Abstract
Despite obvious differences in morphology, substratum chemistry and the electrolyte in which they form, accumulations of iron corrosion products have the following characteristics in common: stratification of iron oxides/hydroxides with a preponderance of α-FeOOH (goethite) and accumulation of metals. Bacteria, particularly iron-oxidizing and sulfate-reducing bacteria have been identified in some accumulations. Both biotic and abiotic mechanisms have been used to rationalize observations for particular sets of environmental data. This review is the first to compare observations and interpretations.
Keywords:
Introduction
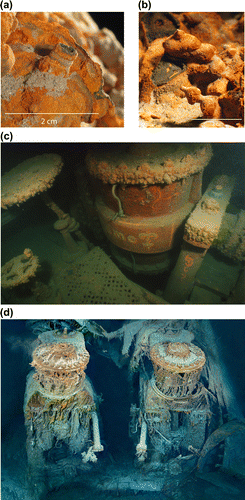
Accumulations of oxidized iron, especially iron corrosion products, are typically referred to by shape, eg tubercles (mounds), whiskers, chimneys and rusticles, and combinations of shapes, eg tubercles with chimneys (Figure a–d). Tubercle formation has been reported for austenitic chromium–nickel alloys, eg 304 and 316 stainless steels (Kobrin Citation1976), carbon steel (Herro Citation1998; Ray et al. Citation2009, 2010) and cast iron (Sarin, Snoeyink, Bebee et al. Citation2004; Sarin, Snoeyink, Lytle et al. Citation2004; Gerke et al. Citation2008, 2010) in chloride-containing waters, freshwater and treated drinking waters. Ballard (Citation1987) observed rust-colored, icicle-like formations on the wreck of the RMS Titanic in the North Atlantic Ocean and coined the term rusticle (Figure d). Since that observation, rusticles have been reported on shipwrecks and other iron substrata in seawater environments (Herdendorf et al. Citation1995; Cook & Peterson Citation2005; Cullimore & Johnston Citation2008).
Figure 1. Examples of iron corrosion product morphologies. Images of (a) chimney and (b) mound shapes from drinking water distribution systems (Gerke et al. (Citation2012); reprinted with the permission of NACE International © 2012); (c) tubercles on engine section from the Comet in Whitefish Bay (Courtesy of Sean Ley, Development Officer, Great Lakes Historical Society); (d) rusticles on engine components of the RMS Titanic (reprinted with the permission of RMS Titanic, Inc. © 2012).
The properties of tubercles and rusticles have been used to estimate the rate of corrosion (Cullimore & Johnston Citation2008), predict the extent of localized corrosion associated with the accumulation (Angell Citation2003), and propose mechanisms for corrosion, especially the potential role of microorganisms in their formation (Borenstein & Lindsay Citation1988). It has also been suggested that the properties of rusticles can ‘provide archival information about ship sinkings’ (Cullimore Citation2010).
The following is a review of available data regarding accumulations of iron corrosion products including the morphology, mineralogy, microbiology and the mechanisms for formation. Use of descriptive terms to denote specific iron corrosion product morphologies is somewhat arbitrary. For example, Cullimore et al. (Citation2002) reported the coexistence of several different oxidized iron formations on deep ocean shipwrecks and used the term ‘rusticle’ to collectively describe them all. Throughout this paper the descriptive terms are those used by the authors in the citations.
Rusticles
Rusticles have been located on ironclad ships and on iron-containing materials on wooden shipwrecks, eg chains, machinery, typically associated with carbon steel and wrought iron (Table ) in marine environments. Carbon steel is commercial iron that contains carbon as an essential alloying constituent in amounts up to ~ 1.7 wt%. Wrought iron is an iron alloy with a very low carbon content (0.1 to 0.25 wt%) that has up to 2.0 wt% fibrous inclusions (slag). To date, accumulations of corrosion products on these same materials on shipwrecks in freshwater environments have not had an icicle shape (Figure c) (2014 email communication from Ley S; unreferenced).
Table 1. Shipwreck information from seawater and freshwater locations.
