Abstract
Background: Acute kidney injury (AKI) is a common complication in patients with severe acute pancreatitis (SAP). Caspase-11-mediated pyroptosis is essential for the progression of multiple diseases, but its role in SAP-induced AKI remains unknown.
Aims: This research investigated whether caspase-11-mediated pyroptosis is involved in SAP-induced AKI and whether inhibiting caspase-11-mediated pyroptosis improves SAP-induced AKI.
Methods: A rat model of SAP with AKI was established by slowly injecting 5% sodium taurocholate into the biliopancreatic duct, then wedelolactone (25 or 50 mg/kg), an inhibitor of caspase-11, was injected through the intra-peritoneum 1 and 6 h after SAP induction. Serum biochemical indexes, including serum amylase, lipase, interleukin (IL)-6, blood urea nitrogen (BUN), tumor necrosis factor (TNF)-α, and creatinine (Cr) in rats, were evaluated using biochemical test kits. Caspase-11 and gasdermin D (GSDMD) expression in the kidney tissues was evaluated by western blotting and immunohistochemical staining. IL-1β and IL-18 levels in kidney tissues were detected by ELISA kits. Furthermore, histopathological alterations of pancreas and kidney were assessed by H&E staining.
Results: The serum biochemical indexes and pyroptosis-related proteins in kidney tissues were significantly increased after SAP induction. Furthermore, wedelolactone decreased the expression of pyroptosis-linked proteins in kidney tissues, reduced serum lipase, amylase, IL-6, TNF-α, BUN, and Cr, and ameliorated the renal and pancreatic histological damage in SAP rats.
Conclusion: Caspase-11-mediated pyroptosis contributes to SAP-induced AKI, and targeting caspase-11-mediated pyroptosis might be a novel treatment strategy for SAP-induced AKI.
Introduction
Severe acute pancreatitis (SAP) is a serious and acute inflammation disorder with increased rates of morbidity and death [Citation1]. The fate of SAP patients is related to multiple organ dysfunction, possibly organ failure, including acute renal failure, acute respiratory failure, and acute cardiovascular failure [Citation2]. Acute kidney injury (AKI) is a frequent SAP-associated multiple organ dysfunction and increases SAP mortality [Citation3]. Unfortunately, the detailed mechanism of AKI in individuals with SAP remains ambiguous; therefore, investigating how SAP causes AKI is essential for identifying new interventions.
The specific mechanism by which SAP causes AKI is complicated, with previous studies suggesting that increased oxidative stress [Citation4], uncontrolled inflammation [Citation5], and severe hypovolemia [Citation6] are related to AKI caused by SAP. Moreover, tubular epithelial cell death could be crucial in the pathophysiology of AKI [Citation7, Citation8]; hence, the destruction of renal tubular epithelial cells may be involved in AKI induced by SAP.
Pyroptosis is a novel type of controlled cell destruction, unlike necrosis and apoptosis, as gasdermin D (GSDMD) serves as the primary executive protein [Citation9]. GSDMD can be cleaved by stimulating inflammatory caspases to generate C-terminal (GSDMD-C) and N-terminal (GSDMD-N) fragments. GSDMD-N attaches to the cell membrane and causes pores, which facilitate pyroptotic cell destruction and promotes the IL-1β and IL-18 leakage [Citation10]. Pyroptosis is triggered by caspase-11-mediated non-canonical and caspase-1-mediated canonical signaling pathways and has been implicated in a range of diseases, including tumors[Citation11], infectious diseases [Citation12], nervous system diseases [Citation13]. Recently, several studies indicated that caspase-11-mediated pyroptosis participates in AKI caused by various pathologic conditions [Citation14, Citation15]. However, whether caspase-11-dependent pyroptosis is implicated in AKI pathogenesis caused by SAP is unknown.
Wedelolactone (Wed), an inhibitor of caspase-11, suppresses caspase-11 expression by suppressing the IκB kinase (IKK) complex [Citation16]. The caspase-11-dependent pyroptosis signaling pathway can be inhibited effectively by Wed [Citation17, Citation18], but it is unclear whether Wed alleviates renal injury caused by SAP.
