Abstract
Background/aims
Sepsis is one of the major problems encountered in intensive care units, causing organ damage and increasing mortality. Suberosin (SBR) is a type of coumarin with antioxidant and anti-inflammatory activities. The goal of this study is to explore the protective effects of SBR on the lungs in a rat model of sepsis.
Methods
Male Wistar rats were utilized in this study. A cecal ligation and puncture (CLP) model was applied to induce sepsis. Rats were separated into six groups with nine animals in each group, including healthy control, SBR, CLP, and CLP + SBR (5, 10, and 20 mg/kg) groups. Superoxide dismutase (SOD), glutathione (GSH) enzyme activities, and malondialdehyde (MDA) level were measured via enzyme-linked immunosorbent assay (ELISA). The messenger RNA (mRNA) expressions of tumor necrosis factor α (TNF-α) and interleukin 1β (IL-1β) were evaluated by real-time polymerase chain reaction (RT-PCR). Histopathological changes in the lungs were investigated with hematoxylin and eosin (H&E).
Results
MDA levels and GSH and SOD enzyme activities were negatively affected in the CLP group, but SBR treatment ameliorated these oxidative stress parameters in the SBR1-3 groups (p< 0.05). The mRNA expressions of TNF-α and IL-1β were increased in the CLP group, and SBR treatment decreased those expression levels in a dose-dependent manner (p < 0.05). Organ damage and necrosis were seen in the CLP group and were alleviated in the SBR3 group. Immunohistochemical (IHC) analysis of lung tissues demonstrated decreased TNF-α and IL-1β immunopositivity in the SBR1-3 groups (p< 0.05).
Conclusions
SBR ameliorated sepsis-related lung injury in a dose-dependent manner. This compound has significant potential as a future agent in the treatment of sepsis.
1. Introduction
Sepsis, also often referred to as systemic inflammatory response syndrome (SIRS), is caused by bacteria or other factors that initiate host immune responses [Citation1]. Acute respiratory distress syndrome (ARDS), which has been considered to be a result of sepsis [Citation2], is characterized by alveolar barrier injury caused by many different events, including the excessive release of proinflammatory cytokines such as tumor necrosis factor α (TNF-α) or interleukin 1β (IL-1β) [Citation3]. These excessive cytokines or chemokines attract macrophages and neutrophils to the infected site and then secrete cytotoxic reactive oxygen species (ROS), consequently making the immune response more severe [Citation4,Citation5]. The presence of infectious factors, prevention of phagocytosis, or acquired resistance to antibiotics may make the host response to infection insufficient. This can cause cytokine storms in patients with sepsis [Citation6]. Cytokines including TNF-α and IL-1β regulate the early phase of the immune response [Citation3].
Despite impressive scientific advances, sepsis-related organ damage and failure still cause mortality in intensive care units. Oxidative stress contributes to inflammation and thus tissue destruction, influencing the course of sepsis cases negatively [Citation7,Citation8]. Under normal conditions, oxidants and antioxidant scavengers are balanced [Citation9], but this changes when the oxidant concentration in the cells increases and disrupts the functioning of various organs [Citation9]. Abnormal oxidant production is also an essential factor in sepsis [Citation10]. In recent studies of animal models, the sepsis-related alteration of oxidative stress markers was shown [Citation7]. Decreased superoxide dismutase (SOD) and glutathione (GSH) enzyme activities and increased malondialdehyde (MDA) levels are used as markers of sepsis [Citation11].
The genus Ferulago W.D.J. Koch of the family Apiaceae has long been used in treatments for ulcers, headaches, and spleen diseases in Asian folk medicine [Citation12,Citation13]. Furthermore, Ferulago species are known for their antimicrobial [Citation14], cytotoxic, immunomodulatory [Citation15], and antioxidant [Citation14] effects. Coumarins, plant-derived secondary metabolites, are crucial pharmaceutical agents as a result of their therapeutic effects on many diseases and disorders including sepsis [Citation8]. Coumarins isolated from Ferulago cassia Boiss. & Heldr. (Apiaceae), including suberosin (SBR), exert biological activities in cells [Citation16]. In a previous study, human umbilical vein endothelial cells (HUVECs) exposed to bacterial lipopolysaccharide (LPS) were used to explore the effects of F. cassia extract. It was concluded that F. cassia time-dependently reduced the antiproliferative effects of LPS in HUVECs at 24, 48, and 72 h [Citation17].
