Losic , D , Pillar , R J , Dilger , T , Mitchell , J G and Voelcker , N H . 2007a . Atomic force microscopy (AFM) characterization of the porous silica nanostructure of two centric diatoms . J Porous Mater , 14 : 61 – 69 .
- Losic , D , Short , K , Mitchell , J G , Lal , R and Voelcker , N H . 2007b . AFM nanoindentations of diatom biosilica surfaces . Langmuir , 23 : 5014 – 5021 .
- Mahapatro , A , Johnson , D M , Patel , D N , Feldman , M D , Ayon , A A and Agrawal , C M . 2006 . Surface modification of functional self-assembled monolayers on 316L stainless steel via lipase catalysis . Langmuir , 22 : 901 – 905 .
- Majumbar , P , Lee , E , Patel , N , Ward , K , Staslien , S , Daniels , J , Chisholm , B , Boudjouk , P , Callow , M E Callow , J A . 2008 . Combinatorial research applied to the development of new surface coatings IX: an investigation of novel antifouling/fouling release coatings containing quaternary ammonium salt groups . Biofouling , 24 : 185 – 200 .
- Mansfeld , F . 2007 . The interaction of bacteria and metal surfaces . Electrochim Acta , 52 : 7670 – 7680 .
- Mansfeld , F , Liu , G , Xiao , H , Tsai , C H and Little , B J . 1994 . The corrosion behavior of copper alloys, stainless steels and titanium in seawater . Corros Sci , 36 : 2063 – 2095 .
- Mantel , M , Rabinovich , Y I , Wightman , J P and Yoon , R H . 1995 . A study of hydrophobic interactions between stainless steel and silanated glass surface using atomic force microscopy . J Colloid Interface Sci , 170 : 203 – 214 .
- Marconnet , C , Dagbert , C , Roy , M and Féron , D . 2008 . Stainless steel ennoblement in freshwater: from exposure tests to mechanisms . Corros Sci , 50 : 2342 – 2352 .
- Martinelli , E , Suffredini , M , Galli , G , Glisenti , A , Pettitt , M E , Callow , M E , Callow , J A , Williams , D and Lyall , G . 2011 . Amphiphilic block copolymer/poly(dimethylsiloxane) (PDMS) blends and nanocomposites for improved fouling-release . Biofouling , 27 : 529 – 541 .
- Maruthamuthu , S , Eashwar , M , Raja , S S and Balakrishnan , K . 1993 . Effects of microfouling and light/dark regimes on the corrosion potentials of two stainless alloys in seawater . Biofouling , 7 : 257 – 265 .
- Mattila , M , Carpen , L , Hakkarainen , T and Salkinoja-Salonen , M S . 1997 . Biofilm development during ennoblement of stainless steel in Baltic Sea water: a microscopic study . Int Biodeterior Biodegr , 40 : 1 – 10 .
- Mitbavkar , S and Anil , A C . 2000 . Diatom colonization on stainless steel panels in estuarine waters of Goa, west coast of India . Indian J Mar Sci , 29 : 273 – 276 .
- Mitbavkar , S and Anil , A C . 2008 . Seasonal variations in the fouling diatom community structure from a monsoon influenced tropical estuary . Biofouling , 24 : 415 – 426 .
- Molino , P J and Wetherbee , R . 2008 . The biology of fouling diatoms and their role in the development of microbial slimes . Biofouling , 24 : 365 – 379 .
- Molino , P J , Hodson , O M , Quinn , J F and Wetherbee , R . 2006 . Utilizing QCM-D to characterize the adhesive mucilage secreted by two marine diatom species in-situ and in real-time . Biomacromolecules , 7 : 3276 – 3282 .
- Molino , P J , Hodson , O M , Quinn , J F and Wetherbee , R . 2008 . The quartz crystal microbalance: a new tool for the investigation of the bioadhesion of diatoms to surfaces of differing surface energies . Langmuir , 24 : 6730 – 6737 .
