Abstract
Cerebral ischemia-reperfusion (I/R) injury (CI/RI) is a severe problem in patients with cerebral ischemia. The current study explored the influences of circular (circ)-Gucy1a2 on neuronal apoptosis and mitochondrial membrane potential (MMP) in the brain tissue of CI/RI mice. Forty-eight mice were randomized into the sham group, transient middle cerebral artery occlusion (tMCAO) group, lentivirus negative control (LV-NC) group, and LV-Gucy1a2 group. Mice were first injected with lentivirus loaded with LV-Gucy1a2 or LV-NC via lateral ventricle, followed by the establishment of CI/RI models 2 weeks later. Twenty-four hours after CI/RI, the neurological impairment of mice was assessed using a 6-point scoring system. The cerebral infarct volume and brain histopathological changes were determined in CI/RI mice through histological staining. In vitro, pcDNA3.1-NC and pcDNA3.1-Gucy1a2 were transfected into mouse primary cortical neurons for 48 hours, followed by the establishment of oxygen-glucose deprivation/reoxygenation (OGD/R) models. The levels of circ-Gucy1a2 in mouse brain tissues and neurons were examined using RT-qPCR. Neuronal proliferation and apoptosis, MMP loss, and oxidative stress (OS)-related indexes in neurons were detected using CCK-8 assay, flow cytometry, JC-1 staining, and H2DFFDA staining. CI/RI mouse models and OGD/R cell models were successfully established. After CI/RI, neurons in mice were impaired and the cerebral infarction volume was increased. circ-Gucy1a2 was poorly expressed in CI/RI mouse brain tissues. Overexpression of circ-Gucy1a2 increased OGD/R-induced neuronal proliferation and mitigated apoptosis, MMP loss, and OS. Overall, circ-Gucy1a2 was down-regulated in brain tissues of CI/RI mice, and overexpression of circ-Gucy1a2 can protect mice from CI/RI.
Introduction
Cerebrovascular diseases are commonly recognized as a severe danger to human life since it causes high morbidity, disability, and relapse rates, 60-80% of which are ischemic [Citation1]. Hypoxia after cerebral ischemia leads to neuronal injury, and neuronal injury is further aggravated in a short time after blood perfusion recovery, which is known as cerebral ischemia-reperfusion (I/R) injury (CI/RI) [Citation2]. CI/RI is regarded as a pathophysiological process of most ischemic cerebrovascular diseases [Citation3]. Meanwhile, CI/RI stimulates neuronal apoptosis and results in hippocampal and cortical damage [Citation4]. Importantly, the regeneration of neurons may be a challenge once they are injured or dead [Citation5]. At present, CI/RI thrombolytic therapy is the most commonly used approach in the clinic [Citation6]. However, reperfusion further aggravates cerebral edema by increasing the generation of reactive oxygen species (ROS), leading to intracellular DNA damage, protein oxidation, oxidative stress (OS), and lipid peroxidation [Citation7]. Therefore, it is prudent to further figure out strategies to mitigate or prevent CI/RI.
Circular RNA (circRNA) is a unique endogenous non-coding RNA without a 5′ cap structure and 3′ tail structure, which is distinct from traditional linear RNA [Citation8]. In recent years, circRNAs have become the focus due to their stability, homology, and expression specificity in tissues/developmental stage [Citation9]. Compelling evidence exists to support the enrichment of circRNAs in mammalian nervous tissues and the involvement in the central nervous system [Citation10, Citation11]. Recent evidence in middle cerebral artery occlusion (MCAO) mouse models suggests that circRNAs participate in neuronal damage following I/R [Citation12]. For example, the down-regulation of circTLK1 in the brain tissue of mice with focal CI/RI significantly mitigates the cerebral infarction volume, reduces neuronal damage, and improves neurological deficits [Citation13]. Down-regulation of circFoxO3 could defend HT22 cells against glutamate-induced oxidative damage via the inhibition of mitochondrial apoptosis [Citation14]. Additionally, an existing study found poorly expressed circ-Gucy1a2 in oxygen-glucose deprivation/reperfusion (OGD/R)-treated neurons [Citation11]. However, whether circ-Gucy1a2 affects apoptosis and mitochondrial membrane potential (MMP) of neurons in CI/RI mice has not been verified. Therefore, we investigate the potential effects of circ-Gucy1a2 on apoptosis and MMP in CI/RI mice, hoping to confer theoretical support for the treatment methods for CI/RI.
Materials and methods
Ethics statement
All animal protocols and procedures were ratified by the Medical Ethics Committee of Sun Yat-sen Memorial hospital and conducted following the National Guidelines for laboratory animals. Adequate measures were taken to minimize the number of included mice and their pain.
Experimental animals
Male C57BL/6J mice (7-8 weeks old, weighing 22-24 g) supplied by Changsheng Biotechnology (Liaoning, China) [License No. SCXK (Liao) 2020-0002] were given ad libitum access to food and water and raised at 22-25 °C with 12 hours of light/dark cycles.