Structure and mineralogy
In describing accumulations of iron corrosion products on the RMS Titanic, Cullimore et al. (Citation2002) reported hanging, plate-like, tubercle, and whorled formations. Cullimore and Johnston (Citation2008) examined eight deep ocean shipwrecks in five different locations and concluded that the form of the accumulated corrosion products ‘… appears to be dictated at least in part by the nature and form of the steel surfaces…upon which the rusticle has become attached and subsequently grown.’ According to Cullimore and Johnston (Citation2008), all rusticles have the following characteristics: iron oxides and hydroxides, porosity with extensive water channels that may be connected to or closed to the outside environment, distinct microbial communities and bioaccumulation of ions from seawater. They referred to, ‘a cortex that is highly crystallized and commonly dominated by ferric forms of oxide and hydroxide, and outer surfaces are frequently laminar possessing laterally deployed structures’ (Figure ).
Figure 2. Schematic of rusticles from the RMS Titanic, adapted from Pellegrino (Citation2000).
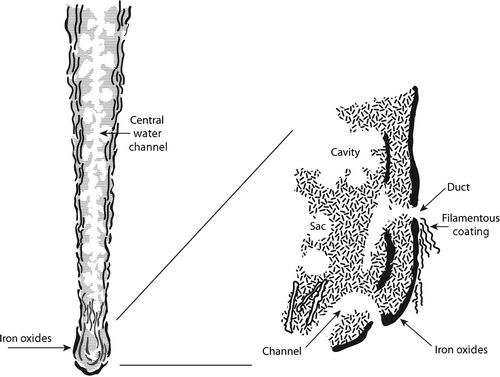
Stoffyn-Egli and Buckley (Citation1992) used environmental scanning electron microscopy coupled with energy dispersive X-ray spectrometry and powder X-ray diffraction (XRD) to examine rusticles from the RMS Titanic. They reported, ‘Rusticles are formed of a brittle iron oxy-hydroxide shell … with a smooth dark red outer surface (toward seawater) and an orange rough inner surface (toward the center of the rusticle). The core of the rusticle and the inner surface of the shell is [sic] made of a reticular framework of spherical aggregates.’ Stoffyn-Egli and Buckley (Citation1992) described the aggregates as α-FeOOH (goethite) and the surface as γ-FeOOH (lepidocrocite). They also examined flakes of iron corrosion products from the RMS Titanic and demonstrated that the flakes had the same bulk mineralogy as the rusticles, ie a mixture of α-FeOOH and γ-FeOOH. However, Stoffyn-Egli and Buckley (Citation1992) also reported Fe2O3 (hematite), FeCO3 (siderite), PbCO3 (lead carbonate) and PbS (galena) in association with the flakes. The source of the Pb was confirmed to be from a Pb-based paint. Based on the mineralogical data, Stoffyn-Egli and Buckley (Citation1992) concluded that, despite the presence of minerals indicative of differing redox potentials within rusticles, there was no evidence of extreme (undefined) reducing conditions in the rusticles from the RMS Titanic.
In contrast, Long et al. (Citation2004) provided mineralogical evidence for reducing conditions within rusticles from the RMS Titanic. Using Mossbauer spectroscopy they identified small particles of α-FeOOH, traces of SiO2 (quartz) and Fe(OH)2 (green rust). Green rust is the result of the reaction of iron with water in reducing environments and is stable only in the absence of oxygen. In chloride containing waters the formula for green rust is typically presented as [Fe2+3 Fe3+(OH)8]+ [Cl⋅H2O]–.
Cook and Peterson (Citation2005) provided additional evidence of reducing environments in rusticles. Using Mossbauer spectroscopy and powder XRD, they reported that rusticles on wrought iron surfaces of the USS Monitor had an outer casing of FeCO3, γ-FeOOH and α-FeOOH. They described fluid-filled cores in rusticles that contained unstable ‘Corrosion Magnetite’ [sic], at pH 3. Corrosion magnetite (Fe2+Fe3+2O4) results from aqueous corrosion in contrast to magnetite (Fe3O4) that forms at high temperature, eg mill scale (2014 email communication from Cook DC; unreferenced). Cook and Peterson (Citation2005) suggested that corrosion magnetite was the result of aqueous corrosion under anaerobic conditions.
