Abstract
Purpose
In vivo models are anatomically comparable to humans allowing to reproduce the patterns and progression of the disease and giving the opportunity to study the symptoms and responses to new treatments and materials. This study aimed to establish a valid and cost-effective in vivo rat model to assess the effects of implanted shoulder hemiarthroplasty materials on glenoid articular cartilage wear.
Methods
Eight adult male Wistar rats underwent right shoulder hemi-arthroplasty. A stainless steel metal bearing was used as a shoulder joint prosthesis. X-rays were performed one week after surgery to verify correct implant position. Additional X-rays were performed 30 and 60 days post-implantation. Animals were sacrificed 24 weeks after implantation. All specimens were evaluated with micro-CT for cartilage and bone wear characteristics as well as histologically for signs of osteoarthritis. Samples were compared to the non-operated shoulders.
Results
All animals recovered and resumed normal cage activity. All X-rays demonstrated correct implant positioning except for one in which the implant was displaced. Histologic evaluation demonstrated arthritic changes in the implanted shoulder. Decreased Trabecular thickness and Trabecular Spacing were documented among the implanted parties (p < .05). Bone Mineral Density and Tissue Mineral Density were reduced in the operated shoulder although not significantly (p = .07).
Conclusions
This study demonstrated significant glenoid cartilage wearing in the operated shoulder. Furthermore, the presence of an intra-articular hemiarthroplasty implant diminished underlying glenoid bone quality. This novel, in vivo-model will enable researchers to test implant materials and their effects on cartilage and bone tissue in a cost-effective reproducible rat model.
Introduction
The glenohumeral joint is a highly mobile joint stabilized by both active and passive structures, including the rotator cuff muscles, the glenohumeral ligament and the glenoid labrum [Citation1]. Complex fracture of the proximal humerus pose a challenge to the orthopedic surgeons, and may be treated in a conservative and operative manner including open reduction and internal fixation, total and partial shoulder replacement [Citation2].
Hemiarthroplasty is a common orthopedic procedure used to replace various joints including the hip, shoulder, knee, and small joints. Despite the development of better biomaterials and surgical techniques, tissue wear following hemiarthroplasty remains a major concern for orthopedic surgeons [Citation3]. New recovery methods following arthroplasty, such as the fast-track recovery, and material developments in orthopedics have created a need for in vivo models which serve to explore the effects of these innovative materials on periarticular tissues [Citation4].
Apart for incongruent joint mating surfaces, the main cause of cartilage wear is generally considered to be the tribology of the joint material surfaces [Citation5]. Pyrolytic carbon prothesis demonstrate a modulus of elasticity that resembles that of hyaline cartilage, and are therefore considered to offer favorable joint wear properties [Citation6]. Testing new materials used for arthroplasty bearing surfaces requires valid in vivo models that are generally expensive and somewhat impractical. Some models using a free interpositional arthroplasty concept for the shoulder, in which the humeral head is exchanged by a ball shaped prosthesis, have already proved successful and been termed “The snooker ball arthroplasty” [Citation7,Citation8]. Kramer et al. [Citation9] described a rat model for the development of shoulder rotator cuff arthropathy related to massive rotator cuff tears. However, rat models for the development of hemiarthroplasty-related shoulder osteoarthritis have yet to be described.
The primary goal of this study was to design a valid, cost-effective, reproducible and clinically relevant in vivo rat model that will allow: (1) for the implantation of hemiarthroplasty prosthetic bearing surface materials and to evaluate their effect on the glenoid cartilage wear and bone quality; (2) to evaluate the degree of glenoid cartilage wear as well as bone quality after shoulder joint hemiarthroplasty with a stainless-steel ball bearing prosthesis.
Materials and methods
Study design
This study was accepted by the local Institutional Animal Care and Use Committee (approval number 12-3-16). Eight adult male Wistar rats (250–300 g) at the age of 3 months were obtained for use in this study (Harlan Laboratories Ltd, Israel). Rats were housed in pairs in a conventional facility. The animals were preserved on a 12-hr light/12-hr dark cycle at 21–22 °C and acclimatized for 7 days prior to the experiments. The rats were allowed ad libitum access to food and water.