Therefore, this study aimed to ascertain if caspase-11-dependent pyroptosis participated in the pathogenic mechanism of AKI in rats with SAP and tested the impact of Wed on renal tissue damage in rats with SAP to reveal a potential pathogenic mechanism behind SAP-induced AKI, which could be used in the development of a novel treatment strategy for renal injury caused by SAP.
Materials and Methods
Animals
Mature Wistar male rats (age, seven weeks; weight, 220–250 g) were purchased from Qingdao University's (Qingdao, China) animal center. The rats were housed in a controlled climate setting with a 12 h/12 h light/dark cycle and were freely permitted to eat and drink. All experiments were approved by the Qingdao University Institutional Animal Care and Use Committee in compliance with the National Institutes of Health standards for laboratory animal usage.
SAP in Rats
Rats were divided into four groups at random (8 rats in each): sham control, SAP, SAP + 25 mg/kg Wed (Wed-L), and SAP + 50 mg/kg Wed (Wed-H) group. Before the experiment, all the rats fasted for 12 h. The SAP rat model was constructed as described previously [Citation19] using 5% sodium taurocholate (1 mL/kg) introduced gradually into the biliopancreatic duct after rats were intraperitoneally sedated with 3% pentobarbital sodium (20 mg/kg). The rats in the sham group were administrated an equivalent amount of normal saline. In addition, Wed (MedChemExpress) was injected through the intra-peritoneum (i.p.) at a dosage of 25 or 50 mg/kg 1 and 6 h after SAP induction. All rats in the sham and SAP groups received the exact vehicle dosing.
All rats were anesthetized again at 24 h after surgery. Blood samples were collected by puncture of the postcava, and the serum was separated via centrifugation and frozen at −80 °C until analysis. Furthermore, kidney and pancreas tissue samples were rapidly fixed using paraformaldehyde (4%) before Hematoxylin & Eosin (H&E) staining and immunohistochemical assessment. Excess tissues were stored at −80 °C for further analysis.
Serum Lipase and Amylase
Serum amylase and lipase levels were assessed by standardized assay kits (Jiancheng Biotech, Nanjing, China) in compliance with the manufacturer's guidelines.
Renal Function Assessment
Renal function was estimated by serum creatinine (Cr) and blood urea nitrogen (BUN) using standard assay kits (Jiancheng Biotech, Nanjing, China).
Elisa
The serum levels of TNF-α and IL-6 and the renal tissue levels of IL-1β and IL-18 were quantified by commercially available ELISA kits (BOSTER Biological Technology Co., Ltd, Wuhan, China) according to the manufacturer's guidelines.
H&E Staining
Fixed renal and pancreatic tissues were impregnated in paraffin before 5-μm-thick slices were cut and H&E stained for visualization under a light microscope. The histology grades of the pancreas and kidney were assessed independently by two observers according to previously established scoring criteria.
IHC Staining
The expression of caspase-11 and GSDMD were assessed by immunohistochemical analysis. The paraffin-embedded sections were processed by routine methods, including dewaxing, hydrating, antigen extracting, and serum blocking. Next, the samples were incubated with antibodies against caspase-11 (Novus Biologicals) and GSDMD (Affinity Biosciences) at 4 °C overnight. After rinsing the tissue sections with PBS, the corresponding secondary antibody was applied and incubated at 37 °C for 1 h before being visualized with diaminobenzidine and a microscope (Olympus, Tokyo, Japan) at 200× magnification. Image-Pro Plus program 6.0 (Media Cybernetics, Rockville, MD, USA) was used to analyze the integrated optical density of positive staining.