Microorganisms
Cullimore and Johnston (Citation2008) and Stoffyn-Egli and Buckley (Citation1992) used a liquid culture technique (BART, Hacht, Loveland, CO, USA) to identify communities of bacteria associated with rusticles. Cullimore and Johnston (Citation2008) consistently identified iron-related bacteria (both oxidizing [FeOB] and reducing [FeRB]), sulfate-reducing bacteria (SRB) and heterotrophic bacteria associated with rusticles from five different wreck locations. They also reported significant populations of fungi on the outer walls of rusticles. Stoffyn-Egli and Buckley (Citation1995) reported that a variety of bacteria, notably SRB, grew within days of inoculation of the culture medium with seawater extractions from RMS Titanic rusticles. However, in the absence of ‘extreme’ reducing conditions, Stoffyn-Egli and Buckley (Citation1995) concluded that anaerobic SRB were not active in the rusticles they examined from the RMS Titanic. Stoffyn-Egli and Buckley (Citation1992) concluded that the coexistence of minerals with different redox potentials indicated that reactions were either slow or that both FeOB and FeRB were active in microenvironments. Stoffyn-Egli and Buckley (Citation1992) also observed spheres on the outer surface of rusticles that resembled bacteria associated with γ-FeOOH. Herdendorf et al. (Citation1995) identified bacteria with the characteristics of a FeOB (Leptothrix sp.) in rusticles from the SS Central America (Table ), a wooden steamer with iron machinery that had been on the floor of the North Atlantic Ocean in 2,200 m of water for 144 years (5.6 mg l−1 O2).
Adsorption of metals
Cullimore and Johnston (Citation2008) cited concentrations of metals, eg Sr, Pb and Al, as evidence of bioaccumulation in rusticles. They further suggested that concentrations of accumulated metals could be used as a diagnostic tool for forensic analysis of ship cargo. For example, the presence of Sr within rusticles from DMK U-166, a World War II German U-boat and the SS Robert E. Lee, a steam passenger ship, both in the Gulf of Mexico, was interpreted as an indicator of deteriorating munitions and flares that had been on the ships. Lead concentrations > 0.01% were interpreted as indicative that a ship was carrying hydrocarbon fuel with a lead additive. As previously indicated, Stoffyn-Egli and Buckley (Citation1992) attributed the presence of Pb in rusticles from the RMS Titanic to red lead paint, identified on the hull. The presence of Al in some rusticles on the DMK U-166 and the SS Robert E. Lee, known to be carrying 5,000 tons of aluminum ore (bauxite) at the time it sank, was also used to support the notion of bioaccumulation (Cullimore & Johnston Citation2008).
Mechanism for formation and relationship to corrosion
Church et al. (Citation2009) examined six shipwrecks in the Gulf of Mexico. The wrecks have in common that they were all casualties of World War II, sunk in the year 1942. The wrecks are located at water depths ranging from 87 to 1,964 m. Church et al. (Citation2009) observed a relationship between water depth and rusticle formation, with the greatest density of rusticles on the Alcoa Puritan at the deepest location (Table ).
Table 2. World War II shipwrecks in the Gulf of Mexico.Table Footnote*
Cullimore et al. (Citation2002) suggested that some combination of the following conditions were required for rusticle formation: ‘areas where steel is poorly protected with paint, embrittled by stress, electrically charged in any way, involved in rhythmic movement of water over the site, positioned on a temperature gradient, or where available water contains sufficient nutrients to support growth.’ The only description of corrosion on a shipwreck associated with rusticles was provided by Cullimore et al. (Citation2002) as ‘dominated by lateral flaking and dissolution directly under maturing rusticle growth.’ The role of microorganisms in the formation of rusticles and the relationship of rusticles to accelerated corrosion have been addressed briefly by several investigators. Long et al. (Citation2004) concluded: ‘… the slow degradation of the RMS Titanic follows a well known corrosion process…’ Stoffyn-Egli and Buckley (Citation1993) concluded: ‘…that biological activity plays a major role in promoting corrosion of the Titanic…’ Cullimore et al. (Citation2002) described the corrosion related to rusticles as ‘biological extraction’ whereby iron was ‘… biologically extracted from the steel of the ship into the rusticle structures. The iron is then exported into the oceanic environment as red dust … and yellow colloids.’ Cullimore and Johnston (Citation2008) proposed that ‘… significant differentiation in form and function has occurred within the rusticle generated by the microbial consortia.’ They further suggest, ‘This level of differentiation parallels tissue differentiation in the plant and animal kingdom [sic]…’ Cullimore et al. (Citation2002) found that iron was not evenly distributed within the rusticle. However, they did not establish a correlation between iron concentration and bacteria in the rusticles from the RMS Titanic.