Establishment and grouping of CI/RI models in mice
Forty-eight mice were randomized to 4 groups (12 mice/group), including the sham group, transient MCAO (tMCAO) group, lentivirus negative control (LV-NC) group, and LV-Gucy1a2 group. Next, 1.5 μL lentiviral vector (109 TU·mL−1) loaded with LV-Gucy1a2 and empty lentiviral vector (LV-NC) were injected stereotaxically into the lateral ventricles (1.5 mm lateral to bregma, 1.1 mm posterior to bregma, and 2 mm beneath the brain surface) of mice in the LV-Gucy1a2 group and LV-NC group using a micropump (RWD, Shenzhen, China) at 0.2 μL/min, respectively [Citation15]. Animals in the sham and tMCAO groups were injected with normal saline as controls under the same injection conditions. The tMCAO model was established 2 weeks after injection. Lentiviruses of LV-Gucy1a2 and LV-NC groups were designed and synthesized by GenePharma (Shanghai, China). The specific experimental process is shown in .
The modeling of tMCAO was performed to establish the CI/RI mouse models. Following a previous report [Citation16], mice were first anesthetized with 3% isoflurane mixed with 30% O2 and 70% N2O and maintained with 1.5% isoflurane through a mask. The rectal temperature was kept at 37.0 ± 0.5 °C during the operation and postoperative recovery with the help of the temperature-controlled heating pads. After making a midline skin incision (1 cm) on the neck, the right common carotid artery (CA) was separated from the peripheral nerves under a stereo-anatomical microscope and sutured with a 4-0 silk thread. Following exposure and isolation from small arterial branches, the right external CA was ligated with a 6-0 thread, 3 mm from the beginning, and an external carotid arteriotomy was performed. Then, the silicone rubber-coated 6-0 nylon thread was put into the external carotid artery 9 ∼ 10 mm along the internal CA to the bifurcation of the CA and the beginning of the middle CA, causing focal cerebral ischemia. The middle cerebral artery of mice was occluded for 1 hour, followed by 24-hour reperfusion. For the sham group, the mice received the same procedure except the insertion of a nylon thread [Citation16]. After 24 hours of CI/RI, neurological impairment was assessed. Subsequently, the mice were euthanized by inhalation of 100% CO2 at 25% volume/min for 3-4 minutes, and their brains were quickly removed for subsequent analyses. Four mice in each group were used for 2,3, 5-triphenyltetrazole chloride (TTC) staining; four mice were used for Hematoxylin-Eosin (HE) staining; the remaining 4 mice were used for reverse transcription quantitative polymerase chain reaction (RT-qPCR).
Assessment of neurological impairment
Neurological deficits were evaluated in a blinded method using the 6-point scoring system [Citation17]. The neurological injury was scored as follows: 0, no significant deficits; 1, failing to extend the left forepaw adequately if the tail is pulled; 2: left contralateral circling if the tail is pulled; 3: circling or walking to the left; 4: walking only if stimulated; 5: no response to stimulation and with low consciousness.
TTC staining
The brain tissues were collected and made into coronal sections (2-mm-thick), incubated with 2% TTC (Millipore Sigma, NJ, USA) for 25 minutes at 37 °C, and then fixed. The white areas represent the infarct volume. The stained sections were observed and analyzed using ImageJ software. The infarct volume was calculated by integrating the infarct area of all sections and expressed as the percentage of the infarct area [Citation18, Citation19].
HE staining
HE staining was conducted to detect histopathological changes in the brain of tMCAO mice. Briefly, the brain tissues were infused with 0.9% normal saline and fixed with 4% paraformaldehyde. After removal of brain tissues, they were fixed with 4% paraformaldehyde and embedded in paraffin, and sliced into paraffined sections at 5 μm. After routine dewaxing and hydration, the sections were stained with HE staining solution (DH0006, Leagene, Beijing, China) and then stained with hematoxylin for 5 minutes. The excess hematoxylin staining solution on the slides was washed off with distilled water, and the sections were differentiated with 1% alcohol hydrochloride, washed with tap water to make the slices turn blue, and then the sections were stained with eosin and washed with double distilled water. Afterwards, the sections were dehydrated, cleared, and sealed with neutral gum, and observed and photographed using a microscope (Olympus, Tokyo, Japan).
Isolation, culture, transfection, and modeling of neurons
As previously described [Citation20], primary cortical neurons were obtained from day 17 embryos of C57BL/6 mice. Isolated primary cortical neurons were seeded into 24-well plates (1.5 × 105 cells/well) and cultured in the neurobasal medium (Gibco, NY, USA) containing 2% B27 (Gibco), 0.5 mM glutamine and 50 U/mL penicillin/streptomycin in an incubator with 5% CO2 at 37 °C. Half of the medium was changed every 4 days, and the incubation time was 9 to 10 days. In addition, 5 μg/mL arabinosylcytosine was added to inhibit the growth of non-neuronal cells after 72 hours of cell culture.
Primary cortical neurons were grown in 24-well plates (1 × 105 cells/well) overnight before transfection. pcDNA3.1-NC and pcDNA3.1-Gucy1a2 (20 nM, Ribobio, Guangzhou, China) were transfected into cells using LipofectamineTM2000 according to the instructions. At 48 hours after transfection, the oxygen-glucose deprivation/reoxygenation (OGD/R) model was established.
Following previous work [Citation21], the OGD/R model was established. Briefly, primary cortical neurons were washed with glucose-free Dulbecco’s modified Eagle medium (DMEM) (Gibco) and incubated in glucose-free DMEM in an anaerobic chamber containing 5% CO2 and 95% N2 at 37 °C. After OGD/R treatment for 3 hours, the cells were cultured in a normal neurobasal medium and recovered for 3 hours under normoxic conditions. Neurons in the control group were cultured under normal conditions.
