ABSTRACT
Purpose/Aim: Powdered hemostats have been widely adopted for their ease-of-use; however, their efficacy has been limited resulting in applications restricted to low-level bleeds. This study investigates the use of bovine-derived gelatin particles (BGP) as a standalone hemostatic powder and compare BGP to commercially available microporous polysaccharide hemospheres (MPH).
Materials and Methods: The powders were investigated for their hemostatic efficacy in a heparinized pre-clinical bleeding model limited to grade 1 and 2 bleeds on a validated intraoperative bleeding scale, which represents the accepted, clinical use of hemostatic powders.
Results: At 10 minutes, the hemostatic success of lesions treated with BGP were 78% while MPH were 22%. The odds ratio for hemostatic success of BGP relative to MPH was 15.18 (95% CI: 7.37, 31.27). The 95% lower limit of the odds ratio was greater than 1. This indicates that BGP are superior to MPH (p < 0.001). The median time to hemostasis for BGP was 1.6 minutes and MPH was 14.5 minutes. The ratio for time to hemostasis of MPH relative to BGP was 9.23 (95% CI: 6.99, 12.19). This indicates that BGP achieve significantly faster time to hemostasis (p < 0.001).
Conclusions: Characterization of tissue explant ultrastructure, particle size, and swelling revealed differences in the materials. BGP, in addition to absorbing fluid and concentrating clotting factors and platelets, integrate into the clot and stabilize the fibrin matrix. BGP have advantages over MPH in terms of speed and efficacy. BGP are a favorable biomaterial for further research that greatly improve the limited efficacy of powdered hemostats.
INTRODUCTION
The use of intraoperative hemostatic agents has become an accepted standard of care in nearly all surgical specialties.Citation1–4 The increased use of these agents has led to decreases in the rates of blood transfusions, which improve outcomes for patients and produce economic benefit for healthcare providers.Citation4 Among the various classes of hemostatic agents, powdered hemostats have been widely adopted for their ease-of-use; however, their efficacy has been limited to low-level bleeds.Citation5–14 More recently, the benefit of using powdered hemostats intraoperatively to treat low-level bleeds has been demonstrated clinically.Citation15–20
The two predominant materials within this class of hemostatic agents are a polysaccharide-based powder and a fibrinogen-thrombin based powder. While greater clinical evidence exists for the fibrinogen-thrombin based powder, polysaccharide powders are favored due to their fast degradation and minimal inflammatory response.Citation21 The available polysaccharide powder is manufactured into microporous polysaccharide hemospheres (MPH) from plant-derived starches. These materials have porous surfaces, which absorb water and low molecular weight compounds from the blood to concentrate blood solids.Citation5
As the adoption of powdered agents becomes more commonplace, the need for more effective agents becomes necessary, especially as bleeding scenarios in current surgical practice further challenge the limitations of current materials. Within the spectrum of biomaterials being investigated gelatin stands out as a clinically relevant hemostatic material. Though typically combined with a procoagulant, gelatin's swelling, inherent procoagulant activity, and degradation profile make it a useful material alone to use as a powdered hemostat.Citation22
In this study, we investigate the use of bovine-derived gelatin particles (BGP) as a standalone hemostatic powder. The BGP were investigated for their hemostatic potential by evaluating ultrastructure, particle size and swell kinetics compared to a commercially available polysaccharide powder, MPH. Ultimately, the efficacy of these two powders is compared in a heparinized porcine hemostatic model limited to grade 1 and 2 bleeds on a validated intraoperative bleeding scale, which represents the accepted, clinical use of hemostatic powders.
MATERIALS AND METHODS
Powdered Hemostatic Agents
Bovine-derived Gelatin Powder (BGP)
FLOSORB™ (Baxter Healthcare Corporation, Deerfield, IL) is a bovine-derived gelatin powder (BGP). BGP is produced by the reaction of glutaraldehyde with gelatin which is then dried and irradiated for sterility. Bovine-derived gelatin has been shown to be more efficacious than other sources of gelatin and has previously been mixed with procoagulants for improved hemostatic efficacy.Citation12, Citation23, Citation24 The BGP is filled at 1.2 gram (dry weight) into a disposable, self-contained delivery device prior to sterilization and is provided ready-to-use in a sterile packaged configuration. The BGP is applied topically by expelling the powder from this delivery device above the source of bleeding.
Microporous Polysaccharide Hemospheres (MPH)
ARISTA™ AH (Davol Inc., Warwick, RI) is composed of microporous polysaccharide hemospheres (MPH). MPH are produced by the reaction of epichlorohydrin with particles composed of a highly-purified potato starch that are then irradiated for sterility. MPH were selected as a comparator because of their similar delivery and use as a powdered hemostatic agent for which the clinical acceptability and biocompatibility characteristics have been established.Citation15, Citation20 The MPH is provided pre-loaded into a disposable plastic bellows and arrives in a sterile packaged configuration. The 1 gram size was utilized for these studies. The powder is applied topically to the source of bleeding following the manufacturer's instructions for use.