Sepsis remains a major problem in intensive care units, causing multiple organ failure. There is a need to develop therapeutic agents with fewer side effects to reduce the damage caused by sepsis. Various biological agents have been investigated to suppress that damage [Citation7,Citation8]. However, SBR isolated from F. cassia is not yet among them. We hypothesized that, due to the antimicrobial and antioxidant effects of Ferulago species, SBR could reduce sepsis-induced lung injury. We investigated the effects of SBR on sepsis-induced lung damage in a rat model of cecal ligation and puncture (CLP).
2. Materials and methods
2.1. Plant material, Extraction, and Isolation of Suberosin
F. cassia was collected from Konya, Turkey, in 2014 and identified by Prof. Dr. Hayri Duman. The herbarium materials were preserved in the Herbarium of the Faculty of Pharmacy of Ankara University with number AEF 26675. The extraction, fractionation, and isolation of SBR were carried out according to the methods of Karakaya et al. [Citation16].
2.2. Animals and Groups
This study was approved by the Ataturk University Animal Experiments Local Ethics Committee (decision number 77040475-641.04-E.2000006421, dated 27.02.2020), which operates in line with European (EU) Directive 2010/63/EU. Male albino Wistar rats (n = 54) with body weights of 250–300 g were purchased from the Medical Experimental Application and Research Center of Ataturk University. They were housed at 20–24 °C with humidity of 60–80% and 12-h light/dark cycles, receiving pellet feed and water. The rats were separated into six groups with nine animals in each group. The control group underwent surgical procedures without CLP and distilled water was administered as a vehicle. In the SBR group, the highest dose (20 mg/kg) of SBR was administered to healthy rats to determine the pure effects of SBR. In the other groups, the animals underwent CLP, and the SBR1, SBR2, and SBR3 groups were subsequently treated with different doses of SBR. The reason for applying three different doses in this CLP model was to observe the different effects that may occur at the lowest, highest, and intermediate doses of SBR. The groups may be summarized as follows:
2.3. Cecal Ligation and Puncture Model of Sepsis
Rats were used for the animal model as they mimic human sepsis well [Citation20]. Animals were intraperitoneally anesthetized with a 10 mg/kg mixture of xylazine (10 mg/kg) and ketamine (20 mg/kg) [Citation8] and then midline laparotomy was performed.
In the course of laparotomy, 0.5 mL of saline was introduced into the abdominal cavity of the rats. The incision was sutured with 4/0 silk. Only the rats of the CLP, SBR1, SBR2, and SBR3 groups underwent CLP procedures. Since the effects of sepsis are starting to be seen for an hour [Citation21], one hour after the beginning of sepsis, SBR suspended in distilled water was administered intraperitoneally to rats in the SBR1, SBR2, and SBR3 groups at different doses (5, 10, and 20 mg/kg). Rats had ad libitum access to water after the operation. Eighteen hours after surgery, animals were anesthetized with a 10 mg/kg combination of xylazine (10 mg/kg) and ketamine (20 mg/kg) [Citation8] and then killed. Lung samples were obtained for further evaluation.
2.4. Biochemical Analysis
Lung tissue samples of 50 mg from the animals were used for biochemical analysis. The tissues were milled with the TissueLyser II grinding set (QIAGEN, Hilden, Germany). One milliliter of phosphate-buffered saline (PBS) was used to homogenize 50 mg of lung tissue. MDA levels and SOD and GSH enzyme activities were measured in triplicate using a commercial enzyme-linked immunosorbent assay (ELISA) kit according to the manufacturer’s protocol. Results are presented as U/mg protein, nmol/mg protein, and nmol/mg protein for MDA (CAS: 122-31-6, Sigma-Aldrich, St. Louis, MO), GSH (CAS: 27025-41-8, Sigma-Aldrich), and SOD (CAS: 9054-89-1, Sigma-Aldrich), respectively, and are provided as mean ± standard deviation per milligram of protein.
2.5. Gene Expression Analysis
RNA extraction of tissues was performed with the RNeasy Mini Kit (QIAGEN). TissueLyser II (QIAGEN) was used for the homogenization of 20 mg of tissues for RNA extraction. Total RNA was quantified using the Epoch Spectrophotometer System and Take3 Plate (BioTek, Winooski, VT). For complementary DNA (cDNA) synthesis, the RT2 HT First Strand Kit (QIAGEN) was utilized. Messenger RNA (mRNA) expressions of IL-1β and TNF-α were analyzed with the ABI StepOnePlus Real-Time PCR System (Applied Biosystems, Waltham, MA). The rat-specific primers TNF-α (Rn01525860_g1), IL-1β (Rn00580432_m1), and β-actin (Rn00667869_m1) were used in amplifications. The reaction volume was 20 µL, containing 10 µL of Fast Advanced Master Mix (Applied Biosystems), 2 µL of cDNA, 1 µL of TaqMan Primer Perfect Probe Mix, and 7 µL of nuclease-free water. The thermal cycling program was carried out for 2 min at 50 °C, 2 min at 95 °C, 1 s at 95 °C, and 20 s at 60 °C. All reactions were conducted in triplicate. The expression ratios of control and experimental groups were compared and reported as relative folds. The reference housekeeping gene was β-actin. The 2−ΔΔCt method was used to evaluate gene expressions [Citation22].
