Abstract
Background
Surgical site infections (SSI) are common complications after surgery, which cause other complications and increase medical costs. However, the effect of negative-pressure wound therapy (NPWT) for the prevention of SSI at stoma reversal remains inconclusive, with controversial results. This meta-analysis aimed to evaluate the safety and efficacy of NPWT following stoma reversal in colorectal surgery to prevent SSI and other wound complications.
Methods
We conducted a systematic search of the PubMed, EMBASE, and Cochrane Library databases for articles published up to July 2022 and identified relevant studies reporting the NPWT administration following stoma reversal in colorectal surgery compared with non-pressure dressing. The primary outcome was the incidence of SSI, and the secondary outcomes were hematoma, seroma, and length of hospital stay (LOS).
Results
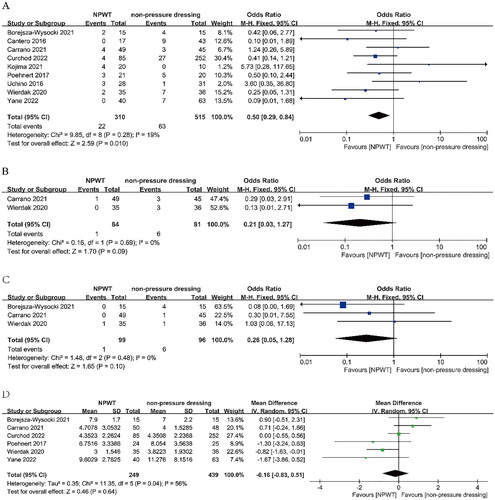
Nine studies were included in the meta-analysis, with 825 patients with (n = 310) or without (n = 515) NPWT. Pooled SSI rate was lower in the NPWT group than in the non-pressure dressing group (OR = 0.50; 95% CI: 0.29, 0.84; P = 0.01). There was no significant effect on hematoma (OR = 0.21; 95% CI: 0.03, 1.27; P = 0.09), seroma (OR = 0.26; 95% CI: 0.05, 1.28; P = 0.1) and LOS (MD = −0.16, 95% CI: −0.83, 0.51; P = 0.64).
Conclusion
The use of NPWT following stoma reversal in colorectal surgery reduced the incidence of SSI. However, this conclusion needs to be interpreted with caution, and further studies should be conducted to confirm in higher-quality RCTs.
Introduction
Patients undergoing stoma reversal in colorectal surgery are at a high risk of developing postoperative complications [Citation1]. Surgical site infection (SSI) is a major problem after stoma reversal, with a reported rate of up to 41% [Citation2]. SSI frequently leads to increased postoperative pain, extended hospital stay, and reduced quality of life [Citation3]. SSI may cause other postoperative complications and significantly increase the mortality rate, causing over $1.6 billion in hospital costs in the US [Citation4–6]. Negative-pressure wound therapy (NPWT) is widely used for treating different infectious complications and has emerged as a promising method for treating wound infections [Citation7, Citation8]. In 2016, the WHO conditionally recommended a new technique of prophylactic NPWT to reduce wound infections (for high-risk wounds alone) [Citation9].
However, the literature addressing the effect of NPWT for the prevention of SSI at stoma reversal remains inconclusive, with controversial results [Citation10, Citation11]. Therefore, this meta-analysis of relevant studies aimed to assess the value of NPWT in preventing SSI following stoma reversal in colorectal surgery.
Materials and methods
Literature search
The meta-analysis was conducted in agreement with PRISMA (Preferred Reporting Items for Systematic reviews and Meta-Analysis) [Citation12] to provide an evidence-based basis for evaluating surgical wound outcomes of ileostomy or colostomy reversal in colorectal surgery with or without application of NPWT. A systematic search of the literature published up to July 2022 was performed by retrieving the PubMed, Embase, and Cochrane Library databases using Medical Subject Heading (MeSH) terms: ostomy, ileostomy, colostomy, and negative-pressure wound therapy.
Selection criteria
The included trials (1) were randomized controlled trials (RCTs), retrospective or prospective observational studies; (2) investigated the effect of applying NPWT following stoma reversal in colorectal surgery compared with non-pressure dressing; and (3) reported SSI (superficial and deep) as an outcome. There were no language restrictions. Two authors independently examined the citations identified in the search against the inclusion criteria, and discrepancies were adjudicated by a third author. Narrative reviews, conference abstracts, animal or laboratory studies and editorials were excluded.
