Abstract
Objective
To compare the recurrence rate and prognosis between minimally invasive surgical (MIS) approach and open surgical approach of endometrial carcinoma (EC) patients with different prognostic risk groups.
Methods
A retrospective analysis of all cases undergoing EC surgery between January 2011 and March 2018 was performed. The patients were grouped according to the management guidelines of EC patients jointly formulated by the ESGO/ESTRO/ESP 2020. Different surgical approaches were compared with regard to tumor characteristics, recurrence rate, disease-free survival (DFS), and overall survival (OS).
Results
A total of 665 patients met the inclusion criteria of which 196 patients underwent MIS (29.5%), and 469 patients underwent open surgery (70.5%). In the MIS group, there was a significant higher rate of recurrence (17.3% vs 6.6%, P = 0.000) compared to the open surgery group. The recurrence rate of MIS was 7.7% (P = 0.000) in the medium-high risk group and 8.2% (P = 0.014) in the high-risk group. Multivariate logistic regression analysis showed that the independent factors influencing recurrence included prognostic risk grouping, surgical approach and lymph vascular space invasion (LVSI) positivity (P < 0.05). K-M survival analysis revealed that in the intermediate and high-risk group of EC, MIS patients had a significantly lower DFS than those undergoing open surgery (P < 0.05), but no significant difference was found in OS.
Conclusions
For patients with EC at moderate and high prognostic risk, MIS is associated with poorer DFS compared to open surgery, but OS was similar across prognostic risk groups. The application of MIS in patients with moderate and high-risk EC needs further research and analysis.
Introduction
The most common malignant tumor in women in developed countries is endometrial carcinoma (EC). Globally, 417,367 new cases of uterine corpus cancer were reported in 2020. The mortality rate associated with EC is about 1.7–2.4 per 100,000 [Citation1, Citation2]. North America and Europe have a 10-fold higher incidence rate of EC than underdeveloped countries, and it is the main cause of reproductive tract cancer-related death among women residing in Europe and the United States [Citation3]. As a result of high-fat, high-calorie diets and sedentary lifestyles, the incidence rate of EC is also increasing every year in Asia, as is the number of women of childbearing age [Citation2]. The main risk factors for EC include obesity, low exercise, insulin resistance, excessive estrogen, and endometriosis [Citation3]. According to guidelines of the European Society of Gynecological Oncology (ESGO), the European Society of Radiation Oncology (ESTRO), and the European Pathological Society (ESP), EC patients were grouped into different prognostic risks groups based on their postoperative pathological conditions. These groups include low-risk, intermediate-risk, intermediate-high-risk, high-risk, and advanced metastasis groups, to determine whether adjuvant treatments are needed, to reduce the recurrence risk of EC [Citation4]. In the early stages of EC, surgery is the main treatment. The National Comprehensive Cancer Network (NCCN) [Citation5] indicates that both minimally invasive surgery (MIS) and open surgery are available as treatment options; however, there is a lack of information about the surgical approaches for intermediate, intermediate-high risk, and high-risk groups.
MIS has many advantages, such as a small incision, less intraoperative bleeding, and faster postoperative recovery. A large number of studies have shown that MIS does not impair recurrence and overall survival in the low-risk group [Citation6, Citation7]. The Laparoscopic Approach to Cervical Cancer (LACC), published in November 2018, showed different results in cervical cancer, that is, patients who underwent MIS had lower DFS and OS rates than patients who underwent open surgery, which greatly challenged the viability of MIS [Citation8]. The LACC trial reopens the debate about the surgical approach for EC, especially for EC patients of intermediate risk, intermediate-high risk, and high-risk categories.
This study aims to compare the effects of different surgical approaches on postoperative recurrence and estimate of overall survival of EC patients with different prognostic risks.
Materials and Methods
Ethical Considerations
This retrospective study was approved by the institutional review board of the Ethics Committee of Fujian Medical Cancer Hospital (K2022-064-01) and adhered to the Helsinki Declaration.