2.6. Histopathological Examination
The samples collected for histopathological analysis were fixed with 10% formalin solution and then embedded in paraffin blocks sliced into sections of 4 µm in thickness. These tissue samples were stained with hematoxylin and eosin (H&E) and viewed by light microscopy (Leica DM1000, Leica Microsystems, Wetzlar, Germany) to assess changes including thickness of the alveolar septum, desquamation and degeneration in the epithelium, peribronchial cell infiltration, and venous hyperemia. Slides were scored with respect to immunopositivity as negative (−), mild (+), moderate (++), severe (+++), or very severe (++++) [Citation23].
2.7. Immunohistochemical Examination
For immunoperoxidase examination, sections prepared on adhesive (poly-L-lysin) slides were treated with xylol and alcohol series. After being washed with PBS, these sections were incubated with 3% H2O2 for 10 min to inactivate the endogenous peroxidase. In order to collect antigens from tissues, samples were treated with antigen retrieval solution for 2 × 5 min at 500 W in a microwave oven and then left to cool. Tissues were incubated with IL-1β and TNF-α antibodies (Catalog Nos. sc-52012 and sc-52746, Santa Cruz Biotechnology, Santa Cruz, CA) at 37 °C for 30 min. Immunohistochemical (IHC) analysis was performed in accordance with the manufacturer’s protocol (Abcam HRP/DAB Detection IHC Kit) and 3.3′-diaminobenzidine (DAB) was used as the chromogen. Hematoxylin staining was applied. Sections were evaluated according to immunopositivity as none (−), mild (+), moderate (++), severe (+++), or very severe (++++) [Citation8].
2.8. Statistical Analysis
All data were analyzed using GraphPad Prism version 5 software (GraphPad Software, La Jolla, CA). The Shapiro–Wilk W test and skewness tests were used to evaluate the normal distribution of the data. Values of p > 0.05 were considered to reflect normal distribution. Differences among the groups were analyzed via one-way ANOVA for normally distributed data and Kruskal–Wallis tests for non-normally distributed data. The Levene test was utilized to check for homogeneity of variance and results of p> 0.05 were considered to reflect homogeneity. The Tukey test and Dunn test were used as post hoc tests to compare the groups. Values of p< 0.05 were considered statistically significant.
3. Results
3.1. Oxidant and Antioxidant Levels of Lung Tissues
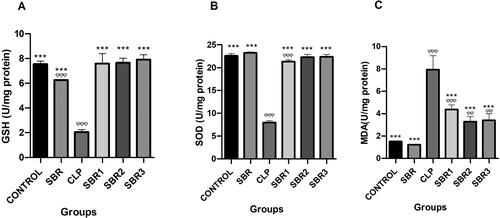
The GSH enzyme activity of the CLP group was significantly decreased compared to the control group (p< 0.0001) (). SBR administration ameliorated the GSH enzyme activity in the SBR1, SBR2, and SBR3 groups (p< 0.0001). The SOD enzyme activity of the CLP group was significantly lower than that of the control group () (p< 0.0001). However, SBR treatment increased SOD enzyme activity in the SBR1, SBR2, and SBR3 groups (p< 0.0001). The mean MDA level of the CLP group was significantly higher than that of the control and SBR1 groups (p< 0.0001) (). SBR administration significantly lowered the MDA levels in the SBR1, SBR2, and SBR3 groups (p< 0.05).
Figure 1. Evaluations of oxidative stress and antioxidant alterations in the lungs of septic rats treated with suberosin. (A) GSH activities of all experimental groups. φ: Compared to the control group; *: compared to the CLP group. (B) SOD activities of all experimental groups. φ: Compared to the control group; *: compared to the CLP group. (C) MDA levels of all experimental groups. φ: Compared to the control group; *: compared to the CLP group.