Data extraction and quality assessment
Data were extracted by two authors independently. Discrepancies were adjudicated by a third author. Baseline characteristics included the following: author, year, study type, number of patients, patient demographics, stoma types, antibiotic information, NPWT, and control information, skin closure method, SSI diagnostic criteria, and follow-up period. The primary outcome was the incidence of SSI in patients undergoing stoma reversal in colorectal surgery, with or without postoperative administration of NPWT. The secondary outcomes included hematoma, seroma, and length of hospital stay (LOS).
The Cochrane Risk of Bias tool was used to assess the risk of bias of the included RCTs by two authors independently and any difference was adjudicated by a third author. Retrospective or prospective observational studies were assessed using the Newcastle-Ottawa scale [Citation13, Citation14].
Statistical analysis
All statistical analyses were performed using the Cochrane Collaboration tool Review Manager 5.3. Dichotomous outcomes are reported as odds ratios (OR) with 95% confidence intervals (CI) while continuous outcomes are reported as mean difference (MD) with 95% CI. Continuous outcomes were reported as median and interquartile range, and values were calculated as mean and standard deviation using the method described by Luo et al. [Citation15].
Statistical heterogeneity was quantified using the chi-squared test. A random-effects model was used when I2 was > 50% and P < 0.1. Otherwise, a fixed-effects model was used. Publication bias was not assessed due to the small number of included studies. Sensitivity analysis was conducted to explore the potential sources of heterogeneity by performing a meta-analysis after excluding one study at a time. Statistical significance was set at p < 0.05.
Results
Study selection
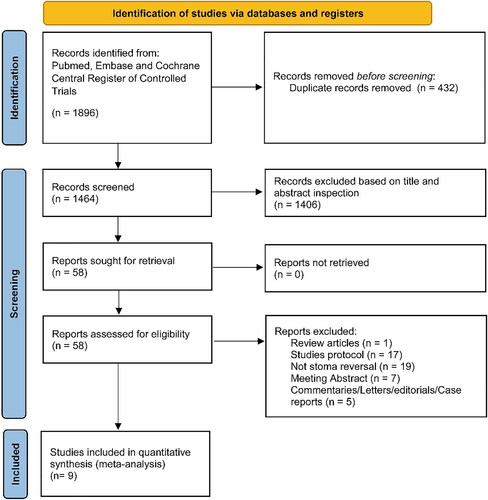
A flow chart of literature screening is shown in . The initial database search captured 1896 records across the three databases. After removing duplicates (n = 432), the remaining 1,464 studies were screened, and 1,406 studies were excluded because of low relevance according to the title and abstract. A total of 58 records were excluded after full-text screening, leaving 4 RCTs [Citation16–19] capturing 254 patients and 5 observational studies [Citation10, Citation20–23] with 571 patients (310 patients in the NPWT group and 515 patients in the non-pressure dressing group) included in the meta-analysis.
Study characteristics and quality assessment
The characteristics of the included studies are summarized in . Three studies included patients who underwent ileostomy or colostomy [Citation17, Citation20, Citation22], while the other studies included patients who underwent ileostomy [Citation10, Citation16, Citation18, Citation19, Citation21, Citation23]. Five reports used the NPWT PICO system [Citation10, Citation16, Citation17, Citation19, Citation20], one used the NPWT NANOVA system [Citation18], two used the NPWT Prevena system [Citation21, Citation23], and one study used the NPWTi-d V.A.C. system [Citation22]. Three studies and the NPWT group in Yane’s study used the purse-string suture (PSS) method for subcutaneous suturing with open wounds [Citation16, Citation17, Citation19, Citation22]. The other five and the control group in Yane’s study used linear skin closure [Citation10, Citation18, Citation20–23]. Additionally, two studies used dressings and irrigation as the control group [Citation16, Citation17], while the others used standard dressing as the control group [Citation10, Citation18–23]. Seven studies [Citation10, Citation17, Citation19–23] used the criteria of the Centers for Disease Control and Prevention (CDC) [Citation24] to diagnose SSI, one study [Citation18] used the criteria of CDC and European Center for Disease Prevention and Control (ECDC) [Citation25], while one study defined SSI as pus observed in the wound [Citation16].
Table 1. Characteristics of included studies.