Imaging and Scanning Electron Microscopy (SEM) Characterization
Stereoscopic Images
Powdered hemostatic agents were characterized with low magnification stereomicroscopy under various conditions using a Nikon SMZ1500 Stereomicroscope and digital camera system. To isolate particle pairs for analysis, particles were placed in proximity using a tungsten needle probe. To generate videos of manual manipulation of particles, a tungsten needle probe was used to displace particles while recording.
Dry and Wet Particle SEM Images
Dry powders were mounted on carbon adhesive tape and imaged directly in variable pressure SEM using an FEI Quanta 650 FEG SEM (ESEM).
For wet particle SEM, dry powders were placed on a temperature controlled sample cup in an ESEM with a gaseous secondary electron detector equipped with a pressure limiting aperture, approximately 500 µm in diameter. Water vapor was injected into the chamber to produce a local environment with humidity from ∼10% up to 100% (saturation), thereby, wetting the particles. The particles were equilibrated in the microscope at approximately 50% humidity and 4°C. A frame was captured approximately every 3 seconds, while the humidity was increased up to and past the point of saturation and then decreased. Movies of the still images were generated, as well as before-and-after images of the particles.
Tissue Explant SEM Images
Liver lesions treated with MPH and BGP were excised and immersed in a fixative solution consisting of 2% glutaraldehyde in 0.1 M cacodylate buffer. Explants were sectioned to approximately 5 mm in thickness and glued to platforms using Loctite 404 tissue adhesive. After approximately 10-minutes of curing in a humid environment, these glued tissue sections were further sectioned to a thickness of 500 µm by a Vibratome 3000. These slices were washed three times with 0.1 M cacodylate buffer and processed through serial dehydrations of ethanol in water up to pure ethanol. This was followed by immersion in mixtures of hexamethyldisilazane (HMDS) and ethanol up to pure HMDS. The resulting dehydrated samples were air dried. Dry tissue slices were mounted and coated with Iridium metal. An FEI Quanta 650 FEG SEM was used to examine the samples.
Particle Size Measurements
Powdered hemostatic agents were sized using a Malvern Mastersizer 2000 laser light scattering particle size distribution analyzer (Malvern Instruments, Malvern, UK). For measuring dry particle diameter, powders were added to 98% methanol. For hydrated particle diameter, powders were suspended in 0.9% saline and allowed to swell for 10 minutes at 2 rotations per second. Instrument parameters were set for a target obscuration of 8–12% and 3500 RPM with triplicate measurements. The mean particle diameter and percent swell were calculated. Percent swell was calculated by ((average diameter in saline − average diameter in methanol)/average diameter in methanol)*100%.
Porcine Hepatic Square Model
Animal activities were performed according to the Guide for the Care and Use of Laboratory Animals and applicable United States animal welfare regulations in an AAALAC-accredited institution following Institutional Animal Care and Use Committee approval.Citation25
A total of 13 male, domestic pigs, weighing 28–42 kg at the time of surgery were used. Animals were quarantined for at least 6 days upon arrival and only animals showing no signs of clinical illness were included in the study. Animals were socially housed in pens within a humidity and temperature-monitored room. Pigs received water ad libitum, a standard pig diet once daily, and various cage-enrichment devices as well as dietary enrichment.
Preparation and Monitoring
Pigs were pre-medicated with midazolam (2 mg/kg, IM), ketamine (10 mg/kg, IM), and atropine (0.04 mg/kg, IM) and anesthesia was maintained with sevoflurane (4% delivered in oxygen) and fentanyl citrate (up to 0.02 mg/kg/hour, IV). Fluid therapy (≤ 10 mL/kg/hour, IV) of Lactated Ringers Solution and additional supportive therapy was provided based on blood pressure trends to maintain mean arterial pressure above 70 mmHg. Plasma total protein and hematocrit were measured at least every 20 minutes to monitor for hemodilution.