Western Blotting
Western blotting was employed to identify caspase-11, caspase-11 P20, GSDMD-N, and GSDMD expression in renal tissues. Proteins were retrieved from the renal tissues and quantified using a BCA Protein Assay kit (TransGen, Beijing, China). Then, the proteins were separated on 10% sodium dodecyl sulfate-polyacrylamide gels and transferred to polyvinylidene fluoride (PVDF) before blocking with 5% skimmed milk. The membranes were incubated overnight at 4 °C with the appropriate primary antibodies: caspase-11 (Affinity Biosciences), caspase-11 P20 (Santa Cruz Biotechnology), GSDMD (Proteintech), GSDMD-N (Cell Signaling Technology), and β-actin (Abcam), then rinsed and incubated at 37 °C for 45 min with the corresponding second antibodies. The protein bands were visualized using an enhanced chemiluminescence reagent (Beyotime) and quantified by Image J software. β-actin was utilized for normalization.
Statistical Analysis
The data are shown as mean ± SD and analyzed using GraphPad Prism 8.0 (GraphPad Prism program, CA, USA). Variations between the groups were compared with one-way ANOVA followed by Tukey's post hoc test. A P-value < 0.05 was considered statistically significant.
Results
Rat model of SAP-Induced AKI
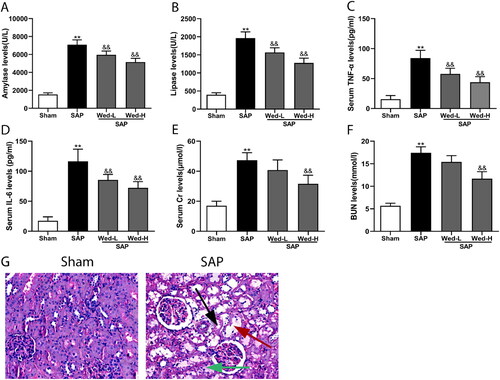
We first evaluated the variations of the serum biochemical indexes in rats after SAP induction. The result showed the serum amylase, lipase, TNF-α, and IL-6 (; p<0.01), as well as serum Cr and BUN levels (; p<0.01), were significantly lower in the sham rat group compared to the SAP group. We next detected the histological changes in the kidney after SAP, H&E staining revealed typical symptoms of renal injury in the SAP rats, like tubular epithelial swelling, dilation of the tubular lumen, and interstitial edema (). These changes indicated that we successfully established the rat model of SAP-induced AKI.
Figure 1. Biochemical indicators in serum and histological abnormalities in the kidneys associated with SAP. (a, b) Lipase and amylase serum activity; (c, d) serum TNF-α and IL-6 levels; (e, f) serum Cr and BUN levels; (g) representative images of H&E-stained kidney sections (original magnification, ×200) indicating tubular epithelial swelling (green arrow), dilation of the tubular lumen (red arrow), and interstitial edema (black arrow). The data are presented as mean ± SD (n = 5). **p < 0.01 vs. the Sham group; &&p < 0.01 vs. the SAP group.
Caspase-11-Mediated Pyroptosis in SAP-Associated Renal Injury
Immunohistochemical staining, western blotting, and Elisa were used to determine whether caspase-11-dependent pyroptosis was involved in SAP-induced AKI. Immunohistochemical staining demonstrated that the expression of caspase-11 in the renal tissue of the SAP group was significantly greater than in the sham group (; p < 0.01). Western blotting demonstrated a similar result, with significantly lower caspase-11 and caspase-11 P20 protein in the sham group compared to the SAP group (, p < 0.01).
Figure 2. GSDMD proteins and caspase-11 expression in the kidney were detected by immunohistochemical staining. (a, b) Representative images of caspase-11 and GSDMD protein immunolabelling (original magnification, ×200). (c, d) Quantitative analysis of caspase-11 and GSDMD expression by Image-Pro Plus software. All data are presented as mean ± SD (n = 5). &&p < 0.01 vs. the SAP group; **p < 0.01 vs. the Sham group.