Surgical procedure
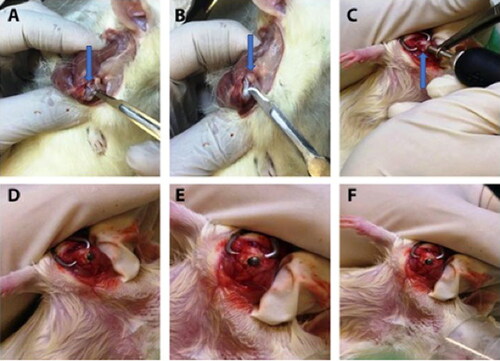
Anasthesia carried out with isoflurane under high flow oxygen. The trans-deltoid lateral approach was used. Skin incision was performed proximal and distal to the lateral border of the acromion, in a parallel axis to the humerus. The middle third (acromial) part of the deltoid muscle was exposed and split between its longitudinal fibers (A + B). The muscle was then retracted to allow exposure of the cranial third of the humerus. The anatomical landmarks (subscapularis tendon, lesser tuberosity, bicipital groove with the bicipital tendon and the greater tuberosity) were identified. Gentle sharp dissection of the anterior fibers of the supraspinatus and the superior fibers of the subscapularis tendons at the rotator interval was performed. Biceps tendon tenotomy followed by dislocation of the humeral head was performed. The center of the humeral head was identified and burred with a dremel tool and conical burr (Skill Tools, Mt. Prospect, Illinois, USA). The burr hole was expanded to create a round cavity with thin cortices in the proximal humeral head at the level of the rotator cuff insertion to the tuberosities (). After measurements of the native humeral head, a 2.7–2.9 mm stainless steel metal bearing ball was inserted into the cavity (, F). Joint reduction was then performed. Stability and joint mobility were tested. Closure of the supraspinatus and subscapularis were performed with 4/0 vicryl. The deltoid was then closed with 4/0 vicryl and the skin with 4/0 nylon sutures.
Figure 1. Surgical procedure. A blue arrow points on the humeral head. A + B. Exposure of the proximal humerus. C. The center of the humeral head is burred through the upper part to achieve a round cavity with thin cortices. The blue arrow points on the humeral head. D. The implant (2.7 mm stainless steel barring) is inserted into the cavity.
Histologic evaluation
Glenohumeral joint harvest and decalcification
Euthanasia of animals took place at 24 weeks post-surgery, followed by amputation of the operated limbs and contralateral control limbs at the clavicle level. Both the clavicle and humerus were dissected, conserving the glenohumeral joint and the adjacent tissue. Upon completion of decalcification, the glenohumeral joints were dehydrated and the anterior aspects of the joints were embedded face down in a neutral position in paraffin. Sectioning was performed in the coronal plane. Seven-micron-thick slices were acquired and mounted on slides for histologic evaluation.
Safranin O staining and modified Mankin score evaluation
Slides were stained with Safranin O and hematoxylin with fast green counterstains to identify proteoglycan matter. Modified Mankin scores (MMSs) for histologic assessment of osteoarthritis was performed by two independent musculoskeletal pathologists. The Mankin scoring system is a widely used scale based on qualitative valuations of four parameters [Citation10]. The four parameters are structure, cellularity, safranin O staining, and tidemark integrity.
X-ray evaluation
X-rays were performed 1 week after the surgery to verify correct implant position. Additional X-rays were conducted at 30 and 60 days post-surgery to evaluate implant stability and radiographic signs of joint narrowing or arthritis.
MicroCT scan evaluation
Glenoid bone density was evaluated using micro-computed tomography (μCT) (Xradia Micro-CT X-ray Tomographic Microscope, Zeiss, Germany). The humeri were conserved in a medium of 4% neutral buffered formalin for 48 h and then stored in 70% ethanol. μCT scans were executed at energy 90 kVp, tube intensity 200 μA, integration time 1000 msec, with an isotropic nominal resolution of 17.2 μm. Trabecular Thickness (Tb.Th, mm), Trabecular Spacing (mm), Tissue Mineral Density (TMD), Bone Volume (BV) and Bone Volume Fraction (Bone Volume (BV)/Total Volume (TV) were measured.