Study Population
All patients who underwent primary surgery between January 2011 and March 2018 were included. During surgery, all patients undergo hysterectomy (radical, modified radical, extra fascial) as well as sentinel lymph node biopsy and pelvic lymph node dissection, paraaortic lymph node dissection. All patients were categorized into two groups viz. MIS (N = 196) and open surgery (N = 469) group. To ensure the operation quality, all the operations included in this study were performed by experienced gynecological oncologists (1) Each surgeon had performed at least 100 operations on malignant tumors (including cervical cancer, EC, and ovarian cancer) every year, (2) no less than 50 MIS operations annually, for at least 3 years. We excluded patients with recurrent EC, distant metastases, palliative surgery, preoperative neoadjuvant chemotherapy or preoperative radiotherapy, and loss of follow-up information.
Study Design and Instruments
The clinical and pathological parameters were retrieved from the electronic medical record system. The included parameters are age, BMI, comorbidity, surgical approach, histopathological type, FIGO stage 2009, grade, tumor size, cervical interstitial involvement, depth of muscularis invasion, LVSI, lymph node status, and postoperative adjuvant therapy and prognostic risk defined by ESGO/ESTRO/ESP2020. DFS is defined as the time from the first treatment to the first progression or the last follow-up; OS is defined as the time from the first treatment to disease-related death or the last follow-up. Outpatient follow-up records were used to determine the recurrence time and location, and after-treatment recurrences were also calculated.
Data and Statistical Analyses
In order to describe the clinical characteristics of the patients, a descriptive statistical method was used. For normally distributed data, mean + standard deviation was used, while the median (range) was used for non-normally distributed data. Chi-square tests or Fisher precise tests were used to compare the frequency distributions among classification variables. In addition, univariate analysis was performed to compare all patient’s data in the MIS group and all patients in the open surgery group. Stepwise (forward: LR) multivariate logistic regression analysis was used to evaluate the factors influencing the recurrence of EC. The OS and DFS of patients grouped according to prognostic risk were compared using the Kaplan-Meier method and log-rank test. The statistical analyses were all performed with SPSS (Version 25.0) and GraphPad Prism 8, and P values < 0.05 were considered statistically significant. The predictive nomograms were established by R language.
Results
A total of 751 patients underwent surgery for EC during the study period, of which 665 patients met the inclusion criteria as per the conditions followed in the study. Among them, 196 patients underwent MIS (29.5%), and 469 patients underwent open surgery (70.5%), with an average age of (53.06 ± 7.76) years. Most of the patients had endometrioid adenocarcinoma (85.4%), 523 patients underwent radical hysterectomy (78.6%), and 142 patients underwent extra fascial hysterectomy (21.4%). Over time, the number of minimally invasive surgeries has increased. In comparison to the MIS group, the open surgery group had a higher BMI (P = 0.016). Compared with the MIS group, the open surgery group had less myometrial infiltration, and only the endometrial infiltration was more than that in the MIS group (12.6% vs 7.7%). Furthermore, the rate of lymph node resection in the MIS group was significantly higher (92.8% vs 83.1%), along with this paraaortic lymph node resection rates were also found to be significantly higher than those in the open group (15.8% vs 3.8%), respectively. In terms of postoperative adjuvant therapy, we found that MIS patients were more likely to suffer from this problem than open surgery patients. In , we summarize the remaining clinical features that did not show any statistical significance.
Table 1. Clinopathological characteristics of patients.
In addition, a higher recurrence rate was observed in the MIS group compared to in the open surgery group (17.3% vs 6.6%) (P = 0.000). There was no significant difference between the low-risk group and the medium-risk group (P = 0.979 and 0.723) among the different prognostic risk groups. However, recurrence rates increased significantly in the MIS group compared to the open surgery group in the moderate-to-high-risk and high-risk groups (P = 0.000 and 0.014), and the rate of distant and lymph node recurrence was significantly higher in the MIS group than in the open surgery group (P = 0.019 and 0.003) (, , Citation2). Likewise, the median follow-up time for the two groups was 62 and 80 months, respectively ().
Figure 1. Recurrence rates of patients with different prognostic risk groups. (A) The MIS group. (B) The open surgery group.