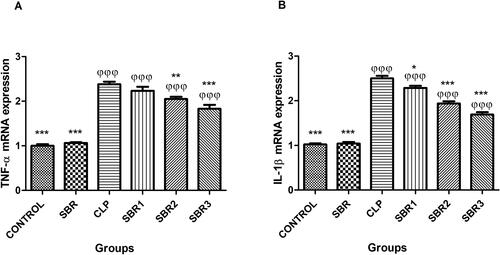
3.2. Molecular Results
TNF-α mRNA expression levels were higher in the CLP, SBR1, SBR2, and SBR3 groups than the control group (p< 0.0001) (). SBR treatment gradually reduced TNF-α expressions in the SBR1, SBR2, and SBR3 groups compared to the CLP group (p< 0.05). IL-1β mRNA expressions were also higher in the CLP, SBR1, SBR2, and SBR3 groups than the control group () (p< 0.0001). SBR administration decreased IL-1β mRNA expression in the SBR1, SBR2, and SBR3 groups in a dose-dependent manner (p< 0.05).
3.3. Histopathological Results
The histopathological findings of the examined lung tissues are presented in . Tissues from the control and SBR groups showed normal histological lung structure (, respectively) and were scored as none (−) with respect to immunopositivity. On the contrary, lung tissues of the CLP group had extremely thick interalveolar septum, marked desquamation, very severe necrosis, and degeneration of the bronchi and bronchial epithelium. Extreme perivascular cell infiltration and venous hyperemia were also observed in the peribronchial space in the CLP group (). Tissues of the CLP group were scored as very severe (++++) and showed significant differences in comparison to the control group (p< 0.0001). The SBR1 group had extreme interalveolar septal thickness, marked polymorph cell infiltration in the peribronchial region, moderate degeneration, desquamation, and very severe venous hyperemia of the bronchi and bronchial epithelium (). This group was scored as severe (+++). There were no significant differences between the SBR1 and CLP groups (p> 0.05). In the SBR2 group, lung tissues revealed moderate interalveolar septal thickness, polymorph cell infiltration in the peribronchial region, and venous hyperemia (). This group was scored as moderate (++). No significant differences between the SBR2 and CLP groups were observed (p> 0.05). The SBR3 group showed relatively less interalveolar septal thickness, degeneration, desquamation of the bronchi and bronchial epithelium, and venous hyperemia (). This group was scored as mild (+). The scores of histological parameters of lung injury increased in the CLP group and decreased in the SBR3 group (p< 0.05). These results demonstrated that SBR treatment limited sepsis-induced lung injury. The histopathological scoring results are evaluated in .
Figure 3. Histopathological examination of lungs of septic rats (magnification 40×). (A) Lung tissue of the control group with normal histological appearance (H&E, bar = 50 µm). (B) Lung tissues of the CLP group with very severe peribronchiolar cell infiltration (empty stars), very severe desquamation in bronchiolar epithelium (arrowheads), and very severe venous hyperemia (H&E, bar = 50 µm). (C) Lung tissue of the SBR1 group with severe peribronchiolar cell infiltration (empty stars), severe interalveolar septum thickening (black stars), moderate desquamation of bronchial epithelium (arrow heads), and severe venous hyperemia (H&E, bar = 50 µm). (D) Lung tissue of the SBR2 group with moderate peribronchiolar cell infiltration (empty stars), moderate interalveolar septum thickening (black stars), moderate desquamation of bronchial epithelium (arrow heads), and moderate venous hyperemia (H&E, bar = 50 µm). (E) Lung tissue of the SBR3 group with mild interalveolar septum thickening (black star), mild desquamation of bronchial epithelium (arrow head), and moderate venous hyperemia (H&E, bar = 50 µm). (F) Lung tissue of the SBR group with normal histological appearance (H&E, bar = 50 µm).
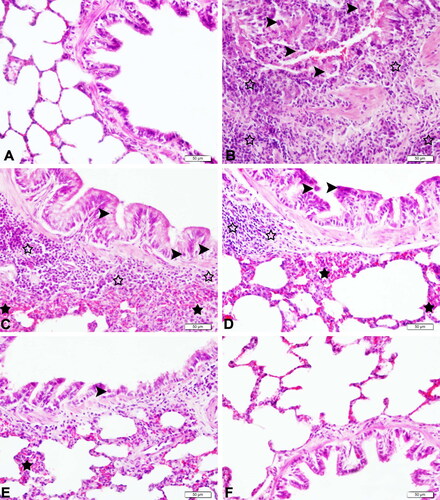
Table 1. Histopathological results and immunohistochemically evaluation of lung tissues.