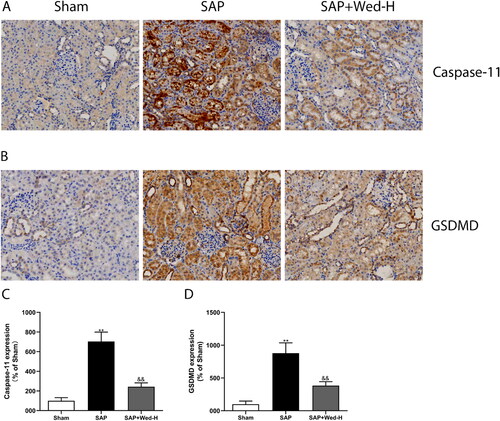
Figure 3. Western blot analysis of pyroptosis core proteins (a). The relative expression of caspase-11, caspase-11 P20, GSDMD, and GSDMD-N was determined by optical densitometry (b-e). All results are presented as the mean ± SD (n = 5). **p < 0.01 vs. the Sham group; &&p < 0.01 vs. the SAP group.
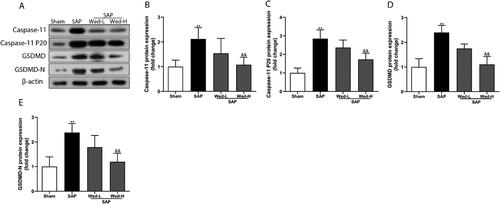
GSDMD is the main effector of pyroptosis activated by caspase-11 and subsequently causes cell death via cellular dilation and lysis [Citation20]. Consistent with the change in caspase-11 expression, immunohistochemistry depicted that the SAP group had significantly higher GSDMD levels than the sham group (, p<0.01). Additionally, as illustrated in , the SAP group had significantly elevated GSDMD and GSDMD-N levels (p < 0.01).
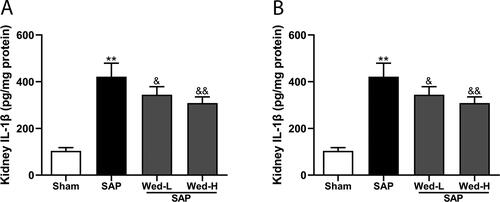
Pyroptosis is characterized by producing mediators, like IL-1β and IL-18. As depicted in , the SAP group had significantly enhanced IL-1β and IL-18 levels in the renal tissues compared to the Sham group (p < 0.01). These data imply that SAP stimulates pyroptosis in the kidney and that non-canonical pyroptosis mediated by caspase-11 may be associated with the renal damage caused by SAP.
Wed Suppresses Caspase-11-Mediated Pyroptosis
To assess the role of non-canonical pyroptosis inhibition in SAP-induced renal injury, different doses of Wed were administered to rats. As displayed in , Wed (50 mg/kg) treatment suppressed GSDMD and caspase-11 expression in the kidney after SAP induction (p<0.01). Furthermore, pyroptosis-related protein expression was notably increased in the SAP group compared to the SAP + Wed-H group (; p < 0.01). Additionally, Wed administration dose-dependently reduced the kidney IL-1β as well as IL-18 levels in the SAP rats (). Overall, these results indicate that Wed treatment effectively suppressed caspase-11-mediated pyroptosis in the rat SAP renal injury model.
Suppression of Caspase-11-Mediated Pyroptosis Attenuates SAP-Induced Renal Damage
To further confirm the function of caspase-11-mediated non-canonical pyroptosis in renal injury associated with SAP and to determine whether suppression of caspase-11-mediated pyroptosis can rescue renal injury, the serum indexes and histopathology of Wed-treated rats were evaluated. As depicted in , in comparison to the SAP groups, Wed treatment significantly reduced serum lipase, amylase, TNF-α, and IL-6 (p < 0.01). Also, serum Cr and BUN measures were significantly diminished after Wed (50 mg/kg) treatment (; p < 0.01), indicating a partial improvement of renal dysfunction.