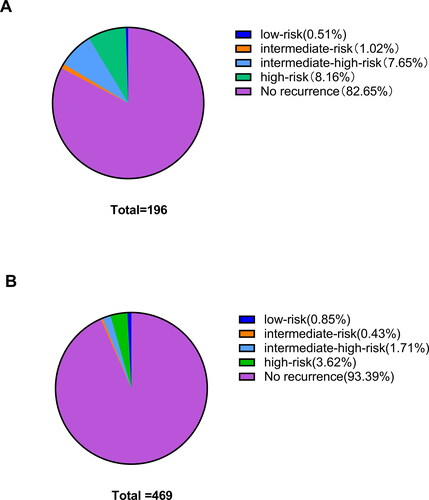
Figure 2. Recurrences site of patients with different surgical approaches. (A) The MIS group. (B) The open surgery group.
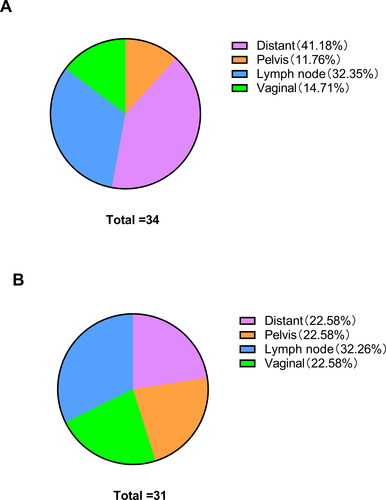
Table 2. Oncologic outcomes of patients.
displays the possible factors affecting the recurrence, including prognostic risk grouping, FIGO stage, surgical approach, depth of myometrial invasion, Lymph node sampling, grade, LVSI, LN metastasis, cervical stromal invasion depth, radiotherapy, and chemotherapy (P < 0.05).
Table 3. Univariate analysis of influence on recurrence.
Table 4. Multivariate logistic regression analysis of recurrence.
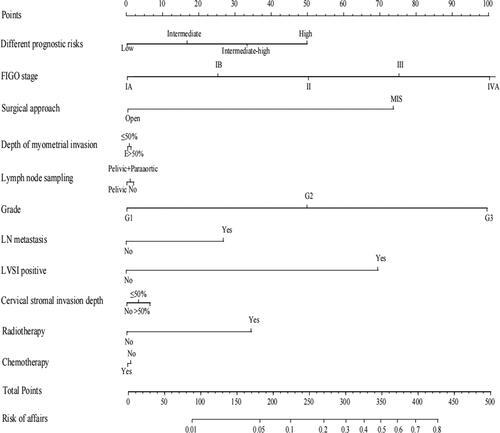
In , recurrence (Yes/No) was taken as a dependent variable, and the prognostic risk was grouped by significant indicators in univariate analysis (low-risk/intermediate-risk/intermediate-high-risk/high-risk), FIGO stage (Ia/Ib/II/III/IVA), surgical approach (MIS/Open), depth of myometrial invasions (≥50%/<50%/E), lymph node sampling (Pelvic/Pelvic + Paraaortic/NO), grade (G1/G2/G3), LN metastasis (No/Yes), LVSI positive (positive/negative), cervical stromal invasion depth (No/<50%/≥50%), radiotherapy (No/Yes), chemotherapy (Yes/No) were used as an independent variable. Stepwise analysis of multivariate logistic regression was conducted (forward: LR). The results indicated that prognostic risk grouping, surgical approach, and LVSI status were independent predictors of relapse (P < 0.05). In prognostic risk grouping, the high-risk group was used as a reference, revealing that the low-risk and intermediate-risk groups had significantly lower recurrence rates (P < 0.05). A low-risk group’s recurrence probability of EC is 0.081 times greater than that of a high-risk group, and an intermediate-risk group is 0.103 times more than that of a high-risk group. In addition, MIS patients faced a 3.893 times greater risk of recurrence than those undergoing open surgery (P < 0.05). Furthermore, negative LVSI demonstrated a 0.245 times risk of recurrence compared to positive LVSI (P < 0.05).We made nomograms to present the prediction models ().