Tubercles
The term tubercle, meaning a small rounded prominence, has been used to refer to iron corrosion products on steel surfaces exposed in treated (ie chlorinated and heated) and untreated, stagnant and flowing, fresh and marine waters. Tubercles have been reported for all steel alloys containing < 6 wt% Mo, including carbon steels, cast iron and austenitic chromium–nickel alloys.
Structure and mineralogy
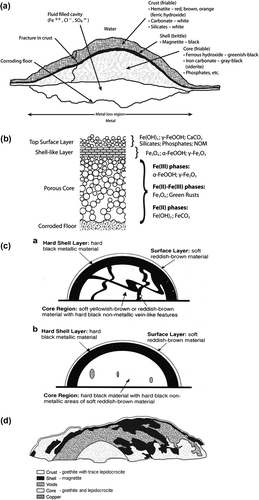
Mound-shaped tubercles are the predominant corrosion product morphology in cast iron pipes in drinking water distribution systems (DWDS). However, other morphologies including flutes or cones, mounds with protruding tubes (Figure a), and freestanding tubes (Figure b) have been observed (Gerke et al. Citation2013). Several authors have described the internal morphologies of tubercles (Herro Citation1998; Sarin, Snoeyink, Lytle et al. Citation2004; Gerke et al. Citation2008; Ray et al. Citation2010) and provided schematics of tubercles (Figure a–d). Herro (Citation1998) indicated that tubercles should contain the following structural features: outer crust, inner shell, core material, fluid cavity and corroding floor (Figure a). Sarin et al. (Citation2001, Citation2004) indicated a surface layer, a shell-like layer and a porous core over a corroding floor (Figure b). The significant difference between the Herro (Citation1998) and the Sarin, Snoeyink, Lytle et al. (Citation2004) models is the absence of a fluid-filled cavity in the Sarin, Snoeyink, Lytle et al. (Citation2004) model. Instead, Sarin, Snoeyink, Lytle et al. (Citation2004) suggested that porosity within the tubercle determined the ease with which ions migrate within the core. In all cases γ-FeOOH and α-FeOOH were the predominant iron minerals in the outer crust and core, separated by a shell of Fe3O4. Veins of Fe3O4 within core regions have also been reported (Figure c), and Gerke et al. (Citation2008) and Ray et al. (Citation2010) described tubercles with multiple cores and cores that extended into the pits that had resulted from localized corrosion (Figure d). Gerke et al. (Citation2013) reported that internal morphologies for other shapes, eg tubes, from DWDS were texturally complex but mineralogically simple, composed of two iron oxide/oxyhydroxide minerals: α-FeOOH and Fe3O4.
Figure 3. Representations of accumulated iron corrosion products. (a) Herro (Citation1998) schematic of tubercle (reprinted with permission of NACE International © 1998); (b) Sarin, Snoeyink, Lytle et al. (Citation2004) tubercle model (reprinted with permission from ASCE © 2004); (c) Gerke et al. (Citation2008) schematics of tubercles from drinking water distribution systems (reprinted with permission from Elsevier © 2008); (d) Ray et al. (Citation2010) schematic of Duluth Superior Harbor tubercle.
Microorganisms
Iron-oxidizing bacteria (Ray et al. Citation2010) and SRB (Lytle et al. Citation2005) have been identified in some, but not all tubercles. Miller and Tiller (Citation1970) indicated that FeOB, together with the ferric hydroxide they produced, could form extensive deposits inside water pipes. Tiller (Citation1982) suggested that FeOB ‘encouraged’ tubercle formation. Using environmental scanning electron microscopy, Ray et al. (Citation2009) identified iron encrusted stalks, a biosignature for FeOB (Chan et al. Citation2011), within the core regions of tubercles on carbon steel pilings in Duluth Superior Harbor (DSH) Lake Superior, that were associated with localized corrosion. Hicks (Citation2007) identified the FeOB Sideroxydans lithotrophicus by sequencing the 16S rDNA in DSH corrosion products. The identification of iron-encrusted stalks produced by FeOB has been limited to core regions and surfaces of tubercles. Microorganisms have not been identified within crust and shell regions.