A baseline activated clotting time (ACT) was measured using a HEMOCHRON Jr. Signature+ Whole Blood Microcoagulation System (ITC, Edison, NJ) prior to the surgical procedure followed by heparin administration (initial 200 U/kg IV bolus). ACT was monitored throughout the surgical procedure and additional heparin was administered as needed to maintain ACT between 180 and 250 seconds, which was selected to mimic clinically relevant coagulopathies associated with chronic disease and heparinization levels recommended for peripheral vascular surgery.Citation26–28
Surgical Model
The surgical model was performed as previously described with refinements.Citation23, Citation29 Access to the liver was facilitated with a mid-line celiotomy and up to 26 square lesions per animal were created on the liver surface using sharp dissection. Each lesion was 1× 1 cm (confirmed by using a metal dye to cut the outlines of the squares) and 0.2 cm to 0.3 cm deep. A validated intraoperative bleeding scale was used to grade bleeding, wherein clinically acceptable hemostasis was defined as Grade 0, “No bleeding.”(30) Bleeding was assessed by a single observer trained to accurately assess the level of bleeding. To represent the accepted, clinical use of hemostatic powders, only lesions with a bleeding grade of 1 or 2 were included in the study. Bleeding grades greater than 2 were treated with an alternate means and excluded. Following creation and scoring of initial bleed levels, lesions were treated with either BGP or MPH. Treatment of individual lesions was prescribed in a block-randomized fashion across animals with as many lesions created per animal as clinically and experimentally justified. The investigator was blinded to the treatment until after the lesion was created and scored. Utilizing the included delivery devices, BGP and MPH were delivered directly to the appropriate bleeding sites. A saline-moistened gauze was held approximate to the bleeding site for 2 minutes. The amount delivered was visually targeted to completely cover a 1 cm × 1 cm lesion and to be equal between the two materials. The mass of material delivered was quantified by weighing each device prior to and immediately following application. Each device was used to treat one lesion.
Hemostasis Evaluation
After removing the gauze, each lesion was evaluated qualitatively for hemostasis at 2, 3, 4, 5, 6, 7 and 10 minutes after application using the validated bleeding scale. Following the 10-minute hemostasis evaluation, lesions were irrigated with saline, and visually assessed for removal of excess material not incorporated in the blood clot. Following evaluation of multiple lesions, animals were humanely euthanized with pentobarbital while under deep surgical anesthesia after the final hemostasis evaluation.
Statistical Analysis
Calculations were performed with SAS® software version 9.4 (SAS Institute Inc., Cary, NC) and R software version 3.4.0 (Foundation for Statistical Computing, Vienna, Austria).Citation31 The primary endpoint of this prospective randomized study, hemostatic success at 10 minutes after application, was evaluated as to superiority of BGP relative to MPH. A logistic regression model was fit using PROC GENMOD in SAS with hemostatic success as the dependent variable, treatment group as the independent variable, baseline (pre-treatment) bleeding score as the covariate and lesion pair within animal as random effect. An unstructured covariance matrix was used to model the correlation of the responses from lesion pairs within an animal.
An accelerated failure time (AFT) model was used to model the difference in interval-censored time to hemostasis between BGP and MPH. The model included the fixed effects covariates article (BGP or MPH) and pre-treatment bleeding score. This model was fit using R function survreg of R package survival.Citation32 Random effects for animal and lesion pair within animal were included using R function frailty (options distribution=”gaussian” and method=”aic”) of the same R package. The difference in time to hemostasis between BGP and MPH was assessed by their ratio, and the corresponding 95% confidence interval (CI) of the ratio and the two-sided p-value for the alternative hypothesis of difference in time to hemostasis between BGP and MPH were calculated.
RESULTS
Imaging and SEM Characterization
Stereoscopic imaging reveals a difference in color between BGP, which are off-yellow granules, and MPH, which are a white fine powder (, ).
FIGURE 1 FLOSORB, bovine-derived gelatin particles (A, BGP), is a more course powder than ARISTA AH, microporous polysaccharide hemospheres (F, MPH). The FLOSORB granules are of irregular shape and the ARISTA AH are round (B, G, and Supplemental Figure S1). FLOSORB particles shown before (B, C) and after (D, E) hydration, reversibly swell uniformly (Supplemental Video V1). ARISTA AH particles shown before (G, H) and after (I, J) hydration swell irregularly then fuse (Supplemental Video V2). When manipulated, the FLOSORB particles remain separate (Supplemental Video V3) while the ARISTA AH particles form a cohesive sheet (Supplemental Video V4). Scale bars are 500 µm.
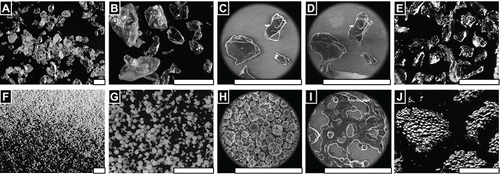
SEM reveals that BGP granules are irregularly shaped and relatively larger than MPH spheres (, ). Further magnification reveals the BGP having angular facets and both smooth and rough surface morphology (). At the same magnifications, MPH are shown to be approximately spherical with an undulated surface morphology (). Wet SEM show that particles swell proportional to the relative humidity and reach a stable size, when the relative humidity of water vapor reaches saturation and liquid water droplets condense onto the stage and granules (Supplemental Video V1). The BGP granules expanded uniformly, while the MPH lost their undulated surface morphology and became smooth spheres. Following decreasing humidity and evaporating unbound water, the BGP granules returned to their original shape (, ), while the MPH spread and lost their spherical structure (, ) (Supplemental Video V2). Additionally, hydrated then dried BGP particles were found to remain as individual granules (, Supplemental Video V3), while MPH particles were observed to fuse into cohesive sheets (Supplemental Video V4). Examination of MPH particles in isolation reveals merging and deformation of the particles during hydration (, Supplemental Video V5).