Cell counting kit (CCK8) assay
The isolated neurons were seeded into 96-well plates and added with 10 μL CCK8 (Beyotime, Shanghai, China) reaction solution in each well at 37 °C for 60 minutes, followed by measurement of the absorbance at 450 nm.
Flow cytometry
Neuronal apoptosis was examined using a flow cytometer (FACSCalibur; BD Biosciences, CA, USA). Cell suspension (100 uL) was collected and stained with the Annexin V-FITC and propidium (Beyotime) and then incubated for 20 minutes in dark and evaluated by flow cytometry.
The reduction of MMP (Δψm) is considered a sign of cell apoptosis [Citation22]. The depolarization of Δψm was determined by JC-1 dye (BD Bioscience, NJ, USA) to reflect cell apoptosis. After treatment, the cells were incubated with 5 μM JC-1 in dark conditions at 37 °C for 20 minutes. The fluorescence intensity was analyzed by flow cytometry.
Determination of MMP
The MMP was determined using the JC-1 staining (Beyotime). After collecting neurons, the isolated mitochondria were purified following the kit protocol (SM0020, Solarbio, Beijing, China). The cells were added with 5 μg/mL JC-1 working solution (G009-2, Yaji Biotechnology, Shanghai, China) at 37 °C for 30 minutes to monitor changes in MMP. The excitation wavelength was 488 nm and the emission wavelength was measured at 590 nm and 525 nm. The red and green fluorescence intensities were examined using Image-Pro Plus software (Media Cybernetics, USA). Normal mitochondria emit strong red fluorescence, while those with reduced MMP emit green fluorescence. The loss of MMP was manifested as an upregulated ratio of green to red fluorescence intensity.
Determination of ROS
As previously described [Citation14], ROS levels were measured using the H2DCFDA kit (Invitrogen). In brief, the cells were loaded with 10 µmol/L H2DCFDA for 20 minutes at 37 °C in dark conditions. The fluorescence intensity was measured at 485/530 nm excitation/emission wavelength.
Detection of superoxide dismutase (SOD) and malondialdehyde (MDA)
The SOD and MDA levels in cells were examined using the SOD and MDA kits (Jiancheng Biology, Nanjing, China) strictly following the provided instructions. The absorbance was measured at 450 nm for SOD levels and 530 nm for MDA contents using a microplate reader (Bio-Rad Laboratories, CA, USA).
RT-qPCR
Total RNA content was isolated from brain tissue or neurons using the TRIzol reagent (Invitrogen, CA, USA). Subsequently, the obtained RNA was reverse transcribed into cDNA in accordance with the protocol of the reverse transcriptase kits (Takara, Dalian, China). RT-qPCR was subsequently conducted on the ABI 7500 rapid real-time PCR system (ABI, Foster City, CA, USA) using the SYBR Green real-time PCR Master Mix (Takara) and miScript SYBR Green PCR kit (Qiagen, German). Reaction conditions were as follows: 40 cycles of 94 °C for 30 s, 60 °C for 30 s, and 72 °C for 30 s. With β-actin as an internal parameter, the relative expression was computed by the 2-△△ct method. shows primer sequences.
Table 1. RT-qPCR primer sequences.
Statistical analysis
Data analyses and plotting were carried out using SPSS 21.0 (IBM Corp. NY, USA) and GraphPad Prism 8.01 (GraphPad Software, CA, USA). Data were expressed as mean ± standard deviation. Data comparisons between two groups were performed by the t test, data comparisons among groups were conducted by one-way ANOVA, and Tukey’s test was adopted for the post hoc test. P < 0.05 was indicative of statistical significance.
Results
circ-Gucy1a2 was poorly expressed in brain tissues of CI/RI mouse
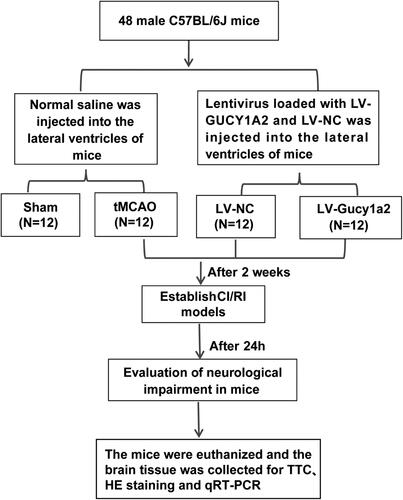
In an effort to measure the circ-Gucy1a2 expression in brain tissues of CI/RI mice, we first established the CI/RI mouse model using tMCAO and measured the neurobehavioral score and cerebral infarction volume of mice 24 h after reperfusion. We discovered significant differences in neurobehavioral scores and cerebral infarction volume between the sham and tMCAO groups (P < 0.01) (). After detection of the histopathological changes in the brain of tMCAO mice by HE staining, we found that the neurons of tMCAO mice lost the complete cellular structure, with contracted nucleus and cytoplasm (). Together, the aforementioned findings validated the successfully established mouse CI/RI models. Furthermore, RT-qPCR showed lower levels of circ-Gucy1a2 in brain tissues of tMCAO mice (P < 0.01) ().