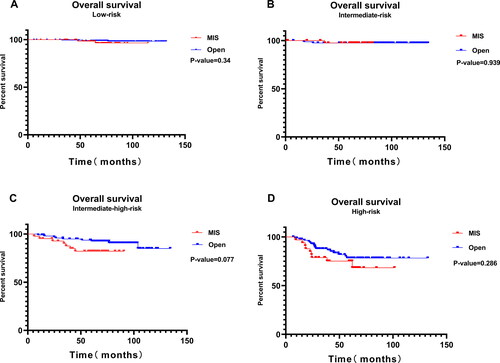
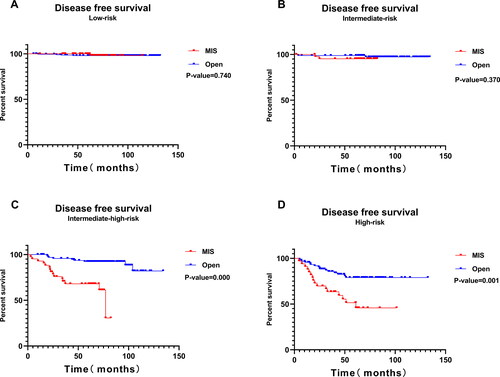
and Citation5 show the K-M survival analysis of the MIS and the open surgery groups across different studied prognostic risk groups. OS did not differ significantly between the two groups among different prognostic risk groups (P > 0.05). There was no significant difference observed in DFS between the low-risk group and intermediate-risk group (P > 0.05). However, the DFS in the MIS group was significantly lower than in the open surgery group for intermediate-high-risk and high-risk patients, respectively (P < 0.05).
Discussion
This retrospective study evaluated the clinical characteristics and pathological results of EC patients who underwent either minimally invasive surgery or open surgery; and for the first time, recurrence rates of EC patients were compared based on the prognostic risk factors formulated by ESGO/ESTRO/ESP 2020. In all the included patients, the rate of recurrence was significantly higher in the MIS group than in the open surgery group, especially in patients with intermediate or higher prognostic risk. MIS was associated with poor DFS in the intermediate-high risk and high-risk groups. On the other hand, there was no evidence that MIS adversely affected OS. The results of this study are in line with those of some previous studies [Citation9, Citation10].
At present, many studies reported the benefits of MIS in early EC which include less intraoperative blood loss, less postoperative pain, less postoperative complications, faster postoperative recovery of daily activities, shorter hospital stay, and better postoperative quality of life for patients [Citation11–13]. Most importantly, in terms of DFS or OS, there are no significant differences between MIS and open surgery [Citation6, Citation7]. The LACE study published by Janda et al. in JAMA in 2017 is a randomized controlled multicenter study, which included 760 early EC patients who received MIS or open hysterectomy treatment. It was found that disease-free survival was 81.3% in TAH and 81.6% in TLH over a 4.5-year follow-up, and there was no difference in overall survival rate. As a result, a laparoscopic hysterectomy is a viable option for patients with stage I endometrial cancer. In addition, in the LAP2 trial, Walker et al. randomly assigned clinical stage I to IIA patients to MIS and open surgery groups at a 2: 1 ratio, and found that both groups had the same 5-year overall survival rate of 89.8%. The results are consistent with those found in our study of low-risk EC patients. The definition of prognostic risk, the research objects, and the conclusions differ for EC above the intermediate prognostic risk group Minimally invasive treatment methods for patients with early high-grade EC had similar survival rates and fewer complications than laparotomies, according to a published study [Citation14]. In another study of 229 patients with uterine-confined disease (stage IA disease with high-grade tumors or stage IB and II disease), no significant difference in DFS and OS was observed between the MIS and open surgery groups [Citation15]. Kim et al. defined stage I B and stage II EC as moderate-risk groups, including 206 patients. In the stage, IB EC patients, recurrence rate, DFS, and OS were not significantly different between MIS and open surgery patients. However, patients undergoing MIS experienced a significantly lower disease-free survival rate than those undergoing open surgery for stage II EC (P = 0.012) [Citation16]. For these studies, mixed factors such as pathological type, LVSI status, and postoperative adjuvant therapy may lead to poor recurrence rate and survival rate, thus masking the effect of the surgical approach on survival rate. Therefore, patients with similar prognostic risks provide a better comparison for studying the effect of the surgical approach on recurrence risk. Therefore, in our study, the patients were grouped based on the prognostic risk factors formulated by ESGO/ESTRO/ESP in 2020; few unfavorable uterine risk factors were incorporated, reducing the influence of these factors on survival outcomes.
