Abstract
Background
The purpose of this study was to evaluate the effect of single-incision plus one port laparoscopic surgery (SILS + 1) for myomectomy.
Methods
We retrospectively analyzed data from patients who underwent laparoendoscopic single-site myomectomy (LESS-M group, n = 40) and SILS + 1 (SILS + 1-M group, n = 40) for myomectomy at our hospital from October 2018 through December 2020. The patients’ baseline demographic information and clinical data were compared between the two groups.
Results
The results showed that no significant difference in basic characteristics or between the number, size, and location of uterine myomas between the two groups (p < 0.05). However, the surgery was more difficult and the total operating time was significantly longer in the LESS-M group compared to the SILS + 1-M group (83.5 ± 14.2 vs. 108.2 ± 18.1 min, p = 0.001). Moreover, the estimated intraoperative blood loss (113.4 ± 46.5 vs. 211.4 ± 60.3 ml, p = 0.001) and changes in hemoglobin level (13.1 ± 7.6 vs. 18.2 ± 6.0, p = 0.001) were significantly lower in the SILS + 1-M group compared to the LESS-M group. In addition, no serious intraoperative or postoperative complications occurred after surgery in either group. The clinical outcomes in the SILS + 1-M group were associated with a significant reduction in total surgical time compared to the LESS-M group (p < 0.05).
Conclusion
SILS + 1 for myomectomy is popular in clinics, with a satisfactory clinical effect.
Background
Uterine leiomyomas, also called uterine fibroids or myomas, are the most common benign gynecological tumors of women of reproductive age [Citation1]. Although many patients remain asymptomatic, 30–40% develop various symptoms, including irregular vaginal bleeding, pelvic pain, infertility, and pelvic mass, solely due to the size and location of the uterine myomas [Citation2]. Moreover, long-term irregular vaginal bleeding may occur in some patients, which may lead to severe anemia [Citation3,Citation4]. An estimated 26% of patients with uterine leiomyomas become symptomatic and require medical intervention. Although hysterectomy is the most common surgical option in such cases, myomectomy is adopted for women who require uterine-sparing during treatment [Citation5]. This study was undertaken to evaluate the gynecologic endoscopic surgical techniques, including resectoscopic myomectomy, laparoscopic myomectomy, and laparoscopy assisted vaginal hysterectomy, used in the treatment of uterine myomas, with the aim to improve pregnancy outcomes and fewer postoperative complications.
The risk of uterine fragment dispersion, with the subsequent appearance of pelvic adenomyotic masses and parasitic leiomyomas, was first described in 2006 and remains a risk that may be avoided by extensive peritoneal lavage and careful removal of all fragments [Citation6]. Similarly, in 2014, upon the discovery of a postoperative case of intraabdominal leiomyosarcoma dissemination, the Food and Drug Administration (FDA) in the USA issued a warning statement discouraging the use of mechanical morcellation [Citation7]. The incident sent shockwaves through the medical community given that the removal of the specimen using mechanical morcellators is a key step in laparoscopic myomectomy [Citation8]. These studies suggest reducing the surgical difficulty of laparoscopic myomectomy procedures.
With the increased experience originating from technological advances, incisions may become smaller, leading to quicker recoveries and shorter hospital stays. In addition, with the ability to finely execute laparoscopy procedures with just one small incision, laparoendoscopic single-site (LESS) has become a popular surgical procedure in gynecologic oncology. The surgical procedures are varied and include endometrial cancer staging, ovarian cancer staging, retroperitoneal pelvic lymph node dissection, risk-reducing extrafascial hysterectomy/bilateral salpingo-oophorectomyand (BSO) alone, ovarian cystectomy, and BSO for complex adnexal masses [Citation9]. Although the LESS myomectomy (LESS-M) procedure eschews mechanical morcellators, thereby reducing the likelihood of spreading of uterine myomas, the technical difficulty in suturing and tying the incision limits its widespread application [Citation10].
Therefore, to minimize abdominal trauma and technical difficulties, single-incision plus one port laparoscopic surgery (SILS + 1), which includes an additional port in the right lower quadrant to SILS for sigmoid and rectal cancer, has gained increasing attention from colorectal surgeons. The whole procedure is conducted in the absence of mechanical morcellators, with removal of the specimen being performed through the umbilical incision. Here, we compared the conventional LESS-M with the suggested dual incision operation and observed a fine difference between the two procedures.