FIGURE 3 FLOSORB granules (BGP) swell uniformly when hydrated, and dehydration reveals that granules return to their original size and shape (Supplemental Video V1). In contrast, ARISTA AH particles (MPH) swell non-uniformly when hydrated, and dehydration reveals that particles fuse into aggerates with adjacent particles (Supplemental Video V5). Still images are shown before hydration ∼20%RH (A, D), at saturation >100% RH (B, C), and after dehydration ∼20% RH (C, F). Water droplets are seen condensing on the particles and stage (B, E). Scale bars are 200 µm.
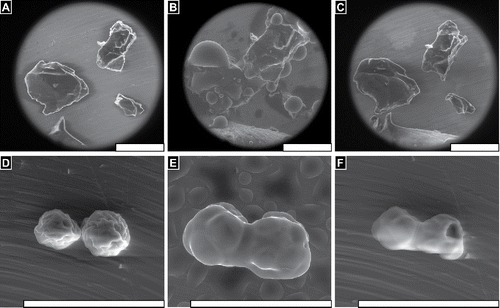
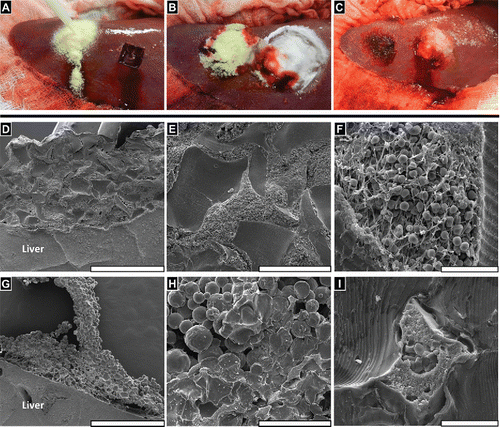
FIGURE 4 FLOSORB (BGP) provided faster and sustained hemostasis over 10 minutes relative to ARISTA AH (MPH) in a heparinized porcine hepatic bleeding model. (A, Left) Application of FLOSORB to a grade 1 bleeding site and (A, Right) an untreated grade 1 lesion (ooze or intermittent flow, estimated >1 - 5 mL/minute rate of blood loss); (B) Cessation of bleeding 2–minutes after treatment and approximation with saline-moistened gauze (FLOSORB, left; ARISTA AH, right); (C) FLOSORB-treated lesion at 10 -minutes after application (left) and a ARISTA AH-treated lesion continuing to bleed (right); (D) FLOSORB granules adhere to liver surface while (G) ARISTA AH particles leave voids between clot material and tissue surfaces. (E) FLOSORB granules maintain jagged morphology and are surrounded by clot material while (H) ARISTA AH particles show both spherical (dry) morphology and irregularly melded (wet) morphology with a noticeable absence of clot material. (F) Clot material near FLOSORB Granules contains fibrin and red blood cells while (I) clot material entrapped in melded ARISTA AH lacks visible fibrin fibers and is poor in red blood cells. Scale bars: D, G are 1mm; E, H are 200µm; and F, I are 20 µm.
Particle Size Measurement
BGP had a dry mean diameter of 325.7 ± 2.7 µm and a wet mean diameter of 480.7 ± 3.9 µm yielding a 47.6% swell. Whereas, MPH had a dry mean diameter of 76.0 ± 0.5 µm and a wet mean diameter of 106.6 ± 0.1 µm yielding a 40.2% swell. In addition, the distributions of particle size differed in the range of particle sizes between BGP and MPH. The average range of particle diameters (10%–90%) for BGP was 128.0 ± 1.6 to 565.0 ± 4.4 µm dry and 184.0 ± 0.3 to 836.8 ± 9.6 µm wet. The range of particle dimeters for MPH was 49.3 ± 0.3 to 107.2 ± 0.7 dry and 44.2 ± 1.8 to 165.7 ± 0.2 µm dry.
Hemostatic Efficacy
Both groups had a similar number of grade 1 (73 of 89 for BGP and 80 of 92 for MPH) and grade 2 bleeds (16 of 89 and 12 of 92, respectively). Applied masses of materials, determined by the difference in weights of each device prior to and immediately following application, were found to be 0.60 g ± 0.12 g (Mean ± SD) for BGP and 0.76 ± 0.06 g for MPH. Excess material, defined as material not incorporated in the blood clot, was successfully irrigated away from all treatment sites in both treatment groups after 10 minutes.