Usher et al. (Citation2014) used pyrosequencing to identify and enumerate bacteria and archaea in tubercles from a 70-year old carbon steel railway line submerged 0.5 m in seawater. The dominant microorganisms in their samples were related to methanogenic archaea, capable of extracting electrons directly from steel. The work by Usher et al. (Citation2014) is the first to use pyrosequencing to evaluate microbial communities in any iron corrosion product and to propose direct electron uptake from an iron substratum as a mechanism for marine corrosion.
Tubercle formation on austenitic chromium–nickel alloys, especially 300 series stainless steels, has been investigated extensively and in most cases the formation has been attributed to FeOB without any demonstration of cells or biomineralogy (Lutey Citation2001). Tubercles on these alloys results in aggressive localized corrosion that is controlled by the alloying elements. All 300 series stainless steels have a nominal 18 wt% Cr and 8 wt% Ni alloy mixture. The accumulation of corrosion products on the surface of these alloys in oxygenated, chloride-containing waters causes under-deposit corrosion that initiates a series of events, leading to aggressive corrosion, ie fixation of a small anode surrounded by a large cathode, dissolution of metal at the anode, acidity under the tubercle and ingress of chloride. The structure, mineralogy and microbiology for tubercles on 300 series stainless steel have never been examined in detail.
Adsorption of metals
Synchrotron-based μ-X-ray fluorescence mapping and μ-X-ray absorption near edge structure have been used to map the distribution of metal cations of V, Sr, Pb, Ni, Cr, Cu, Zn, and Mn in surface regions of tubercles in DWDS (Gerke et al. Citation2010, 2012, 2013, Citation2014). These observations were not defined as bioaccumulation. Abiotic accumulation of cations from water to iron oxides is a well-characterized phenomenon. Farley et al. (Citation1985) described abiotic, hydrous metallic oxides as dominant sorbents of cations in natural water. They were among the first to provide a vocabulary for the discussion of abiotic surface precipitation reactions. ‘Adsorption’ is used to denote monolayer coverage of cations to oxide surfaces and ‘sorption’ is used in a more general way to indicate all processes that transfer cations from solution to the solid phase. Farley et al. (Citation1985) reported that as adsorption took place, new hydroxide surfaces formed, resulting in multi-layer sorption processes. Langley et al. (Citation2009) described two features that influenced sorption to fine-grained iron oxides: (1) large surface area-to-mass ratios (several hundred m2 g−1) and (2) highly reactive protonation/de-protonation reactions of hydroxyls on the mineral surface. Cations can be sorbed as a result of inner or outer sphere complexes, incorporation into vacancies or substitutions within iron mineral structures.
Some FeOB extrude abiotic polymeric structures, eg stalks or sheaths, upon which they deposit Fe3+ derived from their metabolism (oxidation of Fe2+ to Fe3+). The result is bacteriogenic iron oxides (BIOS), fine-grained (2–500 nm) iron minerals that are typically mixed with live bacteria, cellular debris and extracellular polymeric substances. BIOS have the same physico-chemical properties as reported for other fine-grained iron oxides. BIOS act as sorbents of dissolved metal ions and enrichments of Cu, Pb, Cd, Al, Cr, Zn, Mn and Sr have been reported (Dong et al. Citation2003; Martinez & Ferris Citation2005). Chan et al. (Citation2009) concluded that polymer-directed iron hydroxide mineralization is a general phenomenon that can occur in any system containing acidic polysaccharides and iron. The dominant mineral phase in BIOS from both fresh and marine waters is typically 2-line ferrihydrite (Fe3+2O3⋅0.5H2O) (Langley et al. Citation2009). Ferrihydrite is a poorly ordered mineral that transforms into α-FeOOH and/or Fe2O3 over time (Cornell et al. Citation1987). Once deposited, BIOS carry negative charges so that such a process could continue indefinitely without any biological activity. The process has not been described as bioaccumulation.