Our data show that the median follow-up time of MIS is significantly shorter than that of open surgery, indicating that the number of MIS operations has been increasing over time. The overall recurrence rate in our study cohort was slightly higher compared to the stage I-II EC statistics in previous studies [Citation6, Citation7, Citation17], possibly due to the inclusion of patients with non-endometrioid histology and FIGO stage III in our study. However, in terms of prognostic risk grouping, there was no substantial difference found between the MIS and the open group. Therefore, we can conclude that the uterine high-risk factors do not affect the recurrence risk between the two groups. The MIS group had a slightly higher depth of myometrial invasion than the open surgery group (39.3% vs. 31.1%), which may have led to a higher postoperative radiotherapy rate in the MIS group (38.3% vs. 27.1%), but this cannot account for the higher rate of recurrence in the MIS group than in the open surgery group. The results indicate that the recurrence rate of the low-risk group was similar between the MIS group and the open surgery group; however, for patients with moderate prognostic risk and above, the MIS group had a significantly higher recurrence rate than patients with open surgery. Further, patients with intermediate-to-high-risk or high-risk prognoses had a poorer DFS in the MIS group compared to the open surgery group. We then analyzed whether the difference between recurrence rate and DFS could be explained by the following factors. First, the safety of uterine manipulators is controversial. Previous LACC studies on cervical cancer [Citation8] have raised the discussion about whether MIS is beneficial to gynecological malignant tumors, and exploratory studies on the safety of uterine manipulators have been carried out in some early CC studies [Citation18, Citation19]. The uterine manipulator may cause malignant tumor cells to spread through blood vessels and lymphatic vessels, resulting in tumor metastasis and recurrence. LVSI status has been proved to be an important factor in poor prognosis in EC [Citation20–22]. Additionally, LVSI status was found to be an independent factor influencing EC recurrence, so it is worth considering whether the use of uterine manipulators in EC surgery is associated with a higher incidence of LVSI. Studies conducted by Iserte et al. also reported that the use of uterine manipulators has been associated with poor tumor outcomes in EC patients undergoing minimally invasive surgery [Citation23]. There have been some MIS methods used in early CC without uterine manipulators [Citation24, Citation25]. It is possible that these methods can be applied to EC surgery too. Secondly, the pneumoperitoneum using carbon dioxide in MIS and the associated pneumoperitoneum pressure may cause tumor cells to diffuse in the abdomen and pelvic cavity. As early as 1999, Volz and coworkers have found in animal experiments that pneumoperitoneum can damage the peritoneum, thus inducing the growth of melanoma cells in the peritoneum [Citation26]. Under low pressure (4 mmHg) heat humidification conditions with standard CO2, Gunusen et al. found inflammation, DNA damage, oxidative stress, and histopathological changes in the ovaries and peritoneum of female rats [Citation27]. However, these studies are limited to animals, and further prospective studies are needed to clarify the effects of Carbon dioxide pneumoperitoneum on gynecological malignant tumors. There are also a few new and improved MIS techniques being performed to treat malignant tumors, such as Vaginal-assisted gasless, low-pressure humidified pneumoperitoneum, etc. However, these new techniques require higher surgical skills. Finally, in vivo vaginal resection in step Trendelenburg position during MIS may also lead to tumor overflow to the abdominal and pelvic cavity, which is more common in EC patients with cervical involvement. Preliminary prospective studies with small samples have found that the presence of tumor cells in the vagina during surgery may lead to vaginal recurrence in patients with EC [Citation28]. Interestingly, our data indicate that the rate of vaginal recurrence after EC is significantly lower than in previous studies, which may be attributed to adequate vaginal preparation before operation and the higher rate of radical hysterectomy (RH) surgery to remove longer vaginal tissue. Although there is evidence that RH does not improve the overall survival rate of early EC, it can reduce the rate of vaginal recurrence [Citation29].
For the first time, the prognostic risk plan formulated by ESGO/ESTRO/ESP in 2020 was used to integrate the clinical disease characteristics of EC patients for comparative analysis. This reduced the influence of uterine risk factors and allows a focused comparison of different surgical approaches in medium-risk and high-risk EC groups. In contrast, the rate of LN resection or dissection in the open surgery group was lower than in the MIS group (7.1% vs. 16.4%), which may have led to missing the stage III diagnosis after surgery. By performing a proper preoperative imaging evaluation and following up postoperatively, this risk can be minimized. In contrast, our study included a large number of participants. After excluding tumor recurrence, advanced metastasis, and lost follow-up samples, the data showed that the study patients were evenly distributed among different prognostic risk groups, and the types of hysterology between the groups did not differ significantly. More than 80% of lymph nodes were sampled, ensuring as much consistency as possible in the study samples.