Table 2. Histopathological and immunohistochemical changes in lung injury.
3.4. Immunohistochemical Results
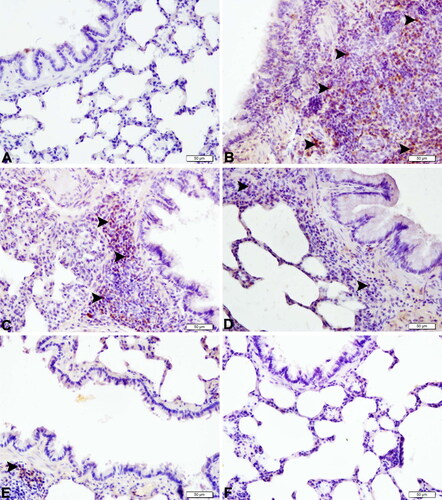
IHC staining results for the lung tissues are shown in and . The IHC staining results are provided in . IHC analysis of the control and SBR groups revealed negative findings for TNF-α (, respectively) and IL-1β (, respectively). In the CLP group, increased TNF-α and IL-1β immunopositivity was observed in peribronchial-bronchiole polymorph and mononuclear cells and perivascular and alveolar regions compared to the control group (p< 0.0001) ( and , respectively). In the SBR1 group, severe immunopositivity was seen in peribronchial-bronchiole inflammatory cells for TNF-α () and in the perivascular and alveolar regions for IL-1β (). In the SBR1 group, TNF-α and IL-1β immunopositivity was increased compared to the control group (p> 0.0001). In the SBR2 group, moderate immunopositivity was observed in peribronchial-bronchiole inflammatory cells for TNF-α () and in some alveolar surfaces of perivascular regions for IL-1β (). The immunopositivity of TNF-α and IL-1β was increased in the SBR2 group compared to the control group (p> 0.05). In the SBR3 group, the immunopositivity of both TNF-α () and IL-1β () was significantly attenuated compared to the CLP group (p < 0.05). Immunohistochemical scoring results are summarized in .
Figure 4. Expressions of TNF-α in lung tissues of septic rats (magnification 40×). (A) Lung tissue of the control group with negative TNF-α protein expression (IHC-P, bar = 50 µm). (B) Lung tissue of the CLP group with very severe TNF-α protein expression (arrowheads) (IHC-P, bar = 50 µm). (C) Lung tissue of the SBR1 group with severe TNF-α protein expression (arrowheads) (IHC-P, bar = 50 µm). (D) Lung tissue of the SBR2 group with moderate TNF-α protein expression (arrowheads) (IHC-P, bar = 50 µm). (E) Lung tissue of the SBR3 group with mild TNF-α protein expression (arrowhead) (IHC-P, bar = 50 µm). (F) Lung tissue of the SBR group with negative TNF-α protein expression (IHC-P, bar = 50 µm).
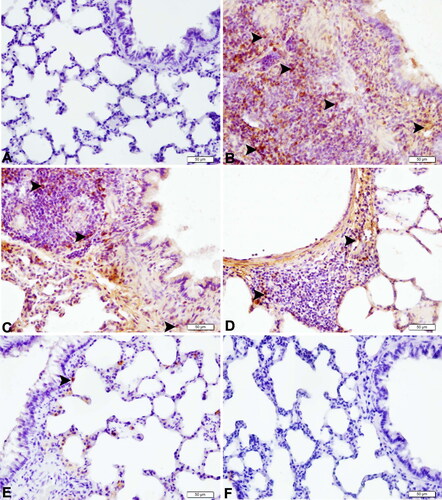
Figure 5. Expressions of IL-1β in lung tissues of septic rats (magnification 40×). (A) Lung tissue of the control group with negative IL-1β protein expression (IHC-P, bar = 50 µm). (B) Lung tissue of the CLP group with very severe IL-1β protein expression (arrowheads) (IHC-P, bar = 50 µm). (C) Lung tissue of the SBR1 group with severe IL-1β protein expression (arrowheads) (IHC-P, bar = 50 µm). (D) Lung tissue of the SBR2 group with moderate IL-1β protein expression (arrowheads) (IHC-P, bar = 50 µm). (E) Lung tissue of the SBR3 group with mild IL-1β protein expression (arrowhead) (IHC-P, bar = 50 µm). (F) Lung tissue of the SBR group with negative IL-1β protein expression (IHC-P, bar = 50 µm).