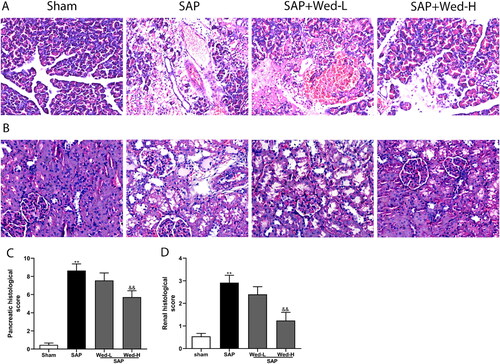
There were no evident pathological alterations associated with renal and pancreatic injury in the sham group (); however, the pancreatic tissues were significantly damaged, with an obvious necrotic area, infiltration of inflammatory cells, and hemorrhage in the SAP group. Wed (50 mg/kg) administration significantly improved the pancreas histological injuries and scores (, p < .01). Additionally, illustrates typical renal injury in the SAP group, which was ameliorated by Wed (50 mg/kg) (; p<0.01).
Figure 5. Effects of Wed treatment on the pancreatic and kidney histological abnormalities in rats following SAP. Representative images of kidney and pancreas sections stained with H&E (original magnification, ×200). (a) Pancreas pathology; (b) kidney pathology; (c, d) pancreatic and renal tissue pathological grades. The data are presented as the mean ± SD (n = 5). **p < 0.01 vs. the Sham group; &&p < 0.01 vs. the SAP group.
Discussion
This study demonstrated that caspase-11-dependent pyroptosis is critical for renal injury generated by SAP and that inhibition of caspase-11-mediated pyroptosis ameliorated SAP-associated renal damage and dysfunction.
SAP patients commonly experience AKI, with a 10-fold increase (74.7% vs.7%) [Citation21] in mortality for patients with renal failure following SAP. Unfortunately, there are no effective therapies for AKI since the AKI pathogenesis in the SAP setting is poorly understood. Therefore, further study of the pathogenesis of AKI associated with SAP has great significance. We observed increased serum measures of Cr and BUN and changes in renal morphology in the SAP rat model, indicating that SAP development induced renal dysfunction and injury.
Pyroptosis is essentially an intrinsic immune reaction and contributes to the clearance of exogenous invasion by causing local inflammatory reactions [Citation20]. However, severe pyroptosis may lead to an uncontrolled local inflammatory reaction, which may cause tissue damage and even organ failure. Caspase-11 can trigger the non-canonical pyroptosis pathway when activated by lipopolysaccharide [Citation22]. Recent studies have proved that caspase-11-mediated pyroptosis involves cisplatin, contrast, or sepsis-induced renal injury [Citation14, Citation15, Citation23]. A significant increase in caspase-11 and caspase-11 P20 protein in the renal tissue of the SAP rat model was observed in the current study. GSDMD, a crucial downstream target of caspase-11, is involved in pyroptosis and causes rapid lytic cell death by promoting cell pore formation [Citation24]. Here, we also observed the upregulation of GSDMD as well as GSDMD-N expressions in the renal tissue of SAP rats. In summary, these observations revealed that caspase-11-mediated pyroptosis occurs in the renal injury induced by SAP. Furthermore, it is hypothesized that pyroptosis occurs mostly in renal tubular epithelia because GSDMD and caspase-11 were highly expressed in renal tubular epithelia.
Pyroptosis is characterized by the production of pro-inflammatory molecules like IL-1β and IL-18, which contribute to the etiology of AKI [Citation25, Citation26]. The present study revealed that kidney IL-1β as well as IL-18 levels were increased in SAP rats.
The involvement of caspase-11-mediated pyroptosis in renal injury generated by SAP prompted us to evaluate the potential therapy targeting caspase-11-mediated pyroptosis in AKI caused by SAP. Wed, an inhibitor of caspase-11, has been demonstrated to suppress caspase-11-dependent pyroptosis in many studies [Citation17, Citation18]. Recently, Wed was demonstrated to protect renal podocytes against doxorubicin-induced injury [Citation27] and mitigate lung injury caused by acute pancreatitis [Citation28]. Therefore, we determined whether Wed may counter renal injury produced by SAP by inhibiting caspase-11-dependent pyroptosis. Following Wed therapy, the expression of caspase-11, caspase-11 P20 protein, and GSDMD-N protein, a pyroptosis executor, all decreased in the kidney tissues in the SAP group and were associated with enhanced renal function and alleviation of renal histological injury. Thus, caspase-11-mediated pyroptosis happens in the SAP-induced renal injury model, and inhibiting caspase-11-dependent pyroptosis can attenuate renal injury.