Figure 2. Circ-gucy1a2 was poorly expressed in brain tissues of CI/RI mice. tMCAO was performed to establish the CI/RI mouse model. A: Neurobehavioral score was used to detect neurological impairment in mice, N = 12; B: TTC staining was used to observe the volume of cerebral infarction in CI/RI mice induced by tMCAO (marked by the yellow line), N = 4; C: HE staining was used to detect the pathological changes of mouse brain tissue, N = 4; D: RT-qPCR was used to detect the expression of circ-Gucy1a2 in brain tissues of mice, N = 4. Data were expressed as mean ± standard deviation, and the t test was used for data comparison between the two groups. ** P < 0.01.
Overexpression of circ-Gucy1a2 reduced the cerebral infarction volume in CI/RI mice
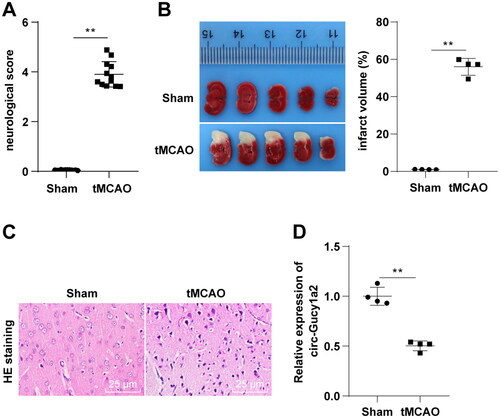
To verify the role of circ-Gucy1a2 in CI/RI mice, we injected the LV-Gucy1a2 lentiviral vectors and LV-NC lentiviral vectors into the lateral ventricles of mice, followed by the establishment of CI/RI mouse model 2 weeks later. RT-qPCR showed higher expression of circ-Gucy1a2 in the LV-Gucy1a2 group than the LV-NC group (P < 0.01) (), indicating that we successfully overexpressed circ-Gucy1a2. Subsequently, the neurobehavioral score and cerebral infarction volume were measured in tMCAO mice and the results noted that overexpression of circ-Gucy1a2 reduced the neurobehavioral score () and the cerebral infarction volume of mice (). Besides, we detected the histopathological changes in the brain of tMCAO mice by HE staining and discovered that overexpression of circ-Gucy1a2 alleviated the neuronal damage after CI/RI in mice (P < 0.01) ().
Figure 3. Overexpression of circ-Gucy1a2 reduced cerebral infarction volume in CI/RI mice. The LV-Gucy1a2 lentiviral vector and LV-NC lentiviral vector were injected into the lateral ventricles of mice to overexpress circ-Gucy1a2. Two weeks later, CI/RI mouse models were established. A: The expression of circ-Gucy1a2 was detected by RT-qPCR, N = 4; B: Neurobehavioral score was used to detect neurological impairment in mice, N = 12; C: TTC staining was used to observe the volume of cerebral infarction in CI/RI mice induced by tMCAO, N = 4; D: HE staining was used to detect the pathological changes of mouse brain tissue, N = 4. One-way ANOVA was used for data comparison among groups and Tukey’s test was used for the post hoc test. * P < 0.05, ** P < 0.01.
Overexpression of circ-Gucy1a2 alleviated OGD/R-induced neuronal apoptosis
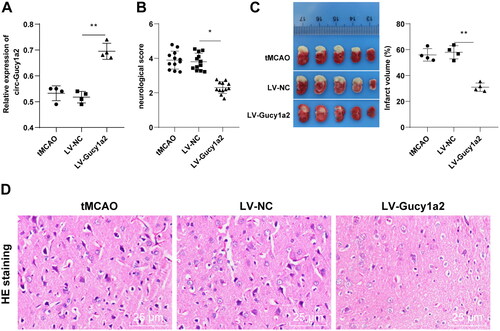
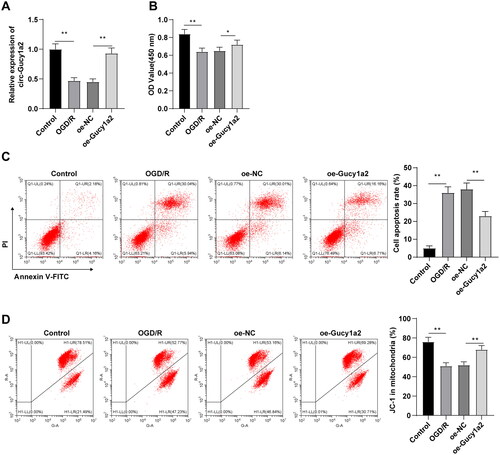
To verify the effects of circ-Gucy1a2 on neuronal proliferation and apoptosis of CI/RI mice, pcDNA3.1-NC and pcDNA3.1-Gucy1a2 were transfected into neurons, and OGD/R models were established 48 hours after transfection. Transfection efficiency was detected using RT-qPCR, which revealed that circ-Gucy1a2 was successfully overexpressed in neurons (P < 0.01) (). CCK-8 assay discovered that the proliferation of neurons in the OGD/R group was lowered relative to the control group (P < 0.01); but promoted in the oe-Gucy1a2 group in comparison with the oe-NC group (P < 0.05) (). In short, overexpression of circ-Gucy1a2 improved the OGD/R-induced neuronal proliferation. Additionally, flow cytometry elicited a higher apoptosis rate of neurons in the OGD/R group and lower JC-1 than those in the control group (P < 0.01); the apoptosis rate of neurons in the oe-Gucy1a2 group was lower and the JC-1 was higher than those in the oe-NC group (P < 0.01) (). Taken together, overexpression of circ-Gucy1a2 could mitigate OGD/R-induced neuronal apoptosis.