Hemostatic Success
Representative images show the targeted application of both BGP and MPH (). Immediate hemostatic success after a 2-minute approximation for BGP-treated lesions was 88% (78 of 89 lesions) and for MPH-treated lesions was 65% (60 of 92 lesions) (). At the end of the 10-minute observation period, hemostatic success of BGP-treated lesions fell to 78% (69 of 89) while MPH-treated lesions fell to 22% (20 of 92). Controlling for differences in pre-treatment bleeding level, the odds ratio for hemostatic success of BGP relative to MPH was 15.18 (95% CI: 7.37 to 31.27). The 95% lower limit of the odds ratio was greater than 1. This indicates that BGP are superior to MPH (p < 0.001). Over the 10-minute observation period, re-bleeding (defined as a lesion that was initially scored as successful then found to bleed at 10 minutes) was observed in 14% (13 of 89) BGP-treated lesions and 43% (40 of 92) MPH-treated lesions. When BGP failures occurred, blood flowed through the applied test article. For the majority of MPH failures, blood flowed out from under a contiguous mass of MPH (, ).
FIGURE 5 FLOSORB (BGP; white bars) provided superior hemostatic success over 10 minutes relative to ARISTA AH (MPH; black bars) and sustained hemostasis over 10 minutes. Superiority was determined by logistic regression with an odds ratio of for hemostatic success of FLOSORB relative to ARISTA AH was 15.18 (95% CI: 7.37 to 31.27). The 95% lower limit of the odds ratio was greater than 1. This indicates that FLOSORB is superior to ARISTA AH (p < 0.001).
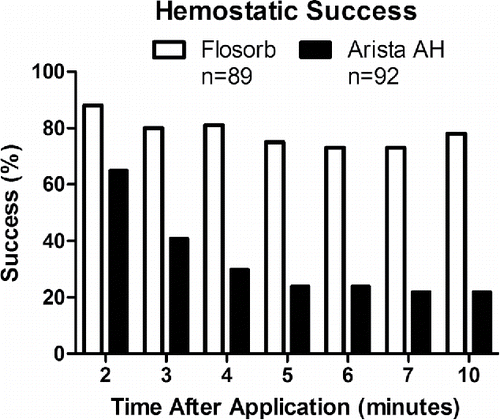
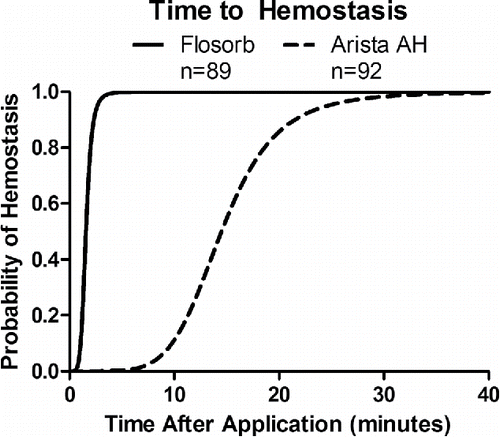
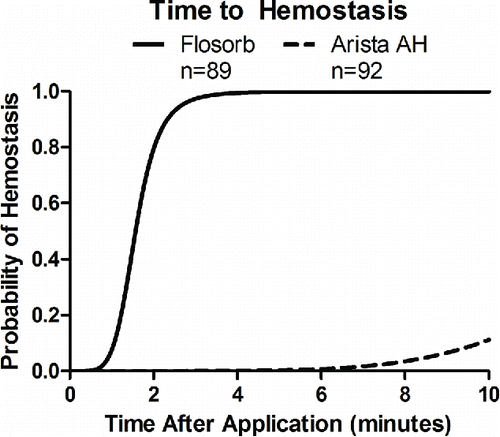
FIGURE 6 Statistical model-estimated probability of hemostasis over time with FLOSORB (BGP; solid line) compared to ARISTA AH (MPH; dashed line) controlled for pre-treatment bleeding level (mild to moderate). Time to hemostasis was 9.23 times faster (95% CI: 6.99 to 12.19) with FLOSORB. The median time to hemostasis (50%) for FLOSORB was inferred from this model to be 1.57 minutes with hemostasis likely to be complete (99%) at 3.65 minutes. The median time to hemostasis (50%) for ARISTA AH was inferred form this model to be 14.5 minutes with hemostasis likely to be complete (99%) at 33.7 minutes (Supplemental Figure S2).
Time to Hemostasis
Controlling for differences in pre-treatment bleeding level, the median time to hemostasis for MPH was 14.5 minutes and 1.6 minutes for BGP (Supplemental Figure S1). For MPH, the median time to hemostasis was inferred from the AFT model since less than 50% of MPH treated lesions achieved hemostasis within the 10-minute observation period. Likewise, for BGP, the median time to hemostasis was inferred from the AFT model since greater than 50% of BGP treated lesions achieved hemostasis before the 2-minute observation. The ratio for time to hemostasis of MPH relative to BGP was 9.23 (95% CI: 6.99 to 12.19) (). This indicates that BGP achieves significantly faster time to hemostasis (p < 0.001).