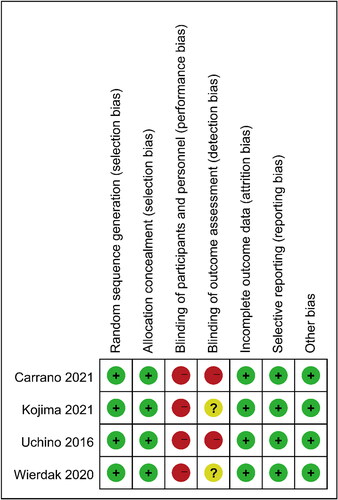
Quality assessment showed that four RCTs were considered to have low-to-moderate risk (). Owing to the nature of the study, blinding may not be achievable for patients, surgeons, and evaluators across studies. Four observational studies were ranked as high quality according to the Newcastle-Ottawa scale, while one was of medium quality ().
Table 2. Quality assessment for observational studies based on Newcastle-Ottawa scale.
Primary outcome
All included RCTs reported the rate of SSI (both superficial and deep). The meta-analysis showed that the SSI rate was significantly reduced after stoma reversal in the NPWT group than in the non-pressure dressing group (OR = 0.50; 95% CI: 0.29, 0.84; P = 0.01), with low heterogeneity (I2 = 19%) ().
Secondary outcome
Two trials (n = 165) reported the rate of hematoma formation. The pooled result revealed no significant difference in the rate of hematoma between the NPWT and non-pressure dressing groups (OR = 0.21; 95% CI: 0.03, 1.27; P = 0.09), without heterogeneity (I2 = 0%) ().
Three trials (n = 195) reported the rate of seroma formation. The pooled result revealed no significant difference in the seroma rate between the NPWT and non-pressure dressing groups (OR = 0.26; 95% CI: 0.05, 1.28; P = 0.1), without heterogeneity (I2 = 0%) ().
Six trials (n = 688) reported LOS. The pooled result revealed no significant difference in LOS between the NPWT and non-pressure dressing groups (MD = −0.16, 95% CI: −0.83, 0.51; P = 0.64), with high heterogeneity (I2 = 56%) ().
Sensitivity analysis
After excluding one study at a time, the sensitivity analysis demonstrated that the primary outcome was stable. Heterogeneity was mainly derived from two studies [Citation16, Citation19] and it disappeared after excluding them, with statistically significant primary outcomes (OR = 0.37; 95% CI: 0.20, 0.67; P = 0.001; I2 = 0).
Discussion
The SSI rate is high in clinical practice, and ongoing RCTs reflect its importance [Citation26]. Many authors have evaluated the NPWT role in various surgeries, determining whether NPWT wounds have better outcomes in terms of SSI, and different studies have yielded different results. Some surgeons were concerned about the potential for localized infection due to the creation of a closed space by NPWT itself [Citation19]. Previous meta-analyses have shown that NPWT reduced the incidence of postoperative SSI, despite the heterogeneity in some results. However, these meta-analyses mainly focused on exploring the effect of NPWT on clean-contaminated wounds [Citation27, Citation28]. Stoma closure is classified as a contaminated wound, suggesting a highly contaminated wound and possible infection [Citation29]. Our meta-analysis demonstrated that NPWT after stoma reversal in colorectal surgery reduced the SSI rate, without difference in hematoma, seroma, and LOS in the NPWT group compared to the non-pressure dressing group. However, these results need to be interpreted with caution owing to the inclusion of observational studies and the small number of included studies.
Mclean et al. [Citation30] first reported the clinical application of NPWT approximately 60 years ago, and the technique was established in the 1990s [Citation31]. Standard wound dressings are effective in separating the incision from the hospital environment but are unable to increase wound stability or improve lymphatic drainage [Citation27]. Animal models have demonstrated that NPWT enhanced clearance of lymph from subcutaneous dead spaces in a porcine model, enhanced microvascular blood flow and oxygen levels in wounds [Citation32, Citation33]. Glass et al. reported that NPWT may lead to extracellular matrix remodeling, angiogenesis, and granulation tissue deposition by promoting a shift toward a molecularly anti-inflammatory phenotype [Citation34]. However, one of the main disadvantages of NPWT is it is expensive. According to the U.S. Food and Drug Administration, the cost of a single use of NPWT device ranges from an average of $200 to $500 per unit, and future studies need to consider health costs as the core issue [Citation28]. Heard et al. suggested that NPWT may be cost-effective in preventing SSI in women with obesity after cesarean section [Citation35]. Given that the included studies were not designed to address this question, we were unable to conduct a cost-effectiveness analysis. Another drawback of NPWT is device-induced adverse events, such as local skin irritation. Gillespie et al. demonstrated that women with obesity had a considerable increased risk of blistering in the NPWT group after cesarean section [Citation36]. High rates of adverse events, such as blisters and bleeding have been reported in some studies when using NPWT, and some studies have stopped prematurely because of such events [Citation37, Citation38]. Although no related adverse events were reported in the included studies, this finding should be reported as a safety outcome for future studies.