Mechanisms for formation and relationship to corrosion
The presence of tubercles on carbon steel and cast iron cannot be used to conclude localized corrosion directly under the tubercles or a role for bacteria in their formation (Ray et al. Citation2010). Some tubercles cover a fluid filled cavity (Herro Citation1998), others have increased porosity at the base of the core associated with localized corrosion (Sarin, Snoeyink, Bebee et al. Citation2004), some are filled with corrosion products (Ray et al. Citation2009) and still others have no obvious relationship to localized corrosion (Gerke et al. Citation2008). Abiotic mechanisms have been proposed for the formation of tubercles. Menzies (Citation1970) described tubercle formation at breaks or discontinuities in iron oxide scales exposed in an oxygenated environment. ‘Anodic dissolution takes place and as metal ions concentrate in the solution the solubility product of the solid hydroxide is exceeded locally and hydroxide precipitates out as a hemispherical membrane which surrounds and covers the original discontinuity. These results in effective screening of the anodic area from available oxygen and the metal at the discontinuity remains anodic.’ Herro (Citation1998), working with tubercles in cooling waters, concluded that differential aeration cells caused tuberculation, suggesting that oxygen-deficient regions below the accumulated corrosion products were anodic sites, while surrounding areas were cathodic. Herro indicated that tubercles grew as a result of both internal (anodic) and external (cathodic) reactions, ie anodic dissolution of metal resulted in the accumulation of Fe2+ and Fe3+ ions and cathodic reactions outside the tubercle increased the pH and caused the precipitation of carbonate and other species whose solubility decreases with increasing pH.
Comprehensive physico-chemical data of corrosion products from mounds, mounds with protruding tubes and freestanding tubes were examined in detail (Tang et al. Citation2006; Tamura Citation2008; Teng et al. Citation2008; Peng et al. Citation2010; Swietlik et al. Citation2012) and used to rationalize a proposed mechanism of formation. In summary, regardless of form or size the physico-chemical characteristics for all corrosion products were similar (Gerke et al. Citation2012). In cast iron pipes exposed to aerated, chlorinated drinking water, metallic iron is oxidized to Fe2+ by dissolved oxygen in the water, released into the water and further oxidized to Fe3+, forming Fe(OH)3, which accumulates on the inner wall of the cast iron pipe at locations that are not necessarily associated with localized corrosion. The Fe(OH)3 dehydrates to form FeOOH. Below a critical O2 concentration FeOOH is reduced to Fe3O4. Gas production at the cathode causes breaks (vents) in the crust. Growth of the vents into tubes is the result of dynamic templating on cathodically produced gas bubbles. Static templating, a process where precipitating material accumulates on artificial or natural substrata whose shape controls the shape of the precipitating material, is also possible.
Some mechanisms for mound formation indicate a spatial relationship between anodic sites and mounds and a connection between the height of the mound and the depth of the resulting pit (Angell Citation2003). That relationship is not always obvious in DWDS, where mounds can accumulate at locations with little to no evidence of pit formation.
Conclusions
Despite the obvious differences in morphology, many of the characteristics of rusticles developing on iron substrata in deep, oxygenated waters around the world are common to tubercles formed under a variety of surface conditions, eg stratification of iron oxides/hydroxides with a preponderance of α-FeOOH and accumulation of metals. Both FeOB and SRB have been reported in some, but not all accumulated iron corrosion products. Mineralogical data suggest that the morphologies of both tubercles and rusticles provide the possibility for reducing environments. Abiotic mechanisms have been proposed for tubercle formation and for adsorption of metal cations on tubercle surfaces. In contrast, biotic mechanisms have been proposed for rusticle formation and cation adsorption, eg biological extraction and bioaccumulation. To date, identification of microorganisms in tubercles has been limited to surfaces and core regions. The precise locations of microorganisms associated with rusticles have not been established.
Acknowledgements
Program element 0601153 N. Role of Iron-Oxidizing Bacteria in Metal Bio-Corrosion in Marine Environments. Program Manager, Dr Linda Chrisey. NRL publication JA/7303 – 14-2194.
References
- Angell P. 2003. Predictive model for non-microbiologically influenced corrosion tuberculation. Technical Report 1003442. Available from http://www.epri.com/abstracts/Pages/ProductAbstract.aspx?ProductId=000000000001003442
- Ballard RD. 1987. The discovery of the Titanic. New York, NY: Warner Books.