Statistical analysis
All statistical comparisons were performed between the implanted and control groups at an identical time point. µCT comparisons were made using Student’s t-tests with significance set a p <. 05. SPSS software (IBM SPSS 21) was used for data analysis. All variables are presented as mean ± standard deviation.
Results
The mean operative time for all rat hemiarthroplasty procedures was 15 min.
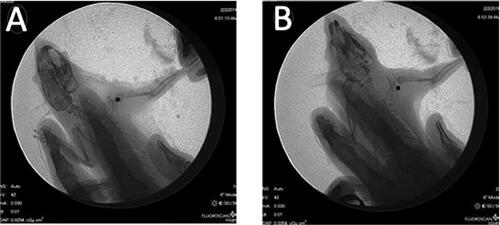
All animals recovered and resumed normal cage activity. All rats demonstrated normal gait patterns and food consumption postoperatively. In addition, there were no signs of infection or any significant injuries in any of the animals’ shoulders. One-week post implantation, X-ray examination was performed to ensure implants position (C). In one rat the implant was displaced, this was believed to be secondary to iatrogenic calcar fracture as evidenced on X-ray imaging ().
Figure 2. Representative post-operative X-rays. A. Implants are positioned correctly. B. Implant is displaced.
Histology
At 24 weeks post prosthesis implantation, histologic evaluation demonstrated severe arthritic changes in the implanted rat shoulders as compared to normal controls. H&E staining analysis of cartilage from a normal rat shoulder presented a well-preserved morphological structure with no signs of cartilage degradation. Subchondral bone and tidemark integrity were well seen. Furthermore, the surface of healthy hyaline cartilage appears white, shiny, elastic and firm (, B). Severe articular destruction was noted in all specimens. Severe OA cartilage demonstrated deep surface clefts, disappearance of cells from the tangential zone, cloning, and a lack of cells in the intermediate and radial zones, which are not arranged in columns. Implanted shoulders revealed loss of safranin-O staining as well as a loss of tidemark integrity (, D).
Figure 3. A. Control HE, B. Safranin O—Control, C. Implant HE, D—Safranin O—Implant. (A—Normal cartilage tissue; B—Arrow points on the deep zone of the cartilage tissue; C, D—Severe OA damage, cartilage and subchondral bone destruction.
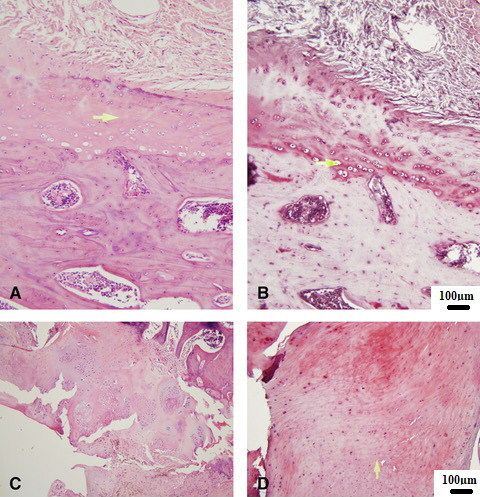
A—Normal cartilage tissue (control).
B—Arrow points on the deep zone of the cartilage tissue (control).
C, D—Severe OA damage, cartilage and subchondral bone destruction.
Micro CT
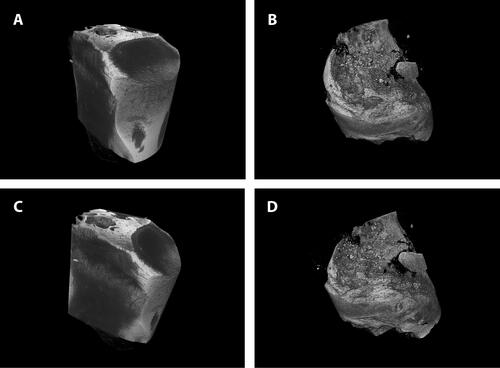
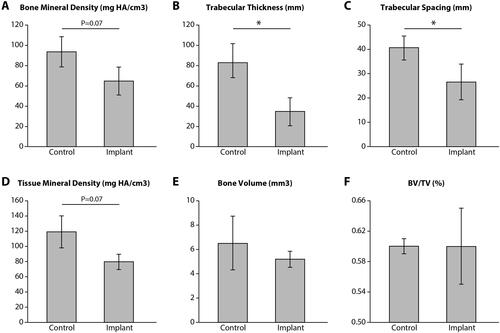
Glenoid bone trabecular thickness (Tb.Th, mm) was significantly lower in the implanted group as compared to controls (34.3 mm vs. 82.6 mm, p<.05). Trabecular Spacing (mm) was also significantly lower in the implanted shoulders (26.7 mm vs. 40.7 mm, p <.05). Additionally, bone mineral density (BMD) and tissue mineral density (TMD) were lower (not significantly −80 mgHA cm3 vs. 119 mgHA cm3, p=.07) in the implanted shoulders as compared to the control group. No significant differences were detected in terms of bone volume and BV/TV (). shows representative micro–computed tomography specimens for the implantation group and matched controls (A—Control, B—Implant; C—Control, and D—Implant).