We must, however, acknowledge some limitations in our study. Firstly, this is a retrospective study and selection bias is inevitable. (1) Despite a large number of samples included, the pathology types of most cases are endometrioid adenocarcinoma (85.4%), and selection bias is inevitable. (2) Patients were not randomly divided into MIS or open groups, as that is not ethically feasible. (3) Patient information was collected retrospectively. Secondly, much research shows that the molecular subgroup is the prognostic biomarker and therapy target for EC patients. The OS and PFS varied significantly in the different molecular subgroups. POLEmut EC has the best and p53abn EC has the worst prognosis [Citation30–32]. Since the medical insurance didn’t cover the testing costs for molecular subtypes, not all patients had this examination done, and this part of the information was incomplete. Thus, the information about EC patients’ The Cancer Genome Atlas (TCGA) molecular subtypes [Citation33] and Endometrial Cancer (ProMisE) molecular classification system [Citation30] was excluded. We expect to include a sufficient number of patients with genetic testing in our research in the near future. Thirdly, the median follow-up time in the MIS group is shorter, which limits the evaluation of long-term tumor safety, and thus a longer follow-up time is needed to assess OS results. Fourth, obesity, low exercise, insulin resistance, excessive estrogen, and endometriosis are all potential risk factors for EC. Despite many studies have shown that the increase in BMI and obesity were two potential risk factors risk factors of endometrial carcinogenesis, whether they were the risk factors for recurrence remains controversial, the evidence is weak and the potential mechanism is also unclear. In our study, we also observed that BMI and comorbidity didn’t affect the recurrence of EC patients. Furthermore, univariate Cox regression analyses indicated that comorbidity was not a independent prognostic risk factor of DFS and OS (DFS: P = 0.468) (OS: P = 0.864). So as BMI (DFS: P = 0.996) (OS: P = 0.986). In order to understand the each comorbidity role clearly, such as excess body fat, diabetes, and hypertension. It is better to list them separately. Finally, the complication rate of different surgical approaches was not investigated, which may have an impact on DFS and OS.
In spite of the limitations of our study, the results suggest that patients with poor prognostic risk have worse DFS with MIS than with open surgery. Clinicians and patients must weigh the advantages and disadvantages of different surgical approaches on the basis of preoperative evaluation, including imaging analysis, vaginal examination, and multidisciplinary discussion (MDT) when necessary. The purpose of our study is to optimize and utilize evidence-based, risk-adaptive clinical treatment methods to treat EC patients, in order to avoid unnecessary recurrence risk. Of course, the advantages of MIS cannot be underestimated. MIS is the optimal surgical method for EC patients in the low-risk group. The results of this research should be further confirmed with a multi-center prospective study. Next, we plan to collect the gene detection data to explore the recurrence and survival differences among EC groups with different prognostic risks integrated with molecular typing.
Author Contributions
Bin Liu and Jie Lin contribute equally to this work. Bin Liu, Jie Lin and Wei Chen contributed to the study conception and design, drafting of the manuscript and analysis and interpretation of the data. Wenju Liu, Weitin Chen and Wanzhen Lin contributed to the acquisition of the data, interpretation of the analysis results, and critical revision of the manuscript for important intellectual content. Bin Liu is responsible for the overall content as the guarantor. All authors read and approved the final manuscript.
Acknowledgments
We thank all the staff in Department of Gynecology, Affiliated Fujian Cancer Hospital, Clinical Oncology School of Fujian Medical University for their contribution on our research.
Disclosure statement
No potential competing interest was reported by the author(s).
Data availability statement
Data are available upon reasonable request. The data that supporting the findings of this study is available from the corresponding author upon reasonable request.
Additional information
Funding
References
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660.
- Aimagambetova G, Terzic S, Lagana AS, Bapayeva G, la Fleur P, Terzic M. Contemporary fertility-sparing management options of early stage endometrioid endometrial cancer in young nulliparous patients. JCM. 2021;11(1):196. doi:10.3390/jcm11010196.