- Borenstein SW, Lindsay PB. 1988. Microbiologically influenced corrosion failure analysis. Mater Perform. 27:51–54.
- Chan CS, Fakra SC, Edwards DC, Emerson D, Banfield JF. 2009. Iron oxyhydroxide mineralization on microbial extracellular polysaccharides. Geochim Cosmochim Acta. 73:3807–3818.
- Chan CS, Fakra SC, Emerson D, Fleming EJ, Edwards KJ. 2011. Lithotrophic iron-oxidizing bacteria produce organic stalks to control mineral growth: implications for biosignature formation. ISME J. 5:717–727.
- Church RA, Warren DJ, Irion JB. 2009. Analysis of deepwater shipwrecks in the Gulf of Mexico: artificial reef effect of six World War II shipwrecks. Oceanography. 22:50–63.
- Cook DC, Peterson CE. 2005. Corrosion of submerged artifacts and the conservation of the USS Monitor. AIP Conf Proc. 765:91–96. Available from http://dx.doi.org/10.1063/1.1923640
- Cornell RM, Giovanoli R, Schindler PW. 1987. Effect of silicate species on the transformation of ferrihydrite into goethite and hematite in alkaline media. Clay Clay Miner. 35:21–28.
- Cullimore DR. 2010. Practical atlas for bacterial indentification. 2nd ed. Boca Raton, FL: CRC Press.
- Cullimore DR, Johnston LA. 2008. Microbiology of concretions, sediments and mechanisms influencing the preservation of submerged archaeological artifacts. Int J Hist Archaeology. 12:120–132.
- Cullimore DR, Pellegrino C, Johnston L. 2002. RMS Titanic and the emergence of new concepts on consortial nature of microbial events. Rev Environ Contam T. 173:117–141.
- Dong D, Hua X, Li Y, Zhang J, Yan D. 2003. Cd adsorption properties of components in different freshwater surface coating: the important role of ferromanganese oxides. Environ Sci Technol. 37:4106–4112.
- Farley KJ, Dzombak DA, Morel FMM. 1985. A surface precipitation model for the sorption of cations on metal-oxides. J Colloid Interface Sci. 106:226–242.
- Gerke TL, Little BJ, Luxton TP, Scheckel KG, Maynard JB. 2013. Strontium concentrations in corrosion products from residential drinking water distribution systems. Environ Sci Technol. 47:5171–5177.
- Gerke TL, Little BJ, Luxton TP, Scheckel KG, Maynard JB, Szabo JG. 2014. Strontium adsorption and desorption reactions in model drinking water distribution systems. Aqua. doi:10.2166/aqua.2014.075.
- Gerke TL, Maynard JB, Schock MR, Lytle DL. 2008. Physiological characterization of five iron tubercles from a single drinking water distribution system: possible new insights on their formation and growth. Corros Sci. 50:2030–2039.
- Gerke TL, Scheckel KG, Maynard JB. 2010. Speciation and distribution of vanadium in drinking water iron pipe corrosion by-products. Sci Total Environ. 408:5845–5853.
- Gerke TL, Scheckel KG, Ray RI, Little BJ. 2012. Can dynamic bubble templating play a role in corrosion product morphology? Corrosion. 68:025004-1–025004-7.
- Herdendorf CE, Thompson TG, Evans RD. 1995. Science on a deep-ocean shipwreck. Ohio J Sci. 95:4–224.
- Herro HM. 1998. MIC myths – does pitting cause MIC? Paper no. 98278. Houston, TX: NACE International.
- Hicks RE. 2007. Structure of bacterial communities associated with accelerated corrosive loss of port transportation infrastructure. Final report. Great Lakes Maritime Research Institute. Available from: http://www.glmri.org/research/completedstudies/Tab5.pdf
- Kobrin G. 1976. Corrosion by microbiological organisms in natural waters. Mater Perform. 15:38–43.
- Langley S, Gault AG, Ibrahim A, Takahashi Y, Renaud R, Fortin D, Clark ID, Ferris FG. 2009. Sorption of strontium onto bacteriogenic iron oxides. Environ Sci Technol. 43:1008–1014.
- Long GJ, Hautot D, Grandjean F, Vandormael D, Leighly HP. 2004. A Mossbauer spectral study of the hull steel and rusticles recovered from the Titanic. Hyperfine Interact. 155:1–13.