Figure 4. Micro-computed tomography results. Glenoid bone trabecular thickness (Tb.Th, mm) and trabecular spacing (mm) were significantly lower in the implanted group (p<.05). Additionally, bone mineral density (BMD) and tissue mineral density (TMD) were lower (not significantly—p=.07) in the implanted shoulders as compared to the control group. There were no significant differences in bone volume and BV/TV Graphs show mean ± SD. *P<.05.
Discussion
The primary outcome of this study demonstrates that implanted metal ball bearings in rat shoulders successfully creates a cost-effective shoulder hemiarthroplasty animal model. Furthermore, this model produced significant wearing of rat glenoid cartilage as well as peri-implant arthritic changes which were confirmed with micro CT and histologic evaluation.
Cartilage wear caused by prosthetic implants remains an issue of great concern. Animal models are an essential research tool used to evaluate the material properties of various implants and their effects on cartilage wear rates and the surrounding bone quality. However, many studies addressing prosthetic surface material evaluation performed on small to medium animals require special prosthesis design [Citation11]. The model developed in this study uses a simple 2.7–2.9 mm ball bearing. This shape may facilitate streemlined manufacturing of novel materials for testing. Numerous anatomic and functional differences between human shoulders and those of animals exist, therefore an exact replica of the human shoulder cannot be found in an animal model. However, in light of the close resemblance to human shoulder [Citation12], the rat shoulder is captured as a favorable model to study shoulder pathologies. Additionally, the rat model provides initial safety for new material testing, an accelerated chronology, while the results of novel biologic treatments remain fairly generalizable to humans [Citation13].
A vast majority of available OA animal models are designed to mimic knee OA. Invasive models (surgical and chemical) are mainly used to investigate the pathogenesis of post-traumatic OA and to evaluate the efficacy of new possible treatment modalities for OA. Anterior cruciate ligament transection (ACLT) is a popular model for the investigation of OA in vivo [Citation14]. Ramme AJ. and colleagues [Citation15] created a noninvasive rat model for anterior cruciate ligament (ACL) separation and subchondral micro-damage provocation. Another regularly performed procedure for OA investigation involves the destabilization of the medial meniscus. Chemically induced models of OA eliminate the need for surgery, they are technically non-demanding, and are useful for short-term studies. For example, intra-articular injection of mono-iodoacetate (MIA) in rats, induces chondrocyte death, leading to osteophyte formation, rapid inflammation, chronic pain, hyperalgesia and allodynia [Citation14]. One of the major limitations of the aforementioned models is the relatively rapidity of the processes that may leave a restricted time window for studying various treatments and determining therapeutic effects.