- Terzic M, Aimagambetova G, Kunz J, et al. Molecular Basis of endometriosis and endometrial cancer: Current knowledge and future perspectives. IJMS. 2021;22(17):9274. doi:10.3390/ijms22179274.
- Concin N, Creutzberg CL, Vergote L, et al. ESGO/ESTRO/ESP guidelines for the management of patients with endometrial carcinoma. Int J Gynecol Cancer. 2021;31(1):12–39. doi:10.1136/ijgc-2020-002230.
- Abu-Rustum NR, Yashar CM, Bradley K, et al. NCCN Guidelines(R) Insights: Uterine Neoplasms, Version 3.2021. J Natl Compr Canc Netw. 2021;19(8):888–895. doi:10.6004/jnccn.2021.0038.
- Janda M, Gebski V, Davies LC, et al. Effect of total laparoscopic hysterectomy vs total abdominal hysterectomy on disease-free survival among women with stage i endometrial cancer: A randomized clinical trial. JAMA. 2017;317(12):1224–1233. doi:10.1001/jama.2017.2068.
- Walker JL, Piedmonte MR, Spirtos NM, et al. Recurrence and survival after random assignment to laparoscopy versus laparotomy for comprehensive surgical staging of uterine cancer: Gynecologic Oncology Group LAP2 Study. J Clin Oncol. 2012;30(7):695–700. doi:10.1200/JCO.2011.38.8645.
- Ramirez PT, Frumovitz M, Pareja R, et al. Minimally invasive versus abdominal radical hysterectomy for cervical cancer. N Engl J Med. 2018;379(20):1895–1904. doi:10.1056/NEJMoa1806395.
- Galaal K, Donkers H, Bryant A, Lopes AD. Laparoscopy versus laparotomy for the management of early stage endometrial cancer. Cochrane Database Syst Rev. 2018;10:CD006655. doi:10.1002/14651858.CD006655.pub3.
- Nasioudis D, Frey MK, Chapman-Davis E, Caputo TA, Holcomb K. Outcomes of minimally invasive surgery for patients with endometrial carcinoma involving the cervix. Int J Gynecol Cancer. 2020;30(5):619–625. doi:10.1136/ijgc-2019-001023.
- Miguel L, Silva JCR, Poli Neto OB, et al. A propensity score-matched case-control study of laparoscopy and laparotomy for endometrial cancer. Rev Assoc Med Bras. 2021;67(5):753–758. doi:10.1590/1806-9282.20210194.
- Kotani Y, Murakami K, Fujishima R, et al. Research article quality of life after laparoscopic hysterectomy versus abdominal hysterectomy. BMC Womens Health. 2021;21(1):219. doi:10.1186/s12905-021-01364-8.
- Mourits MJ, Bijen CB, Arts HJ, et al. Safety of laparoscopy versus laparotomy in early-stage endometrial cancer: a randomised trial. Lancet Oncol. 2010;11(8):763–771. doi:10.1016/S1470-2045(10)70143-1.
- Nasioudis D, Heyward QD, Haggerty AF, et al. Surgical and oncologic outcomes of minimally invasive surgery for stage I high-grade endometrial cancer. Surg Oncol. 2020;34:7–12. doi:10.1016/j.suronc.2020.02.015.
- Tanaka T, Ueda S, Miyamoto S, et al. Oncologic outcomes for patients with endometrial cancer who received minimally invasive surgery: a retrospective observational study. Int J Clin Oncol. 2020;25(11):1985–1994. doi:10.1007/s10147-020-01744-4.
- Kim SI, Park DC, Lee SJ, et al. Survival rates of patients who undergo minimally invasive surgery for endometrial cancer with cervical involvement. Int J Med Sci. 2021;18(10):2204–2208. doi:10.7150/ijms.55026.
- Sasada S, Yunokawa M, Takehara Y, et al. Baseline risk of recurrence in stage I-II endometrial carcinoma. J Gynecol Oncol. 2018;29(1):e9. doi:10.3802/jgo.2018.29.e9.
- Chiva L, Zanagnolo V, Querleu D, et al. SUCCOR study: an international European cohort observational study comparing minimally invasive surgery versus open abdominal radical hysterectomy in patients with stage IB1 cervical cancer. Int J Gynecol Cancer. 2020;30(9):1269–1277. doi:10.1136/ijgc-2020-001506.