In addition to pyroptosis, apoptosis [Citation29] and ferroptosis [Citation30] play key roles in SAP-induced renal injury; therefore, the mechanism of AKI caused by SAP is complex and involves mixed sorts of cell death. The relationship between the different cell death pathways remains to be further studied.
In conclusion, caspase-11-dependent pyroptosis occurs in rats with SAP-induced acute renal injury, and inhibition of caspase-11-dependent pyroptosis could attenuate SAP-induced renal tissue damage and preserve renal function. Therefore, targeting caspase-11-dependent pyroptosis might be a novel potential therapy for AKI caused by SAP.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Lee PJ, Papachristou GI. New insights into acute pancreatitis. Nat Rev Gastroenterol Hepatol. 2019;16(8):479–496. doi:10.1038/s41575-019-0158-2.
- Garg PK, Singh VP. Organ failure due to systemic injury in acute pancreatitis. Gastroenterology. 2019;156(7):2008–2023. doi:10.1053/j.gastro.2018.12.041.
- Devani K, Charilaou P, Radadiya D, Brahmbhatt B, Young M, Reddy C. Acute pancreatitis: trends in outcomes and the role of acute kidney injury in mortality – a propensity-matched analysis. Pancreatology. 2018;18(8):870–877. doi:10.1016/j.pan.2018.10.002.
- Shi Q, Liao KS, Zhao KL, et al. Hydrogen-rich saline attenuates acute renal injury in sodium taurocholate-induced severe acute pancreatitis by inhibiting ROS and NF-kappaB pathway. Mediators Inflamm. 2015;2015:685043. doi:10.1155/2015/685043.
- Malmstrom ML, Hansen MB, Andersen AM, et al. Cytokines and organ failure in acute pancreatitis: inflammatory response in acute pancreatitis. Pancreas. 2012;41(2):271–277.
- Nassar TI, Qunibi WY. AKI associated with acute pancreatitis. Clin J Am Soc Nephrol. 2019;14(7):1106–1115. doi:10.2215/CJN.13191118.
- Wang Y, Quan F, Cao Q, et al. Quercetin alleviates acute kidney injury by inhibiting ferroptosis. J Adv Res. 2021;28:231–243.
- Pefanis A, Ierino FL, Murphy JM, Cowan PJ. Regulated necrosis in kidney ischemia-reperfusion injury. Kidney Int. 2019;96(2):291–301. doi:10.1016/j.kint.2019.02.009.
- Shi J, Zhao Y, Wang K, et al. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature. 2015;526(7575):660–665.
- Jorgensen I, Miao EA. Pyroptotic cell death defends against intracellular pathogens. Immunol Rev. 2015;265(1):130–142. doi:10.1111/imr.12287.
- Tan G, Huang C, Chen J, Zhi F. HMGB1 released from GSDME-mediated pyroptotic epithelial cells participates in the tumorigenesis of colitis-associated colorectal cancer through the ERK1/2 pathway. J Hematol Oncol. 2020;13(1):149. doi:10.1186/s13045-020-00985-0.
- Man SM, Karki R, Kanneganti TD. Molecular mechanisms and functions of pyroptosis, inflammatory caspases and inflammasomes in infectious diseases. Immunol Rev. 2017;277(1):61–75. doi:10.1111/imr.12534.
- He Z, An S, Chen J, et al. Neural progenitor cell pyroptosis contributes to Zika virus-induced brain atrophy and represents a therapeutic target. Proc Natl Acad Sci U S A. 2020;117(38):23869–23878.
- Miao N, Yin F, Xie H, et al. The cleavage of gasdermin D by caspase-11 promotes tubular epithelial cell pyroptosis and urinary IL-18 excretion in acute kidney injury. Kidney Int. 2019;96(5):1105–1120.