Shoulder OA models are uncommon and have not been studied as much as knee models. The rat model described by Kramer et al. [Citation9] accurately reproduces rotator cuff muscle degradation following massive rotator cuff tears induced by either infraspinatus and supraspinatus tenotomy or suprascapular nerve injury. With the aid of a modified Mankin scoring system, Kramer demonstrated a significant glenohumeral cartilage damage following both rotator cuff tenotomy and suprascapular nerve injury after a 12 weeks period. However, this model simulates a patterns of cuff tear arthropathy (CTA) rather than OA and may be more applicable as a cuff tear arthropathy model than as one to study shoulder OA. Zingman A. et al. [Citation16] assessed the advancement of CTA in a mice model by inducing massive rotator cuff tears (RCTs). The authors aimed to design a mouse model of CTA that displays pathological modifications similar to that identified in humans, and to develop a novel histopathological scoring system. They demonstrated a progressive and time-dependent pattern of post tear pathologies in the shoulder joint with features similar to human cuff tear arthropathy. Micro-CT evaluation demonstrated acetabularization of the acromion and femoralization of the humeral head [Citation16]. These results are in line with the results of the presented study demonstrating humeral structural bony defects in micro-CT. A recent study by Sheppard et al. [Citation17] presented a novel in vivo mouse model replicating prosthetic shoulder infection. A surgical implant was press-fit into the proximal humerus of mice and inoculated with Staphylococcus bacteria. Their model offers a basis to the investigation of new therapeutics, interventions, and host modifications. This innovative model however has not been proven as a system to determine cartilage wear rate and the response of the local bone environment to shoulder hemiarthroplasty.
In the current study we created a clinically relevant unique rat model that mimics shoulder joint hemiarthroplasty in humans. By comparing the glenoid cartilage damage and bone quality in the replaced shoulder to the native contralateral shoulder, we could evaluate the differential effects of the implant on the glenoid. This study suggests that rat proximal humerus “snooker ball” hemiarthropasty is feasible, reliable, cost-effective, and produces significant osteoarthritic changes within 24 weeks. The simple architecture of the implant (2.7 mm ball) allows comparison and testing of different materials.
This study does have limitations. Although sharing similar shoulder anatomical landmarks, rats are quadrupedal and therefore have different joint loading mechanics [Citation13]. Furthermore, only one time point was assessed in this study. All animals were sacrificed at 24 weeks when the erosions on the glenoid side were progressive. It is probable that the rate of cartilaginous degeneration would be different when comparing different materials. For example, if the wear profile of one material demonstrates a more acute progression profile it would be hard to understand the chronological events without animal sacrifice at various timepoints. Additional limitations consist of the lack of a control group and the relatively small number of investigated rats. Further studies of this model with multiple implant materials and animal sacrifice at various time points will lend insight into the wear patterns and chronology of cartilage and bone wear in shoulder hemiarthroplasty.
Conclusions
The results of this study demonstrated significant glenoid cartilage wearing following shoulder hemiarthroplasty. Furthermore, this study establishes a novel, cost-effective and reproduceable rat model mimicking hemiarthroplasty of the shoulder joint, and permits the testing of new materials and the biological responses of the joint tissues to the implanted materials. This is an effective in vivo model and preclinical tool that serves as an essential step prior to the initiation of clinical trials involving novel materials in humans.
Authors’ contributions
Efi Kazum: Literature search, study design, data analysis, data interpretation, writing. Eran Maman: Literature search, study design, data collection, data analysis, data interpretation, writing. Zachary T. Sharfman: Study design, data collection, data analysis, data interpretation, writing. Reut Wengier: Study design, data collection, data analysis, data interpretation. Osnat Sher: Histological assessment, data interpretation, writing. Amal Khoury: In vivo experiments design, biomechanical testing, data interpretation. Ofir Chechik: Literature search, study design, data analysis, data interpretation, writing. Oleg Dolkart: Literature search, study design, data analysis, data interpretation, writing.
Ethical approval
All procedures were approved by the Institutional Animal Care and Use Committee
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Almajed YA, Hall AC, Gillingwater TH, Alashkham A. Anatomical, functional and biomechanical review of the glenoid labrum. J Anat. 2022;240(4):1–7. doi:10.1111/joa.13582.
- Nelson PA, Kwan CC, Tjong VK, Terry MA, Sheth U. Primary versus salvage reverse total shoulder arthroplasty for displaced proximal humerus fractures in the elderly: a systematic review and meta-analysis. J Shoulder Elb Arthroplast. 2020;4:2471549220949731. doi:10.1177/2471549220949731.
- Carpenter SR, Urits I, Murthi AM. Porous metals and alternate bearing surfaces in shoulder arthroplasty. Curr Rev Musculoskelet Med. 2016;9(1):59–66. doi:10.1007/s12178-016-9319-x.