This study found a preventive effect of NPWT in stoma closure after colorectal surgery on SSI with low heterogeneity, possibly for the following reasons. First, three studies [Citation17, Citation20, Citation22] included patients who underwent colostomy or ileostomy, while others included those who underwent ileostomy. Although ileostomy closure wound is classified as a contaminated wound, the risk of SSI following colostomy closure is fivefold higher than that following ileostomy, considering that ileostomy closure is classified as a small bowel procedure and colostomy tends to harbor more bacteria [Citation29, Citation39, Citation40]. Second, wound closure was performed using the PSS method in four studies [Citation16, Citation17, Citation19, Citation22]. Hsieh et al. demonstrated that PSS was associated with a significant SSI reduction compared to primary closure following stoma reversal [Citation41]. The American College of Surgeons (ACS) guidelines also recommend the PSS method for wound closure following ileostomy reversal to reduce the SSI frequency [Citation42]. Considering the reduced incidence of SSI following ileostomy closure using the PSS method, this is a possible reason for the failure to find a preventive effect of NPWT on SSI in three RCTs [Citation16, Citation17, Citation19]. After excluding these three RCTs, the heterogeneity of the pooled SSI result was eliminated (OR = 0.30; 95% CI: 0.16, 0.60; P = 0.0005; I2 =0). In addition, considering that two of the three studies [Citation16, Citation19] used wound healing time as the primary outcome, this might be a source of heterogeneity. When patients underwent stoma closure using the PSS method, especially in patients with obesity, there were disadvantages, such as unsatisfactory cosmetic results and extended wound healing time [Citation43, Citation44]. These problems can be avoided using a combination of primary closure and NPWT, which needs to be further demonstrated by RCTs [Citation20, Citation23]. Third, patients with benign diseases, such as ulcerative colitis were included in some studies. However, the majority of the patients who underwent colorectal cancer surgery were older population with comorbidities, most received chemoradiation before stoma reversal, and were probably at higher risk for infectious complications, such as SSI.
We found no evidence of benefit of NPWT for secondary outcomes, including hematoma, seroma, and LOS, consistent with other reviews focusing on general abdominal surgery [Citation27, Citation45]. Wierdak et al. reported shortened wound healing time in the NPWT group than that in the standard dressing group (P = 0.03), wherein patients received linear skin closure [Citation18]. In contrast, NPWT did not significantly reduce wound healing time in studies using the PSS method [Citation16, Citation19]. Gao et al. reported that NPWT promoted wound healing in wound infections regardless of surgery type [Citation46]. We did not perform a meta-analysis on wound healing due to the different wound closure methods and the lack of standard deviation in Kojima’s study. Therefore, the role of NPWT in promoting wound healing in stoma closure cannot be determined based on the current results. Thus, we hope that ongoing RCTs will address the current dilemma [Citation26, Citation47].
Our study had several limitations. First, there were only four RCTs and the sample size was small. Second, the SSI definitions and other wound complications were not clearly defined across the included studies. Third, we were unable to determine the optimal NPWT devices or the duration of application and the optimal pressure setting based on the available data. Fourth, we were unable to analyze the specific risks associated with SSI and other wound complications after stoma closure, and future studies are needed to explore this issue. Fifth, the inability to blind the NPWT device may have led to a high detection bias, which may have affected our results. Sixth, one study used NPWT + PSS to compare with primary closure + standard dressings, not PSS + standard dressings [Citation22]. However, the result was still statistically significant after excluding this study (OR = 0.56; 95% CI:0.33, 0.97; P = 0.04). Some heterogeneity arose from the different wound closure methods used in included studies. Future studies need to explore the differences in the effects of NPWT combined with PSS or linear skin closure on wound complications and healing time to provide surgeons with better options. Seventh, the study failed to analyze patient-reported outcomes such as postoperative wound pain, quality of life, and esthetic assessment of the wound. Finally, our meta-analysis failed to analyze the cost-effectiveness.