- Kanao H, Matsuo K, Aoki Y, et al. Feasibility and outcome of total laparoscopic radical hysterectomy with no-look no-touch technique for FIGO IB1 cervical cancer. J Gynecol Oncol. 2019;30(3):e71. doi:10.3802/jgo.2019.30.e71.
- Barnes EA, Martell K, Parra-Herran C, Taggar AS, Donovan E, Leung E. Substantial lymphovascular space invasion predicts worse outcomes in early-stage endometrioid endometrial cancer. Brachytherapy. 2021;20(3):527–535. doi:10.1016/j.brachy.2020.12.006.
- Jaishankar S, Pifer PM, Bhargava R, et al. Is substantial lymphovascular space invasion prognostic for clinical outcomes in type II endometrial cancer? Clin Oncol (R Coll Radiol). 2022;34(7):452–458. doi:10.1016/j.clon.2022.02.018.
- Dou Y, Song K, Fu Y, et al. Risk factors and prognosis of early recurrence in stage I-II endometrial cancer: A large-scale, multi-center, and retrospective study. Front Med (Lausanne). 2022;9:808037. doi:10.3389/fmed.2022.808037.
- Padilla-Iserte P, Lago V, Tauste C, et al. Impact of uterine manipulator on oncological outcome in endometrial cancer surgery. Am J Obstet Gynecol. 2021;224(1):65 e1–65 e11. doi:10.1016/j.ajog.2020.07.025.
- Chen S, Zheng Y, Tong L, Zhao X, Chen L, Wang Y. Laparoendoscopic single-site radical hysterectomy with vaginal closure and without uterine manipulator for FIGO IB1 cervical cancer. J Minim Invasive Gynecol. 2020;27(7):1471–1472. doi:10.1016/j.jmig.2020.01.003.
- Ding B, Guan X, Duan K, Shen Y. Laparoscopic radical hysterectomy with enclosed colpotomy without the use of uterine manipulator for early-stage cervical cancer. J Minim Access Surg. 2021;17(4):570–572. doi:10.4103/jmas.JMAS_146_20.
- Volz J, Köster S, Spacek Z, Paweletz N. The influence of pneumoperitoneum used in laparoscopic surgery on an intraabdominal tumor growth. Cancer. 1999;86(5):770–774. doi:10.1002/(sici)1097-0142(19990901)86:5 < 770::aid-cncr11 > 3.0.co;2-3.
- Gunusen I, Akdemir A, Gurel C, et al. The effects of different pressure pneumoperitoneum models created by standard or heated-humidified CO2 insufflation on ovary and peritoneum: an experimental study in rats. Reprod Sci. 2022;29(4):1197–1208. doi:10.1007/s43032-022-00878-2.
- Stolnicu S, Terinte C, Ioanid N, Silva E. Presence of tumor cells in the vagina during surgical treatment could be the source of vaginal recurrence in patients with endometrial carcinoma - A pilot prospective study. Ann Diagn Pathol. 2020;46:151503. doi:10.1016/j.anndiagpath.2020.151503.
- Nasioudis D, Sakamuri S, Ko EM, et al. Radical hysterectomy is not associated with a survival benefit for patients with stage II endometrial carcinoma. Gynecol Oncol. 2020;157(2):335–339. doi:10.1016/j.ygyno.2020.02.003.
- Talhouk A, McConechy MK, Leung S, et al. A clinically applicable molecular-based classification for endometrial cancers. Br J Cancer. 2015;113(2):299–310. doi:10.1038/bjc.2015.190.
- Leon-Castillo A, Horeweg N, Peters EEM, et al. Prognostic relevance of the molecular classification in high-grade endometrial cancer for patients staged by lymphadenectomy and without adjuvant treatment. Gynecol Oncol. 2022;164(3):577–586. doi:10.1016/j.ygyno.2022.01.007.
- Cosgrove CM, Tritchler DL, Cohn DE, et al. An NRG Oncology/GOG study of molecular classification for risk prediction in endometrioid endometrial cancer. Gynecol Oncol. 2018;148(1):174–180. doi:10.1016/j.ygyno.2017.10.037.
- Kandoth, C, Schultz, N, Cherniack, AD, et al. Integrated genomic characterization of endometrial carcinoma. Nature. 2013;497(7447):67–73. doi:10.1038/nature12113.