DISCUSSION
In this study, BGP were investigated as an alternative powdered hemostatic agent to MPH. BGP were found to be 15.18 times more successful and 9.23 times faster at achieving hemostasis than MPH in an anticoagulated bleeding model. Characterization of the particles comprising these powdered hemostats revealed that the BGP are composed of larger, more irregularly shaped, and more disperse particles than the MPH. These attributes would be expected to allow the BGP particles to pack less regularly than the MPH particles when applied. The non-uniform packing would create voids in which blood could penetrate and clot. Whereas, MPH particles have uniform packing and lack voids for blood to penetrate through. Further, in contrast to BGP which retain their morphology upon hydration, the MPH were found to deform upon hydration. The spherical nature of the MPH were lost and adjacent MPH particles were observed to fuse together. This was observed both in isolation and in tissue explants. Coupled with the tight packing of the MPH, the fusion of adjacent particles could explain why less fibrin matrix was observed within the MPH clots than the BGP clots ().
These properties suggest that the co-affinity of MPH particles could provide mechanical integrity to the clot. As this mechanism requires MPH particles to be in contact with another, it would be disrupted by the presence of fibrin matrix or tissue. In SEM of tissue explants this clot structure was observed. MPH particles aggregate and are not surrounded by similar levels of fibrin or blood cells. Further, voids appear between the MPH aggregation and the liver surface. In contrast, BGP clots show granules surrounded by fibrin and blood cells with uniform attachment to the liver surface.
Thus, these observations suggest a hypothesis that the MPH form a mechanical barrier to blood flow, while the BGP allow blood to interpenetrate. Both powders are hydrated by the surrounding blood to concentrate blood solids with similar swelling of both. However, fibrin matrix is not seen in the mass of MPH particles. Instead, MPH may cause blood to pool behind the material building pressure until the mass of MPH are lifted off the tissue. Indeed, this re-bleeding failure mode was often observed in the bleed model, wherein clots that initially appeared to provide hemostasis ultimately lifted off the bleeding site and allowed blood to flow from underneath.
The instability of these clots is demonstrated in with MPH clots initially showing 65% hemostatic success but dropping to 22% when observed over 10 minutes. In contrast, the BGP clots showed greater stability with initial success of 88% and 78% at 10 minutes. The success and stability of the BGP over the MPH are further supported by the 15-fold greater odds of hemostasis and 9-fold faster rate of hemostatic success.
The performance of BGP in this study was superior to that of MPH. While BGP swell to provide tamponade as demonstrated in the ESEM images, a possible additional mechanism for the difference in performance could be due to molecular recognition of the gelatin. Gelatin has been reported to bind fibronectin to which platelets adhere, spread, and activate via α5β1 (GP Ic-IIa) and αIIbβ3 (GP IIb-IIIa) receptors.Citation33–35 Given the tamponade effect and the ability of gelatin to activate the coagulation cascade, the clot formed by BGP is more robust, with the material allowing blood to percolate through the mass of discrete particles and form fibrin networks that are well-integrated with both the granules and the tissue. This greater clot formation and stability suggests that it may be able to treat higher level bleeds than the low-level bleeds commonly treated by a hemostatic powder.
In contrast, MPH material absorbs tissue fluid to concentrate blood solids;Citation5 it does not contain components that activate the coagulation cascade. Thus, as supported by SEM of treated lesions, the clot formed by MPH lacks fibrin and platelets and has poor integration with tissue. MPH, instead, form an aggregate of melded particles that obstructs blood flow but, ultimately, dislodges and continues to bleed. Thus, impeding MPH's ability to treat bleeds.
A route of further investigation would be to determine if the efficacy of MPH or BGP is dependent on the amount applied.Citation36 Comparisons could be performed between differing application amounts to investigate if additional BGP or MPH provides an incremental benefit over the other material. In this study, equivalent masses of both powders were carefully applied. From a healthcare economics standpoint, addressing the question of per unit efficacy could be informative to surgeons' choice of powdered hemostat. However, the present study demonstrates that with application of equivalent amounts of BGP and MPH, the BGP are superior in terms of speed and efficacy.
We propose that the mechanism of action of the BGP is that in addition to absorbing fluid and concentrating clotting factors and platelets, the BGP activates the coagulation cascade, integrates into a clot, and serves to stabilize the fibrin matrix. The BGP is, therefore, a favorable biomaterial for further research that has advantages over MPH in terms of speed and efficacy. The BGP greatly improves the limited efficacy in low-level bleeds of powdered hemostats.
DECLARATION OF INTERESTS
This study was solely supported by Baxter Healthcare Corporation without influence on the study design; on the collection, analysis, and interpretation of data; on the writing of the manuscript; and on the decision to submit the manuscript for publication. The authors report conflicts of interest. All authors are employees of Baxter Healthcare Corporation. This publication was subject to review by Baxter Healthcare Corpora- tion prior to submission for accuracy and protection of Confidential Information. Baxter Healthcare Corporation may have an interest in intellectual property rights. Studies were designed and performed using sound scientific methods and standardized methods for impartial data collection and comparison. The authors alone are responsible for the content and writing of the article.