- Molino , P J , Childs , S , Eason Hubbard , M R , Carey , J M , Burgman , M A and Wetherbee , R . 2009 . Development of the primary bacterial microfouling layer on antifouling and fouling release coatings in temperate and tropical environments in Eastern Australia . Biofouling , 25 : 149 – 162 .
- Motoda , S , Suzuki , Y , Shinohara , T and Tsujikawa , S . 1990 . The effect of marine fouling on the ennoblement of electrode potential for stainless steels . Corros Sci , 31 : 515 – 520 .
- Muller , D J and Dufrene , Y F . 2008 . Atomic force microscopy as a multifunctional molecular toolbox in nanobiotechnology . Nature Nanotech , 3 : 261 – 269 .
- Murray , R E , Priscu , J C and Cooksey , K E . 1986 . Stimulation of bacterial DNA-synthesis by algal exudates in an attached algal-bacterial consortium . Appl Environ Microbiol , 53 : 1177 – 1182 .
- Olsson , C OA and Landolt , D . 2003 . Passive films on stainless steels-chemistry, structure and growth . Electrochim Acta , 48 : 1093 – 1104 .
- Parot , S , Vandecandelaere , I , Cournet , A , Délia , ML , Vandamme , P , Bergé , M , Roques , C and Bergel , A . 2011 . Catalysis of the electrochemical reduction of oxygen by bacteria isolated from electro-active biofilms formed in seawater . Bioresource Technol , 102 : 304 – 311 .
- Patil , J S and Jagadeesan , V . 2011 . Effect of chlorination on the development of marine biofilms dominated by diatoms . Biofouling , 27 : 241 – 254 .
- Percival , S L , Knapp , J S , Edyvean , R GJ and Wales , D S . 1998a . Biofilm development on stainless steel in mains water . Water Res , 32 : 243 – 253 .
- Percival , S L , Knapp , J S , Edyvean , R GJ and Wales , D S . 1998b . Biofilms, mains water and stainless steel . Water Res , 32 : 2187 – 2201 .
- Pourbaix , M . 1963 . Atlas d'Equilibres Electrochimiques , 644 pp Paris, France : Gauthier-Villars .
- Raman , A and Gawalt , E S . 2007 . Self-assembled monolayers of alkanoic acids on the native oxide surface of SS316L by solution deposition . Langmuir , 23 : 2284 – 2288 .
- Raman , A , Quiñones , R , Barriger , L , Eastman , R , Parsi , A and Gawalt , E S . 2010 . Understanding organic film behavior on alloy and metal oxides . Langmuir , 26 : 1747 – 1754 .
- Rao , T S , Rani , P G , Venugopalan , V P and Nair , K VK . 1997 . Biofilm formation in a freshwater environment under photic and aphotic conditions . Biofouling , 11 : 265 – 282 .
- Ratner , BD , Hoffman , AS , Schoen , FJ and Lemons , JE . 2004 . Biomaterials science: an introduction to materials in medicine , 2nd ed. San Diego (CA) : Academic Press. 864 pp .
- Sato , N . 1990 . An overview on the passivity of metals . Corros Sci , 31 : 1 – 19 .
- Schneider , R , Chadwick , Br , Jankowski , J and Acworth , I . 1997 . Determination of physicochemical parameters of solids covered with conditioning films from groundwaters using contact angles. Comparative analysis of different thermodynamic approaches utilizing a range of diagnostic liquids . Colloids Surf A , 126 : 1 – 23 .
- Schneider , R P . 1996 . Conditioning film-induced modification of substratum physicochemistry-analysis by contact angles . J Colloid Interface Sci , 182 : 204 – 213 .
- Schultz , M P , Bendick , J A , Holm , E R and Hertel , W M . 2011 . Economic impact of biofouling on a naval surface ship . Biofouling , 27 : 87 – 98 .
- Scotto , V and Lai , M E . 1998 . The ennoblement of stainless steels in seawater: a likely explanation coming from the field . Corros Sci , 40 : 1007 – 1018 .
- Scotto , V , Cintio , R D and Marcenaro , G . 1985 . The influence of marine aerobic microbial film on stainless steel corrosion behaviour . Corros Sci , 25 : 185 – 194 .