4. Discussion
Sepsis is a result of the presence of bacteria or other factors that activate patients’ immune responses [Citation1] and it causes organ damage, including damage to the lungs [Citation24]. Hyperactive immune systems are major problem to consider in efforts to prevent sepsis-related organ damage. Uncontrolled inflammatory responses, increased ROS activity, and rising cytokine levels threaten the lives of patients in intensive care units [Citation25,Citation26]. Antioxidant molecules including coumarin [Citation16] may help to prevent increased oxidative stress and limit the cytokine storms seen in cases of sepsis. In this study, we established a sepsis model in rats using CLP to investigate the protective effects of SBR in the lungs of septic rats through evaluations of oxidative stress (SOD, GSH, and MDA) and proinflammatory markers (IL-1β and TNF-α). IHC and histopathological analyses of lung tissues were also performed.
Overproduction of cytokines including TNF-α and IL-1β in lung tissues can cause damage via edema and alveolar hemorrhage [Citation27]. TNF-α and IL-1β are proinflammatory cytokines with crucial roles in cell proliferation, differentiation, and apoptosis [Citation28]. In patients with sepsis and in animal models, TNF-α and IL-1β expressions are seen to be increased [Citation28,Citation29]. In harmony with the literature, mRNA expressions and protein levels of TNF-α and IL-1β were increased in the CLP group compared to the control group in this study.
Many natural molecules including coumarins have anti-inflammatory effects [Citation30] and may suppress proinflammatory cytokine levels. A recent study showed that coumarin isolated from species of the family Apiaceae contained aromatic herbal compounds and limited the expression of TNF-α and IL-β [Citation31]. SBR also showed antibacterial activity [Citation32]. A study designed with a mouse model of sepsis showed that a natural coumarin derivative alleviated acute lung injury by limiting TNF-α production [Citation33]. Another study concluded that SBR suppressed the production of some cytokines, including IL-2 and interferon-γ [Citation34]. In line with these results, it was found in the present study that SBR treatment decreased TNF-α and IL-β expression in rat lung tissues in the SBR1, SBR2, and SBR3 groups compared to the CLP group in a dose-dependent manner. SBR treatment to healthy rats (SBR groups) did not affect TNF-α and IL-1β expression in lung tissues. Therefore, we can say that SBR does not cause changes in TNF-α and IL-1β expression in healthy animals.
Acute lung injury and ARDS are observed frequently in cases of sepsis [Citation3,Citation24]. The lung pathology seen in sepsis entails the damage of alveolar epithelial cells and alveolar capillary endothelial cells, together with alveolar edema and pulmonary accumulation of neutrophils [Citation35]. When an unusual microbial presence is detected, endothelial cells produce proinflammatory cytokines and chemokines that increase the immune response [Citation36]. In our sepsis model (CLP group), increased interalveolar septal thickening, desquamation of the bronchial epithelium, and venous hyperemia were observed. SBR attenuated these alterations in a dose-dependent manner in the SBR1, SBR2, and SBR3 groups. Lung tissues in the SBR group were similar to the control group. These results suggest that SBR may be capable of ameliorating sepsis-related lung damage.
An imbalance between pro-oxidant and antioxidant levels will push cells into a state of oxidative stress. Many pathological conditions including sepsis induce the production of ROS in cells [Citation8,Citation10]. For example, in patients with sepsis and in animal models, the presence of higher levels of oxidative stress markers was demonstrated [Citation8,Citation10]. One previous study used umbelliferone, a metabolite of Ferulago pauciradiata, and demonstrated decreased MDA levels and increased SOD and GSH enzyme activities in umbelliferone-treated septic rats compared to the CLP group [Citation8]. In the present study, lower SOD and GSH enzyme activities and higher MDA levels were observed in the lung tissues of the CLP group. However, those GSH and SOD enzyme activities were attenuated in SBR-treated septic rats. In the CLP group, the mean MDA level increased compared to the control group. However, SBR treatment reduced the heightened MDA levels in the SBR1, SBR2, and SBR3 groups. SBR did not cause any alterations in the SBR group. In this study, it has been shown that SBR has the ability to reduce the effects of impaired oxidant balances in lung tissues.
In conclusion, this is the first study to show the protective effects of SBR isolated from F. cassia in the lungs of septic rats. SBR alleviated sepsis-induced lung injury. This compound may be considered as an alternative therapy for conditions such as impaired oxidative balance and excessive cytokine production in cases of sepsis. Further investigations of SBR with different methods in different diseases will contribute to a better understanding of the effects of this compound.
Acknowledgments
The authors thank Prof. Dr. Hayri Duman for his worthy support for defining plant material.