Figure 4. Overexpression of circ-Gucy1a2 reduced neuronal apoptosis induced by OGD/R. The pcDNA3.1-NC and pcDNA3.1-Gucy1a2 were transfected into cells, and the OGD/R model was established 48 hours after transfection. A: RT-qPCR was used to detect the expression of circ-Gucy1a2 in brain tissue. B: The proliferation ability of neurons was detected by CCK-8 assay; C and D: Apoptosis rate was detected by flow cytometry with Annexin V/PI staining and JC-1 staining. One-way ANOVA was used for data comparison among groups and Tukey’s test was used for the post hoc test. * P < 0.05, ** P < 0.01.
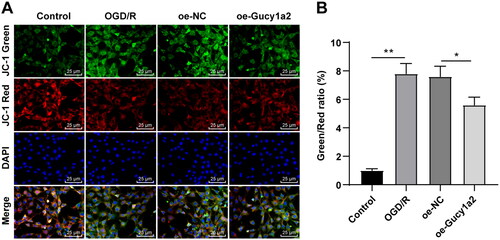
Overexpression of circ-Gucy1a2 alleviated the loss of MMP in neurons induced by OGD/R
Existing evidence suggests that hypoxia and glucose deprivation can lead to mitochondrial damage of cortical neurons [Citation23]. Therefore, we explored the effect of circ-Gucy1a2 on MMP of neurons. JC-1 staining revealed lower MMP in the OGD/R group and a higher ratio of green/red fluorescence intensity than those of the control group (P < 0.05). The MMP of neurons in the oe-Gucy1a2 group was higher and the green/red fluorescence intensity ratio was lower than those in the oe-NC group (P < 0.01) (). Collectively, overexpression of circ-Gucy1a2 can attenuate the loss of MMP in OGD/R-induced neurons.
Figure 5. Overexpression of circ-Gucy1a2 reduced the loss of MMP in neurons induced by OGD/R. A: MMP loss was detected by JC-1 staining. Red fluorescence indicates normal MMP, while green fluorescence indicates damaged mitochondria. B: Quantitative analysis of the ratio of green fluorescence intensity to red fluorescence intensity. One-way ANOVA was used for data comparison among groups and Tukey’s test was used for the post hoc test. * P < 0.05, ** P < 0.01.
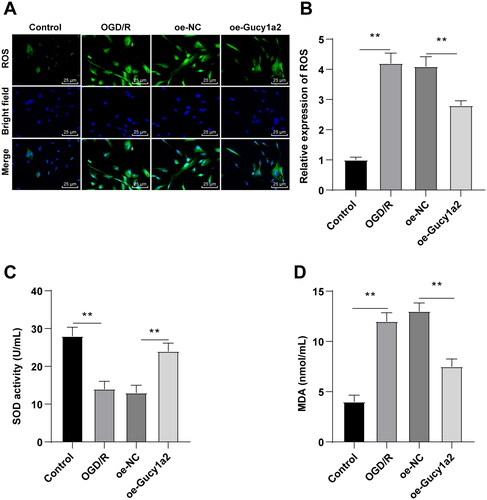
Overexpression of circ-Gucy1a2 reduced the OS in neurons induced by OGD/R
To verify the involvement of circ-Gucy1a2 in CI/RI-induced OS, we detected the levels of OS-related indicators ROS, SOD, and MDA in neurons. ROS level in the OGD/R group was augmented (), SOD activity was diminished (), and MDA content was upregulated (), while overexpression of circ-Gucy1a2 weakened the OS of OGD/R-induced neurons (all P < 0.01).
Figure 6. Overexpression of circ-Gucy1a2 reduced the increase of OS in neurons induced by OGD/R. A: ROS levels in neurons were detected by H2DFFDA staining; B: Quantitative analysis of ROS levels; C: The histogram shows SOD activity; D: The histogram shows MDA concentration. One-way ANOVA was used for data comparison among groups and Tukey’s test was used for the post hoc test. ** P < 0.01.
Discussion
CI/RI may aggravate the initial injury, which is the main contributor to global cerebrovascular death [Citation1, Citation24]. The pathogenesis of CI/RI is perplexing, including neuronal apoptosis, OS, and mitochondrial dysfunction [Citation25, Citation26]. circRNAs are abundant in the mammalian brain and are essential in many neurological diseases and ischemic stroke [Citation27, Citation28]. Circ-DLGAP4 plays a neuroprotective role in Parkinson’s disease through the miR-134-5p/CREB pathway [Citation29]. Circ-HIPK3 alleviates CoCl-induced neuronal apoptosis via the miR-222-3p/DUSP19 axis [Citation30]. Moreover, overexpression of circ-camk4 aggravated neuronal injury induced by CI/RI [Citation11]. There are few studies on circ-Gucy1a2 in CI/RI, and only one recent study reported that circ-Gucy1a2 expression was reduced in OGD/R-treated neurons. By establishing the CI/RI mouse model through tMCAO, we documented decreased circ-Gucy1a2 levels in the brain tissue of tMCAO mice.