Conclusion
The use of NPWT for SSI prevention in surgery is becoming more widespread and has yielded encouraging results. However, there is a lack of unified criteria for its use, which mostly depend on the surgeon’s preference. We found that NPWT following stoma closure in colorectal surgery prevents SSI. Finally, further high-quality RCTs with large-sample are needed before NPWT is recommended as a routine treatment for stoma closure after colorectal surgery.
Author contribution
JZ and QS designed the paper. Data gathering was completed by WX, JG and QF. Data analysis was completed by JZ, SL and ZZ. JZ and QS drafted the manuscript. SL and ZZ accomplished the manuscript revision. All authors read and approved the final manuscript. Junjia Zhu and Qi Sun contributed equally to this paper and were co-first authors.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Chow A, Tilney HS, Paraskeva P, Jeyarajah S, Zacharakis E, Purkayastha S. The morbidity surrounding reversal of defunctioning ileostomies: a systematic review of 48 studies including 6,107 cases. Int J Colorectal Dis. 2009;24(6):1–8. doi:10.1007/s00384-009-0660-z.
- Hackam DJ, Rotstein OD. Stoma closure and wound infection: an evaluation of risk factors. Can J Surg. 1995;38(2):144–148.
- Gabriel A, Gupta S, Orgill DP. Challenges and management of surgical site occurrences. Plast Reconstr Surg. 2019;143(1S):7s–10s. doi:10.1097/prs.0000000000005305.
- Mangram AJ, Horan TC, Pearson ML, Silver LC, Jarvis WR. Guideline for prevention of surgical site infection, 1999. Hospital Infection Control Practices Advisory Committee. Infect Control Hosp Epidemiol. 1999;20(4):250–278. doi:10.1086/501620.
- Badia JM, Casey AL, Petrosillo N, Hudson PM, Mitchell SA, Crosby C. Impact of surgical site infection on healthcare costs and patient outcomes: a systematic review in six European countries. J Hosp Infect. 2017;96(1):1–15. doi:10.1016/j.jhin.2017.03.004.
- de Lissovoy G, Fraeman K, Hutchins V, Murphy D, Song D, Vaughn BB. Surgical site infection: incidence and impact on hospital utilization and treatment costs. Am J Infect Control. 2009;37(5):387–397. doi:10.1016/j.ajic.2008.12.010.
- Morykwas MJ, Argenta LC, Shelton-Brown EI, McGuirt W. Vacuum-assisted closure: a new method for wound control and treatment: animal studies and basic foundation. Ann Plast Surg. 1997;38(6):553–562. doi:10.1097/00000637-199706000-00001.
- Webster J, Liu Z, Norman G, et al. Negative pressure wound therapy for surgical wounds healing by primary closure. Cochrane Database Syst Rev. 2019;3(3):Cd009261. doi:10.1002/14651858.CD009261.pub4.
- Allegranzi B, Zayed B, Bischoff P, et al. New WHO recommendations on intraoperative and postoperative measures for surgical site infection prevention: an evidence-based global perspective. Lancet Infect Dis. 2016;16(12):e288–e303. doi:10.1016/S1473-3099(16)30402-9.
- Borejsza-Wysocki M, Bobkiewicz A, Francuzik W, et al. Effect of closed incision negative pressure wound therapy on incidence rate of surgical site infection after stoma reversal: a pilot study. Wideochir Inne Tech Maloinwazyjne. 2021;16(4):686–696. doi:10.5114/wiitm.2021.106426.
- Okuya K, Takemasa I, Tsuruma T, et al. Evaluation of negative-pressure wound therapy for surgical site infections after ileostomy closure in colorectal cancer patients: a prospective multicenter study. Surg Today. 2020;50(12):1687–1693. doi:10.1007/s00595-020-02068-6.
- Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. doi:10.1136/bmj.b2700.
- Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi:10.1136/bmj.d5928.
- Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–605. doi:10.1007/s10654-010-9491-z.
- Luo D, Wan X, Liu J, Tong T. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat Methods Med Res. 2018;27(6):1785–1805. doi:10.1177/0962280216669183.