Disclosure statement
The authors declare that they have no conflict of interest.
References
- Blanco J, Muriel-Bombín A, Sagredo V, et al. Incidence, organ dysfunction and mortality in severe sepsis: a Spanish multicentre study. Crit Care. 2008;12(6):1. doi:10.1186/cc7157.
- Rubenfeld GD, Caldwell E, Peabody E, et al. Incidence and outcomes of acute lung injury. N Engl J Med. 2005;353(16):1685–9. 10.1056/NEJMoa050333.
- Matthay MA, Ware LB, Zimmerman GA. The acute respiratory distress syndrome. J Clin Invest. 2012;122(8):2731–2740. 10.1172/JCI60331.
- Yang HL, Huang PJ, Liu YR, et al. Toona sinensis inhibits LPS-induced inflammation and migration in vascular smooth muscle cells via suppression of reactive oxygen species and NF- κ B signaling pathway. Oxid Med Cell Longev. 2014;2014:901315–901316. 10.1155/2014/901315.
- Kim MH, Kim JN, Han SN, Kim HK. Ursolic acid isolated from guava leaves inhibits inflammatory mediators and reactive oxygen species in LPS-stimulated macrophages. Immunopharmacol Immunotoxicol. 2015;37(3):228–235. 10.3109/08923973.2015.1021355.
- Russell JA. Management of sepsis. N Engl J Med. 2006;355(16):1699–1713. 10.1056/NEJMra043632.
- Cinar I, Sirin B, Aydin P, et al. Ameliorative effect of gossypin against acute lung injury in experimental sepsis model of rats. Life Sci. 2019;221:327–334. 10.1016/j.lfs.2019.02.039.
- Kutlu Z, Celik M, Bilen A, et al. Effects of umbelliferone isolated from the Ferulago pauciradiata Boiss; Heldr. Plant on cecal ligation and puncture-induced sepsis model in rats. Biomed Pharmacother. 2020;127:110206. 10.1016/j.biopha.2020.110206.
- Gutteridge JMC, Mitchell J. Redox imbalance in the critically ill. Br Med Bull. 1999;55(1):49–75. 10.1258/0007142991902295.
- Mantzarlis K, Tsolaki V, Zakynthinos E. Role of oxidative stress and mitochondrial dysfunction in sepsis and potential therapies. Oxid Med Cell Longev. 2017;2017:5985209–5985210. doi:10.1155/2017/5985209.
- Akpinar E, Kutlu Z, Kose D, et al. Protective effects of idebenone against sepsis induced acute lung damage. J Invest Surg. 2022;35(3):560–568. 10.1080/08941939.2021.1898063.
- Başer KH, Demirci B, Demirci F, Hashimoto T, Asakawa Y, Noma Y. Ferulagone: a new monoterpene ester from Ferulago thirkeana essential oil. Planta Med. 2002;68(6):564–567. 10.1055/s-2002-32557.
- Demetzos C, Perdetzoglou D, Gazouli M, Tan K, Economakis C. Chemical analysis and antimicrobial studies on three species of ferulago from Greece. Planta Med. 2000;66(6):560–563. 10.1055/s-2000-8652.
- Basile A, Sorbo S, Spadaro V, et al. Antimicrobial and antioxidant activities of coumarins from the roots of Ferulago campestris (Apiaceae). Molecules. 2009;14(3):939–952. 10.3390/molecules14030939.
- Amirghofran Z, Bahmani M, Azadmehr A, Javidnia K. Anticancer effects of various Iranian native medicinal plants on human tumor cell lines. Neoplasma. 2006;53(5):428–433. http://www.ncbi.nlm.nih.gov/pubmed/17013538.
- Karakaya S, Koca M, Sytar O, et al. Antioxidant and anticholinesterase potential of Ferulago cassia with farther bio-guided isolation of active coumarin constituents. South African J Bot. 2019;121:536–542. doi:10.1016/j.sajb.2019.01.020.
- Uzunçakmak SK. In vitro Effects of Ferulago cassia Boiss on human umbilical vein endothelial cells induced by lipopolysaccharide. Paper presented at: 5th International Eurasian Congress on Natural Nutrition, Healthy Life & Sport; 2019 Oct 02–06; Ankara-Turkey. 1661.
- Golfakhrabadi F, Abdollahi M, Ardakani MRS, et al. Anticoagulant activity of isolated coumarins (suberosin and suberenol) and toxicity evaluation of Ferulago carduchorum in rats. Pharm Biol. 2014;52(10):1335–1340. 10.3109/13880209.2014.892140.