- Scotto , V , Albiso , G and Marcenaro , G . 1986 . An example of microbiologically influenced corrosion: the behaviour of stainless steel in natural seawater . Bioelectrochem Bioenerget , 16 : 347 – 355 .
- Sekar , R , Nandakumar , K , Venugopalan , V P , Nair , K VK and Rao , V NR . 1998 . Spatial variation in microalgal colonization on hard surfaces in a lentic freshwater environment . Biofouling , 13 : 177 – 195 .
- Sheng , X , Ting , Y P and Pehkonen , S O . 2007 . Force measurements of bacterial adhesion on metals using a cell probe atomic force microscope . J Colloid Interface Sci , 310 : 661 – 669 .
- Sheng , X , Ting , Y P and Pehkonen , S O . 2008 . The influence of ionic strength, nutrients and pH on bacterial adhesion to metals. J Colloid Interface Sci 321:256–264
- Shi , X , Avci , R and Lewandowski , Z . 2002 . Electrochemistry of passive metals modified by manganese oxides deposited by Leptothrix discophora: two-step model verified by ToF-SIMS . Corros Sci , 44 : 1027 – 1045 .
- Shustak , G , Domb , AJ and Mandler , D . 2004 . Preparation and characterization of n-alkanoic acid self-assembled monolayers adsorbed on 316L stainless steel . Langmuir , 20 : 7499 – 7506 .
- Sommer , S , Ekin , A , Webster , D , Stafslien , S J , Callow , M E and Callow , J A . 2010 . A preliminary study on the properties of fouling – release performance of siloxane-polyurethane coatings prepared from poly(dimethylsiloxane) (PDMS) macromers . Biofouling , 26 : 961 – 972 .
- Staats , N , De Winder , B , Stal , L J and Mur , L R . 1999 . Isolation and characterization of extracellular polysaccharides from the epipelic diatoms Cylindrotheca closterium and Navicula salinarum . Eur J Phycol , 34 : 161 – 169 .
- Stadler , R , Fuerbeth , W , Harneit , K , Grooters , M , Woellbrink , M and Sand , W . 2008 . First evaluation of the applicability of microbial extracellular polymeric substances for corrosion protection of metal substrates . Electrochim Acta , 54 : 91 – 99 .
- Stauber , J L and Florence , T M . 1985 . Interactions of copper and manganese: a mechanism by which manganese alleviates copper toxicity to the marine diatom, Nitzschia closterium (Ehrenberg) W. Smith . Aquat Toxicol , 7 : 241 – 254 .
- Stoodley , P , Debeer , D and Lewandowski , Z . 1994 . Liquid flow in biofilm systems . Appl Environ Microbiol , 60 : 2711 – 2716 .
- Strathmann , M , Wingender , J and Flemming , H-C . 2002 . Application of fluorescently labelled lectins for the visualization and biochemical characterization of polysaccharides in biofilms of Pseudomonas aeruginosa . J Microbiol Methods , 50 : 237 – 248 .
- Sundaram , H S , Cho , Y , Dimitriou , M D , Weinman , C J , Finlay , J A , Cone , G , Callow , M E , Callow , J A , Kramer , E J and Ober , C K . 2011 . Fluorine-free mixed amphiphilic polymers based on PDMS and PEG side chains for fouling release applications . Biofouling , 27 : 589 – 602 .
- Sundström , J , Collén , J , Abrahamsson , K and Pedersén , M . 1996 . Halocarbon production and in vivo brominating activity of Eucheuma denticulatum . Phytochemistry , 42 : 1527 – 1530 .
- Sutherland , I W . 2001 . The biofilm matrix – an immobilized but dynamic microbial environment . Trends Microbiol , 9 : 222 – 227 .
- Taylor , G T , Zheng , D , Lee , M , Troy , P J , Gyananath , G and Sharma , S K . 1997 . Influence of surface properties on accumulation of conditioning films and marine bacteria on substrata exposed to oligotrophic waters . Biofouling , 11 : 31 – 57 .