Supplemental Video
Download Zip (13.6 MB)ACKNOWLEDGMENTS
The authors thank Greg Dapper and Ed Osawa for sharing their gelatin expertise, Tim Fulghum and Laurie Stojanovic for providing their technical insights, Piotr Maniak and Jennifer Tung for their involvement, Heather Owen for her technical assistance, and Wolfgang Draxler for his statistical assistance.
Additional information
Funding
REFERENCES
- Bracey A, Shander A, Aronson S, Boucher BA, Calcaterra D, Chu MWA, et al. The use of topical hemostatic agents in cardiothoracic surgery. Ann Thorac Surg. 2017;104(1):353–360. doi:10.1016/j.athoracsur.2017.01.096.
- Wysham WZ, Roque DR, Soper JT. Use of topical hemostatic agents in gynecologic surgery. Obstet Gynecol Surv. 2014;69(9):557–563. doi:10.1097/OGX.0000000000000106.
- Kommu SS, McArthur R, Emara AM, Reddy UD, Anderson CJ, Barber NJ, et al. Current status of hemostatic agents and sealants in urologic surgical practice. Rev Urol. 2015;17(3):150–159.
- Wright JD, Ananth CV, Lewin SN, Burke WM, Siddiq Z, Neugut AI, et al. Patterns of use of hemostatic agents in patients undergoing major surgery. J Surg Res. 2014;186(1):458–466. doi:10.1016/j.jss.2013.07.042.
- Murat FJ, Ereth MH, Dong Y, Piedra MP, Gettman MT. Evaluation of microporous polysaccharide hemospheres as a novel hemostatic agent in open partial nephrectomy: Favorable experimental results in the porcine model. J Urol. 2004;172(3):1119–1122. doi:10.1097/01.ju.0000136001.99920.97.
- Ereth MH, Henderson JL, Schrader LM, Nuttall GA, Oliver WC, editors. Efficacy of microporous polysaccharide hemospheres on liver punch biopsies in porcine model. The Anesthesiology Annual Meeting. San Francisco, CA: American Society of Anesthesiologists; 2003.
- Murat FJ, Le CQ, Ereth MH, Piedra MP, Dong Y, Gettman MT. Evaluation of microporous polysaccharide hemospheres for parenchymal hemostasis during laparoscopic partial nephrectomy in the porcine model. Jsls. 2006;10(3):302–306.
- Ersoy G, Kaynak MF, Yilmaz O, Rodoplu U, Maltepe F, Gokmen N. Hemostatic effects of microporous polysaccharide hemosphere in a rat model with severe femoral artery bleeding. Adv Ther. 2007;24(3):485–492. doi:10.1007/BF02848770.
- Humphreys MR, Castle EP, Andrews PE, Gettman MT, Ereth MH. Microporous polysaccharide hemospheres for management of laparoscopic trocar injury to the spleen. Am J Surg. 2008;195(1):99–103. doi:10.1016/j.amjsurg.2007.03.006.
- Ereth MH, Schaff M, Ericson EF, Wetjen NM, Nuttall GA, Oliver WC, Jr. Comparative safety and efficacy of topical hemostatic agents in a rat neurosurgical model. Neurosurgery. 2008;63(4 Suppl 2):369–372; discussion 72.
- Bjorses K, Holst J. Topical haemostatics in renal trauma-an evaluation of four different substances in an experimental setting. J Trauma. 2009;66(3):602–11. doi:10.1097/TA.0b013e3181823533.
- Emmez H, Tonge M, Tokgoz N, Durdag E, Gonul I, Ceviker N. Radiological and histopathological comparison of microporous polysaccharide hemospheres and oxidized regenerated cellulose in the rabbit brain: A study of efficacy and safety. Turk Neurosurg. 2010;20(4):485–491.
- Humphreys MR, Lingeman JE, Terry C, Castle EP, Andrews PE, Gettman MT, et al. Renal injury and the application of polysaccharide hemospheres: A laparoscopic experimental model. J Endourol. 2008;22(6):1375–1381. doi:10.1089/end.2008.0008.
- Morse DC, Silva E, Bartrom J, Young K, Bass EJ, Potter D, et al. Improved bleeding scores using gelfoam((r)) powder with incremental concentrations of bovine thrombin in a swine liver lesion model. J Thromb Thrombolysis. 2016;42(3):352–359. doi:10.1007/s11239-016-1388-6.
- Bruckner BA, Blau LN, Rodriguez L, Suarez EE, Ngo UQ, Reardon MJ, et al. Microporous polysaccharide hemosphere absorbable hemostat use in cardiothoracic surgical procedures. J Cardiothorac Surg. 2014;9:134.brk doi:10.1186/s13019-014-0134-4.
- Bochicchio GV, Gupta N, Porte RJ, Renkens KL, Pattyn P, Topal B, et al. The finish-3 trial: A phase 3, international, randomized, single-blind, controlled trial of topical fibrocaps in intraoperative surgical hemostasis. J Am Coll Surg. 2015;220(1):70–81. doi:10.1016/j.jamcollsurg.2014.09.019.