Firstly, we injected the LV-Gucy1a2 lentiviral vectors into the lateral ventricles of mice to better understand the role of circ-Gucy1a2 in CI/RI. Upon the overexpression of circ-Gucy1a2, the neurobehavioral score, cerebral infarction volume, and neuronal damage of tMCAO mice were alleviated. Several existing studies have emphasized the importance of circRNAs in CI/RI. For instance, up-regulation of circUCK2 diminished cerebral infarct volume, alleviated neuronal injury, and improved neural defects [Citation31]. Overexpression of circDLGAP4 relieved neurological deficits, reduced infarct volume, and alleviated blood-brain barrier damage in tMCAO mice [Citation32]. But the neuroprotective mechanism of circ-Gucy1a2 in CI/RI has not been studied before. Our results highlighted that up-regulation of circ-Gucy1a2 attenuated cerebral infarction volume and neuronal injury in CI/RI mice.
Moreover, we analyzed the effects of circ-Gucy1a2 on OGD/R-induced neurons. Interestingly, the proliferation ability of neurons treated with OGD/R was reduced and the apoptosis rate of neurons was enhanced. On the contrary, overexpression of circ-Gucy1a2 can bring about an elevation of proliferation and a reduction of neuronal apoptosis. Several prior studies discovered the same effects of other circRNAs. Under the condition of OGD/R, circ_0062166 was up-regulated, which promoted cell proliferation and inhibited cell apoptosis [Citation33]. Similarly, circUCK2 elevation reduced apoptosis in CI/RI through the miR-125b-5p/GDF11 axis [Citation31]. Altogether, these findings and evidence illustrated that restoration of circ-Gucy1a2 can upgrade the proliferation ability of OGD/R-induced neurons and inhibit neuronal apoptosis.
The contribution of hypoxia and glucose deficiency to causing mitochondrial apoptosis in cortical neurons has been reported [Citation34]. Meanwhile, mitochondrial apoptosis is usually accompanied by a decrease in MMP [Citation35]. In the present study, the MMP was found to be decreased and the green/red fluorescence intensity ratio was increased in OGD/R-induced neurons. However, the MMP of neurons was enhanced after overexpression of circ-Gucy1a2, and the green/red fluorescence intensity ratio was reduced significantly. In summary, overexpression of circ-Gucy1a2 can prevent MMP loss in CI/RI mice. Mitochondrial failure leads to MMP loss, which affects ATP production and leads to OS [Citation36, Citation37]. There is evidence to suggest that a high concentration of ROS impairs the mitochondrial membrane, and mitochondrial damage induces ROS generation [Citation38]. In consequence, our findings discovered that ROS levels in the OGD/R group were elevated, SOD activity was reduced, MDA content was increased, and overexpression of circ-Gucy1a2 weakened the OS of neurons. Similarly, overexpression of circ_0003423 can reduce the OS of cerebral microvascular endothelial cells [Citation39]. Overall, our discoveries established that overexpression of circ-Gucy1a2 diminished the ascension of OS in neurons induced by OGD/R.
In conclusion, our findings demonstrated that overexpression of circ-Gucy1a2 inhibited neuronal apoptosis in CI/RI mice, alleviated the loss of MMP, and attenuated the ROS in neurons. This study further enriched the mechanism of circRNAs in CI/RI, providing a possible new target for the treatment of CI/RI. However, there are still some limitations. We only focused on the effects of circ-Gucy1a2 on neuronal apoptosis and MMP in CI/RI mouse brain tissue, but the mechanism of circ-Gucy1a2 regulating other downstream target genes and other circRNA in CI/RI needs further study. In addition, the sample size included in this study was small and the results of this study lack further clinical data.
A study has reported that FUS interacts with circ-Gucy1a2 in the CLIP-seq database. A previous report documented that nuclear FUS overexpression can cause neuronal death [Citation40]. Expression of Wt or ALS-related mutant FUS resulted in mitochondrial disruption in mammalian neuron-like cells, cultured neurons, and FUS transgenic drosophila, reduced MMP, and increased mitochondrial ROS production [Citation41]. Whether circ-Gucy1a2 can affect neuronal apoptosis and MMP in CI/RI mice through interaction with FUS remains to be clarified. In the future, the specific mechanism of circ-Gucy1a2 in regulating neuronal apoptosis and MMP loss in CI/RI mice needs further exploration, and other downstream target genes and the action mechanism of other circRNAs in CI/RI shall be studied in our future endeavors. Moreover, we need to expand the sample size for further exploration.
Authors’ contributions
FW contributes to the definition of learning concepts, learning design, and knowledge content; CM participated in editing and reviewing the manuscript; XLD has contributed to experimental research. QM is responsible for data acquisition and analysis; THW contributed to statistical analysis. All authors read and approved the final manuscript.
Ethics statement
All animal protocols and procedures have been ratified by the Medical Ethics Committee of Sun Yat-sen Memorial hospital and are strictly following the National Guidelines for laboratory animals. Adequate measures were taken to minimize the number of included mice and their pain.
Supplemental Material
Download PDF (71.4 KB)Availability of data and materials
All the data generated or analyzed during this study are included in this published article.
Disclosure statement
The authors declare that they have no competing interests.