- Sajjadi SE, Pestechian N, Kazemi M, Mohaghegh M-A, Hosseini-Safa A. Evaluation of the antimalarial effect of Ferulago angulata (Schlecht.) boiss. Extract and suberosin epoxide against Plasmodium berghei in comparison with chloroquine using in-vivo test. Iran J Pharm Res. 2016;15(3):515–521.
- Hubbard WJ, Choudhry M, Schwacha MG, et al. Cecal ligation and puncture. Shock. 2005;24(1):52–57. doi:10.1097/01.shk.0000191414.94461.7e.
- Zhou L, Gao M, Xiao Z, Zhang J, Li X, Wang A. Protective effect of astaxanthin against multiple organ injury in a rat model of sepsis. J Surg Res. 2015;195(2):559–567. 10.1016/j.jss.2015.02.026.
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2 − ΔΔCT method. Methods. 2001;25(4):402–408. 10.1006/meth.2001.1262.
- Schafer KA, Eighmy J, Fikes JD, et al. Use of severity grades to characterize histopathologic changes. Toxicol Pathol. 2018;46(3):256–265. 10.1177/0192623318761348.
- Ornellas DS, Maron-Gutierrez T, Ornellas FM, et al. Early and late effects of bone marrow-derived mononuclear cell therapy on lung and distal organs in experimental sepsis. Respir Physiol Neurobiol. 2011;178(2):304–314. 10.1016/j.resp.2011.06.029.
- Christ-Crain M, Morgenthaler NG, Struck J, Harbarth S, Bergmann A, Müller B. Mid-regional pro-adrenomedullin as a prognostic marker in sepsis: an observational study. Crit Care. 2005;9(6):R816–24. 10.1186/cc3885.
- Cohen J. The immunopathogenesis of sepsis. Nature. 2002;420(6917):885–891. 10.1038/nature01326.
- Zhao C, Sun J, Fang C, Tang F. 1,8-Cineol attenuates LPS-induced acute pulmonary inflammation in mice. Inflammation. 2014;37(2):566–572. 10.1007/s10753-013-9770-4.
- Mera S, Tatulescu D, Cismaru C, et al. Multiplex cytokine profiling in patients with sepsis. APMIS. 2011;119(2):155–163. 10.1111/j.1600-0463.2010.02705.x.
- Chen J, Xia H, Zhang L, Zhang H, Wang D, Tao X. Protective effects of melatonin on sepsis-induced liver injury and dysregulation of gluconeogenesis in rats through activating SIRT1/STAT3 pathway. Biomed Pharmacother. 2019;117:109150. 10.1016/j.biopha.2019.109150.
- Fylaktakidou K, Hadjipavlou-Litina D, Litinas K, Nicolaides D. Natural and synthetic coumarin derivatives with anti-inflammatory/antioxidant activities. Curr Pharm Des. 2004;10(30):3813–3833. 10.2174/1381612043382710.
- Khan S, Shehzad O, Cheng MS, Li RJ, Kim YS. Pharmacological mechanism underlying anti-inflammatory properties of two structurally divergent coumarins through the inhibition of pro-inflammatory enzymes and cytokines. J Inflamm. 2015;12:47. doi:10.1186/s12950-015-0087-y.
- Tavakoli S, Delnavazi MR, Hadjiaghaee R, et al. Bioactive coumarins from the roots and fruits of Ferulago trifida Boiss., an endemic species to Iran. Nat Prod Res. 2018;32(22):2724–2728. 10.1080/14786419.2017.1375915.
- Jin Y, Qian J, Ju X, et al. Osthole Protects against acute lung injury by suppressing NF- κ B-dependent inflammation. Mediators Inflamm. 2018;2018:4934592–4934512. 10.1155/2018/4934592.
- Chen YC, Tsai WJ, Wu MH, Lin LC, Kuo YC. Suberosin inhibits proliferation of human peripheral blood mononuclear cells through the modulation of the transcription factors NF-AT and NF-κB. Br J Pharmacol. 2007;150(3):298–312. 10.1038/sj.bjp.0706987.
- Ranieri VM, Rubenfeld GD, Thompson BT, et al. Acute respiratory distress syndrome. JAMA. 2012;307:2526–2533. doi:10.1001/jama.2012.5669.
- Faure E, Equils O, Sieling PA, et al. Bacterial lipopolysaccharide activates NF-κB through toll-like receptor 4 (TLR-4) in cultured human dermal endothelial cells. J Biol Chem. 2000;275(15):11058–11063. 10.1074/jbc.275.15.11058.