- Kojima K, Goto M, Nagashima Y, et al. Effectiveness of negative pressure wound therapy for the wound of ileostomy closure: a multicenter, phase II randomized controlled trial. BMC Surg. 2021;21(1):442. doi:10.1186/s12893-021-01446-2.
- Carrano FM, Maroli A, Carvello M, et al. Negative-pressure wound therapy after stoma reversal in colorectal surgery: a randomized controlled trial. BJS Open. 2021;5(6):zrab116. doi:10.1093/bjsopen/zrab116.
- Wierdak M, Pisarska-Adamczyk M, Wysocki M, et al. Prophylactic negative-pressure wound therapy after ileostomy reversal for the prevention of wound healing complications in colorectal cancer patients: a randomized controlled trial. Tech Coloproctol. 2021;25(2):185–193. doi:10.1007/s10151-020-02372-w.
- Uchino M, Hirose K, Bando T, Chohno T, Takesue Y, Ikeuchi H. Randomized controlled trial of prophylactic negative-pressure wound therapy at ostomy closure for the prevention of delayed wound healing and surgical site infection in patients with ulcerative colitis. Dig Surg. 2016;33(6):449–454. doi:10.1159/000446550.
- Curchod P, Clerc D, Jurt J, et al. Closed-wound negative pressure therapy dressing after loop ostomy closure: a retrospective comparative study. Sci Rep. 2022;12(1):7790. doi:10.1038/s41598-022-11856-8.
- Poehnert D, Hadeler N, Schrem H, Kaltenborn A, Klempnauer J, Winny M. Decreased superficial surgical site infections, shortened hospital stay, and improved quality of life due to incisional negative pressure wound therapy after reversal of double loop ileostomy. Wound Repair Regen. 2017;25(6):994–1001. doi:10.1111/wrr.12606.
- Yane Y, Hida JI, Chiba Y, et al. Effectiveness of negative pressure wound therapy with instillation and dwelling after stoma closure: a retrospective and propensity score matching analysis. Sci Rep. 2022;12(1):916. doi:10.1038/s41598-022-05016-1.
- Cantero R, Rubio-Perez I, Leon M, et al. Negative-pressure therapy to reduce the risk of wound infection following diverting loop ileostomy reversal: an initial study. Adv Skin Wound Care. 2016;29(3):114–118. doi:10.1097/01.ASW.0000480458.60005.34.
- Mangram AJ, Horan TC, Pearson ML, Silver LC, Jarvis WR. Guideline for prevention of surgical site infection, 1999. Am J Infect Control. 1999;27(2):97–134. doi:10.1016/S0196-6553(99)70088-X.
- Horan TC, Gaynes RP, Martone WJ, Jarvis WR, Emori TG. CDC definitions of nosocomial surgical site infections, 1992: a modification of CDC definitions of surgical wound infections. Infect Control Hosp Epidemiol. 1992;13(10):606–608.
- Low EZ, Nugent TS, O’Sullivan NJ, et al. Application of PREVENA (Surgical Incision Protection System) in reducing surgical site infections following reversal of ileostomy or colostomy: the PRIC study protocol. Int J Colorectal Dis. 2022;37(5):1215–1221. doi:10.1007/s00384-022-04153-3.
- Sahebally SM, McKevitt K, Stephens I, et al. Negative pressure wound therapy for closed laparotomy incisions in general and colorectal surgery: a systematic review and meta-analysis. JAMA Surg. 2018;153(11):e183467. doi:10.1001/jamasurg.2018.3467.
- Guo C, Cheng T, Li J. Prophylactic negative pressure wound therapy for closed laparotomy incisions after ventral hernia repair: a systematic review and meta-analysis. Int J Surg. 2022;97:106216. doi:10.1016/j.ijsu.2021.106216.
- Culver DH, Horan TC, Gaynes RP, et al. Surgical wound infection rates by wound class, operative procedure, and patient risk index. Am J Med. 1991;91(3b):152s–157S. doi:10.1016/0002-9343(91)90361-Z.
- McLean WC. The role of closed wound negative pressure suction in radical surgical procedures of the head and neck. Laryngoscope. 1964;74:70–94. doi:10.1002/lary.5540740106.
- Durandy Y, Batisse A, Bourel P, Dibie A, Lemoine G, Lecompte Y. Mediastinal infection after cardiac operation. A simple closed technique. J Thorac Cardiovasc Surg. 1989;97(2):282–285.