- Lutey RW. 2001. Treatment for the mitigation of MIC. In: Stoecker JG, editor. A practical manual on microbiologically influenced corroson. Houston, TX: NACE International; p. 9.1–9.30.
- Lytle DA, Gerke TL, Maynard JB. 2005. Effect of bacterial sulfate reduction on iron-corrosion scales. J Am Water Works Ass. 97:109–120.
- Martinez RE, Ferris FG. 2005. Review of the surface chemical heterogeneity of bacteriogenic oxides: proton and cadmium sorption. Am J Sci. 305:854–871.
- Menzies IA. 1970. Introductory corrosion. In: Miller JDA, editor. Microbial aspects of metallurgy. New York, NY: Elsevier; p. 35–60.
- Miller JDA, Tiller AK. 1970. Microbial corrosion of buried and immersed metal. In: Miller JDA, editor. Microbial aspects of metallurgy. New York, NY: Elsevier; p. 61–105.
- Pellegrino C. 2000. Ghosts of the Titanic. New York, NY: Harper Collins.
- Peng CY, Korshin GV, Valentine RL, Hill AS, Friedman MJ, Reiber SH. 2010. Characterization of elemental and structural composition of corrosion scales and deposits formed in drinking water distribution systems. Water Res. 44:4570–4580.
- Ray RI, Lee JS, Little BJ. 2009. Factors contributing to corrosion of steel pilings in Duluth-Superior Harbor. Corrosion. 65:707–717.
- Ray RI, Lee JS, Little BJ, Gerke TL. 2010. The anatomy of tubercles: a corrosion study in a fresh water estuary. Mater Corros. 61:993–999.
- Sarin P, Snoeyink VL, Bebee J, Kriven WM, Clement JA. 2001. Physico-chemical characteristics of corrosion scales in old iron pipes. Water Res. 35:2961–2969.
- Sarin P, Snoeyink VL, Bebee J, Jim KK, Beckett MA, Kriven WM, Clement JA. 2004. Iron release from corroded iron pipes in drinking water distribution systems: effect of dissolved oxygen. Water Res. 38:1259–1269.
- Sarin P, Snoeyink VL, Lytle DA, Kriven WM. 2004. Iron corrosion scales: model for scale growth, iron release, and colored water formation. J Environ Eng-Asce. 130:364–373.
- Stoffyn-Egli P, Buckley DE. 1992. The Titanic 80 years later: initial observations on the microstructure and biogeochemistry of corrosion products. Proceedings of 50th Annual Meeting of the Electron Microscopy Society of America, 27th Annual Meeting of the Microbeam Analysis Society and the 19th Annual Meeting of the Microscopical Society of Canada; August 16–21; Boston, MA. San Francisco, CA: San Francisco Press, 1330–1331.
- Stoffyn-Egli P, Buckley DE. 1993. The Titanic: from metals to minerals. Can Chem News. 45:26–28.
- Stoffyn-Egli P, Buckley DE. 1995. The micro-world of the Titanic. Chem. Br. 31:551–553.
- Swietlik J, Raczyk-Stanislawiak U, Piszora P, Nawrocki J. 2012. Corrosion in drinking water pipes: the importance of green rusts. Water Res. 46:1–10.
- Tamura H. 2008. The role of rusts in corrosion and corrosion protection of iron and steel. Corros Sci. 50:1872–1883.
- Tang ZJ, Hong SK, Xiao WZ, Taylor J. 2006. Characteristics of iron corrosion scales established under blending of ground, surface, and saline waters and their impacts on iron release in the pipe distribution system. Corros Sci. 48:322–342.
- Teng F, Guan YT, Zhu WP. 2008. Effect of biofilm on cast iron pipe corrosion in drinking water distribution system: corrosion scales characterization and microbial community structure investigation. Corros Sci. 50:2816–2823.
- Tiller AK. 1982. Aspects of microbial corrosion. In: Parkins RN, editor. Corrosion processes. London: Applied Science; p. 115–159.
- Usher KM, Kaksonen AH, MacLeod ID. 2014. Marine rust tubercles harbour iron corroding archaea and sulphate reducing bacteria. Corros Sci. 83:189–197.