- Verhoef C, Singla N, Moneta G, Muir W, Rijken A, Lockstadt H, et al. Fibrocaps for surgical hemostasis: Two randomized, controlled phase ii trials. J Surg Res. 2015;194(2):679–687. doi:10.1016/j.jss.2014.12.011.
- Ruitenbeek K, Ayez N, Verhoef C, de Wilt JH, Bottema J, Rijken AM, et al. Safety and efficacy of a novel, dry powder fibrin sealant for hemostasis in hepatic resection. Dig Surg. 2014;31(6):422–427. doi:10.1159/000370006.
- Gupta N, Chetter I, Hayes P, AH OY, Moneta GL, Shenoy S, et al. Randomized trial of a dry-powder, fibrin sealant in vascular procedures. J Vasc Surg. 2015;62(5):1288–1295. doi:10.1016/j.jvs.2015.05.038.
- Schmitz C, Sodian R. Use of a plant-based polysaccharide hemostat for the treatment of sternal bleeding after median sternotomy. J Cardiothorac Surg. 2015;10:59. doi:10.1186/s13019-015-0263-4.
- Antisdel JL, Janney CG, Long JP, Sindwani R. Hemostatic agent microporous polysaccharide hemospheres (mph) does not affect healing or intact sinus mucosa. Laryngoscope. 2008;118(7):1265–1269. doi:10.1097/MLG.0b013e31816c7bc9.
- Schonauer C, Tessitore E, Barbagallo G, Albanese V, Moraci A. The use of local agents: Bone wax, gelatin, collagen, oxidized cellulose. Eur Spine J. 2004;13(Suppl 1):S89–S96. doi:10.1007/s00586-004-0727-z.
- Lewis KM, Atlee HD, Mannone AJ, Dwyer J, Lin L, Goppelt A, et al. Comparison of two gelatin and thrombin combination hemostats in a porcine liver abrasion model. J Invest Surg. 2013;26(3):141–148. doi:10.3109/08941939.2012.724519.
- Oz MC, Cosgrove DM, 3rd, Badduke BR, Hill JD, Flannery MR, Palumbo R, et al. Controlled clinical trial of a novel hemostatic agent in cardiac surgery. The fusion matrix study group. Ann Thorac Surg. 2000;69(5):1376–1382. doi:10.1016/S0003-4975(00)01194-2.
- Council NR. Guide for the Care and Use of Laboratory Animals: Eighth Edition. Washington, DC: The National Academies Press; 2011. 246 p.
- Tripodi A, Mannucci PM. The coagulopathy of chronic liver disease. N Engl J Med. 2011;365(2):147–156. doi:10.1056/NEJMra1011170.
- Jalal DI, Chonchol M, Targher G. Disorders of hemostasis associated with chronic kidney disease. Semin Thromb Hemost. 2010;36(1):34–40. doi:10.1055/s-0030-1248722.
- Mabry CD, Thompson BW, Read RC. Activated clotting time (act) monitoring of intraoperative heparinization in peripheral vascular surgery. Am J Surg. 1979;138(6):894–900. doi:10.1016/0002-9610(79)90318-0.
- Adams GL, Manson RJ, Hasselblad V, Shaw LK, Lawson JH. Acute in-vivo evaluation of bleeding with gelfoam plus saline and gelfoam plus human thrombin using a liver square lesion model in swine. J Thromb Thrombolysis. 2009;28(1):1–5. doi:10.1007/s11239-008-0249-3.
- Lewis KM, Li Q, Jones DS, Corrales JD, Du H, Spiess PE, et al. Development and validation of an intraoperative bleeding severity scale for use in clinical studies of hemostatic agents. Surgery. 2017;161(3):771–781. doi:10.1016/j.surg.2016.09.022.
- Team RC. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2017.
- Therneau TM. A Package for Survival Analysis in s. R package version 2.38. Vienna, Austria: R Foundation for Statistical Computing; 2015.
- Winters KJ, Walsh JJ, Rubin BG, Santoro SA. Platelet interactions with fibronectin: Divalent cation-independent platelet adhesion to the gelatin-binding domain of fibronectin. Blood. 1993;81(7):1778–1786.
- Xu J, Mosher D. Fibronectin and other adhesive glycoproteins. In: Mecham R, editor. The Extracellular Matrix: An Overview. Berlin, Springer Science & Business Media; 2011. pp. 41–75.
- Engvall E, Ruoslahti E, Miller EJ. Affinity of fibronectin to collagens of different genetic types and to fibrinogen. J Exp Med. 1978;147(6):1584–1595. doi:10.1084/jem.147.6.1584.
- MacDonald MH, Wang AY, Clymer JW, Hutchinson RW, Kocharian R. An in vivo comparison of the efficacy of hemostatic powders, using two porcine bleeding models. Med Devices (Auckl). 2017;10(1):273–279.