- Zhang Z, Shao X, Jiang N, et al. Caspase-11-mediated tubular epithelial pyroptosis underlies contrast-induced acute kidney injury. Cell Death Dis. 2018;9(10):983.
- Kobori M, Yang Z, Gong D, et al. Wedelolactone suppresses LPS-induced caspase-11 expression by directly inhibiting the IKK complex. Cell Death Differ. 2004;11(1):123–130.
- Xu S, Liu X, Liu X, et al. Wedelolactone ameliorates Pseudomonas aeruginosa-induced inflammation and corneal injury by suppressing caspase-4/5/11/GSDMD-mediated non-canonical pyroptosis. Exp Eye Res. 2021;211:108750.
- Wang Y, Liu X, Wang Q, Yang X. Roles of the pyroptosis signaling pathway in a sepsis-associated encephalopathy cell model. J Int Med Res. 2020;48(8):300060520949767. doi:10.1177/0300060520949767.
- Huang L, Jiang Y, Sun Z, Gao Z, Wang J, Zhang D. Autophagy strengthens intestinal mucosal barrier by attenuating oxidative stress in severe acute pancreatitis. Dig Dis Sci. 2018;63(4):910–919. doi:10.1007/s10620-018-4962-2.
- Rathinam VAK, Zhao Y, Shao F. Innate immunity to intracellular LPS. Nat Immunol. 2019;20(5):527–533. doi:10.1038/s41590-019-0368-3.
- Kes P, Vucicevic Z, Ratkovic-Gusic I, Fotivec A. Acute renal failure complicating severe acute pancreatitis. Ren Fail. 1996;18(4):621–628. doi:10.3109/08860229609047686.
- Kayagaki N, Stowe IB, Lee BL, et al. Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature. 2015;526(7575):666–671.
- Ye Z, Zhang L, Li R, et al. Caspase-11 mediates pyroptosis of tubular epithelial cells and septic acute kidney injury. Kidney Blood Press Res. 2019;44(4):465–478. doi:10.1159/000499685.
- Aglietti RA, Estevez A, Gupta A, et al. GsdmD p30 elicited by caspase-11 during pyroptosis forms pores in membranes. Proc Natl Acad Sci U S A. 2016;113(28):7858–7863.
- Schrezenmeier EV, Barasch J, Budde K, Westhoff T, Schmidt-Ott KM. Biomarkers in acute kidney injury – pathophysiological basis and clinical performance. Acta Physiol (Oxf). 2017;219(3):554–572. doi:10.1111/apha.12764.
- Rabb H, Griffin MD, McKay DB, Acute Dialysis Quality Initiative Consensus XIII Work Group, et al. Inflammation in AKI: current understanding, key questions, and knowledge gaps. J Am Soc Nephrol. 2016;27(2):371–379.
- Zhu MM, Wang L, Yang D, et al. Wedelolactone alleviates doxorubicin-induced inflammation and oxidative stress damage of podocytes by IkappaK/IkappaB/NF-kappaB pathway. Biomed Pharmacother. 2019;117:109088.
- Fan R, Sui J, Dong X, Jing B, Gao Z. Wedelolactone alleviates acute pancreatitis and associated lung injury via GPX4 mediated suppression of pyroptosis and ferroptosis. Free Radic Biol Med. 2021;173:29–40. doi:10.1016/j.freeradbiomed.2021.07.009.
- Yang X, Zhang X, Lin Z, et al. Chaiqin chengqi decoction alleviates severe acute pancreatitis associated acute kidney injury by inhibiting endoplasmic reticulum stress and subsequent apoptosis. Biomed Pharmacother. 2020;125:110024.
- Ma D, Li C, Jiang P, Jiang Y, Wang J, Zhang D. Inhibition of ferroptosis attenuates acute kidney injury in rats with severe acute pancreatitis. Dig Dis Sci. 2021;66(2):483–492. doi:10.1007/s10620-020-06225-2.