- Tesson , B , Genet , M J , Fernandez , V , Degand , S , Rouxhet , P G and Martin-Jezequel , V . 2009 . Surface chemical composition of diatoms . Chembiochem , 10 : 2011 – 2024 .
- Thompson , E MS , Taylor , A R , Brownlee , C , Callow , M E and Callow , J A . 2008 . The role of nitric oxide in diatom adhesion in relation to substratum properties . J Phycol , 44 : 967 – 976 .
- Videla , H A . 1994 . Biofilms and corrosion interactions on stainless steel in seawater . Int Biodeterior Biodegr , 34 : 245 – 257 .
- Videla , H A and Herrera , L K . 2009 . Understanding microbial inhibition of corrosion. A comprehensive overview . Int Biodeterior Biodegr , 63 : 896 – 900 .
- Vrieling , E G , Beelen , T PM , Van Santen , RA and Gieskes , W WC . 1999 . Diatom silicon biomineralization as an inspirational source of new approaches to silica production . J Biotechnol , 70 : 39 – 51 .
- Wang , W , Zhang , X and Wang , J . 2009 . The influence of local glucose oxidase activity on the potential/current distribution on stainless steel: a study by the wire beam electrode method . Electrochim Acta , 54 : 5598 – 5604 .
- Wargenau , A and Kwade , A . 2010 . Determination of adhesion between single Aspergillus niger spores in aqueous solutions using an atomic force microscope . Langmuir , 26 : 11071 – 11076 .
- Washizu , N , Katada , Y and Kodama , T . 2004 . Role of H2O2 in microbially influenced ennoblement of open circuit potentials for type 316L stainless steel in seawater . Corros Sci , 46 : 1291 – 1300 .
- Werner , D . 1977 . The biology of diatoms. Botanical monographs , 498 pp Berkeley, CA : University of California Press .
- Wetherbee , R , Lind , J L , Burke , J and Quatrano , R S . 1998 . Mini review: The first kiss: establishment and control of initial adhesion by raphid diatoms . J Phycol , 34 : 9 – 15 .
- Wever , R , Tromp , M GM , Krenn , B E , Marjani , A and Van Tol , M . 1991 . Brominating activity of the seaweed Ascophyllum nodosum: impact on the biosphere . Environ Sci Technol , 25 : 446 – 449 .
- Whitehead , K A , Benson , P S and Verran , J . 2011 . The detection of food soils on stainless steel using energy dispersive X-ray and Fourier transform infrared spectroscopy . Biofouling , 27 : 907 – 917 .
- Wigglesworth-Cooksey , B and Cooksey , K E . 1992 . Can diatoms sense surfaces?: State of our knowledge . Biofouling , 5 : 227 – 238 .
- Wigglesworth-Cooksey , B and Cooksey , K E . 2005 . Use of fluorophore-conjugated lectins to study cell-cell interactions in model marine biofilms . Appl Environ Microbiol , 71 : 428 – 435 .
- Wigglesworth-Cooksey , B , Cooksey , K E and Long , R . 1999 . Antibiotic from the marine environment with antimicrobial fouling activity . Environ Toxicol , 22 : 275 – 280 .
- Wigglesworth-Cooksey , B , Berglund , D and Cooksey , K E . 2001 . Cell-cell and cell- surface interactions in an illuminated biofilm: implications for marine sediment stabilization . Geochem Trans , 10 : 75 – 81 .
- Xu , K , Dexter , S C and Luther , G W . 1998 . Voltametric microelectrodes for biocorrosion studies . Corrosion , 54 : 814 – 823 .
- Yuan , S J and Pehkonen , S O . 2007 . Microbiologically influenced corrosion of 304 stainless steel by aerobic PseudomonasNCIMB 2021 bacteria: AFM and XPS study . Colloids Surf B: Biointerfaces , 59 : 87 – 99 .
- Zargiel , K A , Coogan , J S and Swain , G W . 2011 . Diatom community structure on commercially available ship hull coatings . Biofouling , 27 : 955 – 965 .