References
- Zhao B, Li D, Zhang S, He L, Ai Y. Dexmedetomidine attenuates cerebral ischemia-reperfusion injury in rats by inhibiting the JNK pathway. Ann Palliat Med. 2021;10(6):1–10. doi:10.21037/apm-21-1218
- Li L, Zhang Q, Wang Y, et al. Knockdown of lncRNA TUG1 attenuates cerebral ischemia/reperfusion injury through regulating miR-142-3p. Biofactors. 2021;47(5):819–827. doi:10.1002/biof.1765
- Ding Y, Kang J, Liu S, Xu Y, Shao B. The protective effects of peroxisome proliferator-activated receptor gamma in cerebral ischemia-reperfusion injury. Front Neurol. 2020;11:588516. doi:10.3389/fneur.2020.588516
- Zhao JX, Tian YX, Xiao HL, Hu MX, Chen WR. Effects of electroacupuncture on hippocampal and cortical apoptosis in a mouse model of cerebral ischemia-reperfusion injury. J Tradit Chin Med. 2011;31(4):349–355. doi:10.1016/S0254-6272(12)60017-X
- Shan R, Zhou H, Liu X, et al. Neuroprotective effects of four different fluids on cerebral ischaemia/reperfusion injury in rats through stabilization of the blood-brain barrier. Eur J Neurosci. 2021;54(4):5586–5600. doi:10.1111/ejn.15385
- Cai HA, Tao X, Zheng LJ, et al. Ozone alleviates ischemia/reperfusion injury by inhibiting mitochondrion-mediated apoptosis pathway in SH-SY5Y cells. Cell Biol Int. 2020;44(4):975–984. doi:10.1002/cbin.11294
- Kishimoto M, Suenaga J, Takase H, et al. Oxidative stress-responsive apoptosis inducing protein (ORAIP) plays a critical role in cerebral ischemia/reperfusion injury. Sci Rep. 2019;9(1):13512. doi:10.1038/s41598-019-50073-8
- Taulli R, Loretelli C, Pandolfi PP. From pseudo-ceRNAs to circ-ceRNAs: a tale of cross-talk and competition. Nat Struct Mol Biol. 2013;20(5):541–543. doi:10.1038/nsmb.2580
- Song C, Zhang Y, Huang W, et al. Circular RNA Cwc27 contributes to Alzheimer’s disease pathogenesis by repressing Pur-alpha activity. Cell Death Differ. 2022;29(2):393–406. doi:10.1038/s41418-021-00865-1
- Lukiw WJ. Circular RNA (circRNA) in Alzheimer’s disease (AD). Front Genet. 2013;4:307. doi:10.3389/fgene.2013.00307
- Zhang ZH, Wang YR, Li F, et al. Circ-camk4 involved in cerebral ischemia/reperfusion induced neuronal injury. Sci Rep. 2020;10(1):7012. doi:10.1038/s41598-020-63686-1
- Mehta SL, Pandi G, Vemuganti R. Circular RNA expression profiles alter significantly in mouse brain after transient focal ischemia. Stroke. 2017;48(9):2541–2548. doi:10.1161/STROKEAHA.117.017469
- Wu F, Han B, Wu S, et al. Circular RNA TLK1 aggravates neuronal injury and neurological deficits after ischemic stroke via miR-335-3p/TIPARP. J Neurosci. 2019;39(37):7369–7393. doi:10.1523/JNEUROSCI.0299-19.2019
- Lin SP, Hu J, Wei JX, et al. Silencing of circFoxO3 protects HT22 cells from glutamate-induced oxidative injury via regulating the mitochondrial apoptosis pathway. Cell Mol Neurobiol. 2020;40(7):1231–1242. doi:10.1007/s10571-020-00817-2
- Deng L, Guo Y, Liu J, et al. Long noncoding RNA ANRIL knockdown attenuates neuroinflammation following ischemic stroke via suppressing the expression of NF-kappaB in vitro and in vivo. Neurol Res. 2021;43(9):767–777. doi:10.1080/01616412.2021.1934317
- Bai Y, Zhang Y, Han B, et al. Circular RNA DLGAP4 ameliorates ischemic stroke outcomes by targeting miR-143 to regulate endothelial-mesenchymal transition associated with blood-brain barrier integrity. J Neurosci. 2018;38(1):32–50. doi:10.1523/JNEUROSCI.1348-17.2017
- Peng Z, Wang S, Chen G, et al. Gastrodin alleviates cerebral ischemic damage in mice by improving anti-oxidant and anti-inflammation activities and inhibiting apoptosis pathway. Neurochem Res. 2015;40(4):661–673. doi:10.1007/s11064-015-1513-5
- Gao Y, Chen T, Lei X, et al. Neuroprotective effects of polydatin against mitochondrial-dependent apoptosis in the rat cerebral cortex following ischemia/reperfusion injury. Mol Med Rep. 2016;14(6):5481–5488. doi:10.3892/mmr.2016.5936
- Li TH, Sun HW, Song LJ, et al. Long non-coding RNA MEG3 regulates autophagy after cerebral ischemia/reperfusion injury. Neural Regen Res. 2022;17(4):824–831. doi:10.4103/1673-5374.322466
- Liu J, Yu Z, Guo S, et al. Effects of neuroglobin overexpression on mitochondrial function and oxidative stress following hypoxia/reoxygenation in cultured neurons. J Neurosci Res. 2009;87(1):164–170. doi:10.1002/jnr.21826
- Guo D, Ma J, Yan L, et al. Down-regulation of Lncrna MALAT1 attenuates neuronal cell death through suppressing Beclin1-dependent autophagy by regulating Mir-30a in cerebral ischemic stroke. Cell Physiol Biochem. 2017;43(1):182–194. doi:10.1159/000480337