- Kilpadi DV, Cunningham MR. Evaluation of closed incision management with negative pressure wound therapy (CIM): hematoma/seroma and involvement of the lymphatic system. Wound Repair Regen. 2011;19(5):588–596. doi:10.1111/j.1524-475X.2011.00714.x.
- Wackenfors A, Gustafsson R, Sjögren J, Algotsson L, Ingemansson R, Malmsjö M. Blood flow responses in the peristernal thoracic wall during vacuum-assisted closure therapy. Ann Thorac Surg. 2005;79(5):1724–1730. doi:10.1016/j.athoracsur.2004.10.053.
- Glass GE, Murphy GF, Esmaeili A, Lai LM, Nanchahal J. Systematic review of molecular mechanism of action of negative-pressure wound therapy. Br J Surg. 2014;101(13):1627–1636. doi:10.1002/bjs.9636.
- Heard C, Chaboyer W, Anderson V, Gillespie BM, Whitty JA. Cost-effectiveness analysis alongside a pilot study of prophylactic negative pressure wound therapy. J Tissue Viability. 2017;26(1):79–84. doi:10.1016/j.jtv.2016.06.001.
- Gillespie BM, Thalib L, Ellwood D, et al. Effect of negative-pressure wound therapy on wound complications in obese women after caesarean birth: a systematic review and meta-analysis. BJOG. 2022;129(2):196–207. doi:10.1111/1471-0528.16963.
- Chaboyer W, Anderson V, Webster J, Sneddon A, Thalib L, Gillespie BM. Negative pressure wound therapy on surgical site infections in women undergoing elective caesarean sections: a pilot RCT. Healthcare (Basel). 2014;2(4):417–428. doi:10.3390/healthcare2040417.
- Howell RD, Hadley S, Strauss E, Pelham FR. Blister formation with negative pressure dressings after total knee arthroplasty. Curr Orthop Pract. 2011;22(2):176–179.
- Smith RL, Bohl JK, McElearney ST, et al. Wound infection after elective colorectal resection. Ann Surg. 2004;239(5):599–605. doi:10.1097/01.sla.0000124292.21605.99.
- Liang MK, Li LT, Avellaneda A, Moffett JM, Hicks SC, Awad SS. Outcomes and predictors of incisional surgical site infection in stoma reversal. JAMA Surg. 2013;148(2):183–189. doi:10.1001/jamasurgery.2013.411.
- Hsieh MC, Kuo LT, Chi CC, Huang WS, Chin CC. Pursestring closure versus conventional primary closure following stoma reversal to reduce surgical site infection rate: a meta-analysis of randomized controlled trials. Dis Colon Rectum. 2015;58(8):808–815. doi:10.1097/dcr.0000000000000401.
- Ban KA, Minei JP, Laronga C, et al. American college of surgeons and surgical infection society: surgical site infection guidelines, 2016 update. J Am Coll Surg. 2017;224(1):59–74. doi:10.1016/j.jamcollsurg.2016.10.029.
- Lee JT, Marquez TT, Clerc D, et al. Pursestring closure of the stoma site leads to fewer wound infections: results from a multicenter randomized controlled trial. Dis Colon Rectum. 2014;57(11):1282–1289. doi:10.1097/dcr.0000000000000209.
- Reid K, Pockney P, Pollitt T, Draganic B, Smith SR. Randomized clinical trial of short-term outcomes following purse-string versus conventional closure of ileostomy wounds. Br J Surg. 2010;97(10):1511–1517. doi:10.1002/bjs.7151.
- Almansa-Saura S, Lopez-Lopez V, Eshmuminov D, et al. Prophylactic use of negative pressure therapy in general abdominal surgery: a systematic review and meta-analysis. Surg Infect (Larchmt). 2021;22(8):854–863. doi:10.1089/sur.2020.407.
- Gao J, Wang Y, Song J, Li Z, Ren J, Wang P. Negative pressure wound therapy for surgical site infections: A systematic review and meta-analysis. J Adv Nurs. 2021;77(10):3980–3990. doi:10.1111/jan.14876.
- Kim S, Kang SI. The effectiveness of negative-pressure wound therapy for wound healing after stoma reversal: a randomised control study (SR-PICO study). Trials. 2020;21(1):24. doi:10.1186/s13063-019-3925-z.