- Castorina S, Guglielmino C, Castrogiovanni P, et al. Clinical evidence of traditional vs fast track recovery methodologies after total arthroplasty for osteoarthritic knee treatment. A retrospective observational study. Muscles Ligaments Tendons J. 2017;7(3):504–513. doi:10.11138/mltj/2017.7.3.504.
- Szychlinska MA, Leonardi R, Al-Qahtani M, Mobasheri A, Musumeci G. Altered joint tribology in osteoarthritis: reduced lubricin synthesis due to the inflammatory process. New horizons for therapeutic approaches. Ann Phys Rehabil Med. 2016;59(3):149–156. doi:10.1016/j.rehab.2016.03.005.
- Salkeld SL, Patron LP, Lien JC, Cook SD, Jones DG. Biological and functional evaluation of a novel pyrolytic carbon implant for the treatment of focal osteochondral defects in the medial femoral condyle: assessment in a canine model. J Orthop Surg Res. 2016;11(1):155. doi:10.1186/s13018-016-0488-5.
- Garret J, Godeneche A, Boileau P, et al. Pyrocarbon interposition shoulder arthroplasty: preliminary results from a prospective multicenter study at 2 years of follow-up. J Shoulder Elbow Surg. 2017;26(7):1143–1151. doi:10.1016/j.jse.2017.01.002.
- Hudek R, Werner B, Abdelkawi AF, Gohlke F. Pyrocarbon interposition shoulder arthroplasty in advanced collapse of the humeral head. Orthopade. 2017;46(12):1034–1044. Interpositions-Hemiprothese aus Pyrocarbon bei fortgeschrittener Humeruskopfnekrose. doi:10.1007/s00132-017-3495-2.
- Kramer EJ, Bodendorfer BM, Laron D, et al. Evaluation of cartilage degeneration in a rat model of rotator cuff tear arthropathy. J Shoulder Elbow Surg. 2013;22(12):1702–1709. doi:10.1016/j.jse.2013.03.014.
- Mankin HJ, Dorfman H, Lippiello L, Zarins A. Biochemical and metabolic abnormalities in articular cartilage from osteo-arthritic human hips. II. Correlation of morphology with biochemical and metabolic data. J Bone Joint Surg Am. 1971;53(3):523–537. doi:10.2106/00004623-197153030-00009.
- Jie K, Deng P, Cao H, Feng W, Chen J, Zeng Y. Prosthesis design of animal models of periprosthetic joint infection following total knee arthroplasty: a systematic review. PLoS One. 2019;14(10):e0223402. doi:10.1371/journal.pone.0223402.
- Soslowsky LJ, Carpenter JE, DeBano CM, Banerji I, Moalli MR. Development and use of an animal model for investigations on rotator cuff disease. J Shoulder Elbow Surg. 1996;5(5):383–392. doi:10.1016/S1058-2746(96)80070-X.
- Derwin KA, Baker AR, Iannotti JP, McCarron JA. Preclinical models for translating regenerative medicine therapies for rotator cuff repair. Tissue Eng Part B Rev. 2010;16(1):21–30. doi:10.1089/ten.TEB.2009.0209.
- Samvelyan HJ, Hughes D, Stevens C, Staines KA. Models of osteoarthritis: relevance and new insights. Calcif Tissue Int. 2021;109(3):243–256. doi:10.1007/s00223-020-00670-x.
- Ramme AJ, Lendhey M, Raya JG, Kirsch T, Kennedy OD. A novel rat model for subchondral microdamage in acute knee injury: a potential mechanism in post-traumatic osteoarthritis. Osteoarthritis Cartilage. 2016;24(10):1776–1785. doi:10.1016/j.joca.2016.05.017.
- Zingman A, Li H, Sundem L, et al. Shoulder arthritis secondary to rotator cuff tear: a reproducible murine model and histopathologic scoring system. J Orthop Res. 2017;35(3):506–514. doi:10.1002/jor.23383.
- Sheppard WL, Mosich GM, Smith RA, et al. Novel in vivo mouse model of shoulder implant infection. J Shoulder Elbow Surg. 2020;29(7):1412–1424. doi:10.1016/j.jse.2019.10.032.