- Wang K, Chen Z, Huang J, et al. Naringenin prevents ischaemic stroke damage via anti-apoptotic and anti-oxidant effects. Clin Exp Pharmacol Physiol. 2017;44(8):862–871. doi:10.1111/1440-1681.12775
- Tanaka S, Takehashi M, Iida S, et al. Mitochondrial impairment induced by poly(ADP-ribose) polymerase-1 activation in cortical neurons after oxygen and glucose deprivation. J Neurochem. 2005;95(1):179–190. doi:10.1111/j.1471-4159.2005.03353.x
- Zhai Y, Zhu Y, Liu J, et al. Dexmedetomidine post-conditioning alleviates cerebral ischemia-reperfusion injury in rats by inhibiting high mobility group protein B1 group (HMGB1)/toll-like receptor 4 (TLR4)/nuclear factor kappa B (NF-kappaB) signaling pathway. Med Sci Monit. 2020;26:e918617. doi:10.12659/MSM.918617
- Huang Y, Wang Y, Duan Z, et al. Restored microRNA-326-5p inhibits neuronal apoptosis and attenuates mitochondrial damage via suppressing STAT3 in cerebral ischemia/reperfusion injury. Nanoscale Res Lett. 2021;16(1):63. doi:10.1186/s11671-021-03520-3
- Wu MY, Yiang GT, Liao WT, et al. Current mechanistic concepts in ischemia and reperfusion injury. Cell Physiol Biochem. 2018;46(4):1650–1667. doi:10.1159/000489241
- Jin P, Huang Y, Zhu P, Zou Y, Shao T, Wang O. CircRNA circHIPK3 serves as a prognostic marker to promote glioma progression by regulating miR-654/IGF2BP3 signaling. Biochem Biophys Res Commun. 2018;503(3):1570–1574. doi:10.1016/j.bbrc.2018.07.081
- Xiang Q, Kang L, Wang J, et al. CircRNA-CIDN mitigated compression loading-induced damage in human nucleus pulposus cells via miR-34a-5p/SIRT1 axis. EBioMedicine. 2020;53:102679. doi:10.1016/j.ebiom.2020.102679
- Feng Z, Zhang L, Wang S, Hong Q. Circular RNA circDLGAP4 exerts neuroprotective effects via modulating miR-134-5p/CREB pathway in Parkinson’s disease. Biochem Biophys Res Commun. 2020;522(2):388–394. doi:10.1016/j.bbrc.2019.11.102
- Liu Y, Ao S, Zhang H, et al. Circ_HIPK3 alleviates CoCl2-induced apoptotic injury in neuronal cells by depending on the regulation of the miR-222-3p/DUSP19 axis. Biochem Biophys Res Commun. 2021;553:126–133. doi:10.1016/j.bbrc.2021.03.070
- Chen W, Wang H, Feng J, Chen L. Overexpression of circRNA circUCK2 attenuates cell apoptosis in cerebral ischemia-reperfusion injury via miR-125b-5p/GDF11 signaling. Mol Ther Nucleic Acids. 2020;22:673–683. doi:10.1016/j.omtn.2020.09.032
- Bai, Y, Zhang Y, Han B, et al. Circular RNA DLGAP4 ameliorates ischemic stroke outcomes by targeting miR-143 to regulate endothelial-mesenchymal transition associated with blood-brain barrier integrity. J Neurosci. 2020;40:8601. doi:10.1523/JNEUROSCI.2320-20.2020
- Guo Z, Zhang G, Xu B, et al. Circ_0062166 aggravates cerebral ischemia-reperfusion injury through targeting miR-526b-5p/BCL2L13 axis. Brain Inj. 2021;35(10):1245–1253. doi:10.1080/02699052.2021.1972143
- Meng L, Ma H, Meng J, Li T, Zhu Y, Zhao Q. Costunolide attenuates oxygenglucose deprivation/reperfusioninduced mitochondrialmediated apoptosis in PC12 cells. Mol Med Rep. 2021;23(6):1–10. doi:10.3892/mmr.2021.12050
- Zhou Y, Zhou X, Hong T, et al. Lysosome-mediated mitochondrial apoptosis induced by tea polysaccharides promotes colon cancer cell death. Food Funct. 2021;12(21):10524–10537. doi:10.1039/D1FO00987G
- Panickar KS, Anderson RA. Effect of polyphenols on oxidative stress and mitochondrial dysfunction in neuronal death and brain edema in cerebral ischemia. Int J Mol Sci. 2011;12(11):8181–8207. doi:10.3390/ijms12118181
- Wang S, Li Y, Song X, et al. Febuxostat pretreatment attenuates myocardial ischemia/reperfusion injury via mitochondrial apoptosis. J Transl Med. 2015;13:209. doi:10.1186/s12967-015-0578-x
- Liu Y, Shao E, Zhang Z, et al. A novel indolizine derivative induces apoptosis through the mitochondria p53 pathway in HepG2 cells. Front Pharmacol. 2019;10:762. doi:10.3389/fphar.2019.00762
- Yu H, Pan Y, Dai M, Xu H, Li J. Circ_0003423 alleviates ox-LDL-induced human brain microvascular endothelial cell injury via the miR-589-5p/TET2 network. Neurochem Res. 2021;46(11):2885–2896. doi:10.1007/s11064-021-03387-x
- Suzuki H, Matsuoka M. Overexpression of nuclear FUS induces neuronal cell death. Neuroscience. 2015;287:113–124. doi:10.1016/j.neuroscience.2014.12.007
- Deng J, Yang M, Chen Y, et al. FUS interacts with HSP60 to promote mitochondrial damage. PLoS Genet. 2015;11(9):e1005357. doi:10.1371/journal.pgen.1005357