Patients and methods
Study design and participants
We retrospectively analyzed data from October 2018 through December 2020 at our hospital, which sees approximately 2,000 hospitalizations annually for patients diagnosed with gynecological tumors. The inclusion criteria were as follows: women aged between 25 and 55 years who had symptomatic uterine myoma and had an indication for myomectomy; had ≤6 subserous or intramural myomas that were ≤120 mm in diameter; absence of pregnancy or any prior history of myomectomy; and with complete clinical data. The exclusion criteria were as follows: women who had submucosal myoma or other 86 gynecological disease etc., such as ovarian cyst or endometrial hyperplasia; patients with a possibility of malignant uterine myoma, as diagnosed by vaginal ultrasonography and magnetic resonance imaging (MRI), or those with a history of serious psychiatric illnesses or other medical comorbidities within the past 6 months; and incomplete clinical data. The study was approved by the Research Ethics Board of the First Affiliated Hospital of Henan Polytechnic University (KY2021-04-025).
SILS + 1-M
A 1.5–1.7 cm longitudinal incision was made at the umbilicus, followed by placement of the incision protective sleeve into the abdominal cavity, which was connected to the single-site port and a CO2 device to produce a pneumoperitoneum. Subsequently, 5-mm and 10-mm trocars were accessibly placed and used as the routes for the working channels and laparoscope. The surgeon made one 5-mm incision in the left lower abdomen to working channels. Two 5 mm trocars were engaged to remove the myomas, followed by the closure of the uterine incision through continuous suturing using double-layer 1-0 Vicryl suture. A pelvic drainage tube was placed through a 5-mm incision on the left lower abdomen () and was removed 2 days postoperatively. The abdominal incision is shown in .
Figure 1. (A, B) The condition of the incision after SILS + 1-M operation and drainage tube extraction was improved. (C) Incision condition after LESS-M operation. (D, F) A two-port entry system with a transumbilical single-port technique and an additional 5-mm trocar was used for the extraction of myomas through the transumbilical single-port site. (E) The myoma was removed and the myometrium incision was sutured. SILS + 1-M: single-incision plus one port laparoscopic myomectomy, LESS-M: laparoendoscopic single-site myomectomy.
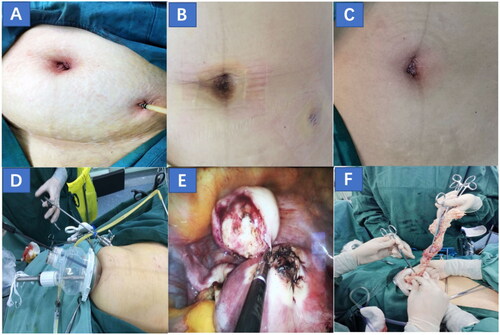
LESS-M
A 2.0–2.5 cm longitudinal umbilical incision was made by the surgeon () and an incision protective sleeve was inserted, which was then connected to a single-site port and CO2 device to produce a pneumoperitoneum. Two 5-mm trocars were used to reduce collisions during surgery and one 10-mm trocar was accessibly placed and used as the routes for the working channels and laparoscope. The myomas were removed through the umbilical incision after excision and a 1-0 Vicry thread was used to suture the uterine incision.
Operative technique
SILS + 1-M and LESS-M were performed by surgeons with comparable surgical skills and experience, which were gained through at least 1,000 cases of conventional multiport laparoscopic surgery and ten cases of LESS. Relevant examinations conducted after postoperative hospitalization, including gynecological examination followed by transvaginal ultrasonography and MRI, revealed no contraindications for laparoscopic surgery or general anesthesia in either of the groups. Cephalosporin, a second-generation antibiotic, was given to both groups 24 h before and after the operation. After performing general anesthesia, patients were placed in the Trendelenburg position, before inserting a Foley catheter and fixing a simple uterine manipulator into the uterine cavity to reduce the difficulty of the operation. Subsequently, carbon dioxide was injected to achieve an intraabdominal pressure of 12–14 mmHg. Commercial single-port systems and a 30-degree laparoscope, as well as all traditional laparoscopic instruments, were used during the operation. Pituitrin and 0.9% saline were injected into the overlying myometrium or serosal layer at a 1:20 ratio to reduce intraoperative blood loss. A longitudinal or transverse incision was made on the overlying myometrium using a pair of monopolar scissors, and 5-mm claw forceps were used to perform myoma enucleation. After excision, the myomas were enclosed in an appropriately sized specimen bag, before closing the uterine muscle. The myomas were manually morcellated using a knife and removed via the umbilical incision (). Next, the abdominal cavity was washed with normal saline and medical chitosan was applied to prevent pelvic adhesions. Finally, the peritoneum, fascia, and subcutaneous tissue were closed using a 2-0 Vicry suture, and the incision was closed with skin adhesive. Enoxaparin was administered to prevent thrombosis for at least 12 h after the operation.
Outcome measures
The patients’ demographic information, including age, body mass index (BMI) (kg/m2), previous surgical and medical history, and clinical indication, as well as the location, size, and number of myomas were recorded for both groups.
The outcomes related to intraoperative and postoperative characteristics, including the total operation time, time taken to remove the specimen, estimated blood loss, degree of surgical difficulty, postoperative pain score, and hemoglobin change, were compared between the two groups. The number of cases of hospitalization due to operative complications in each group were also taken into account.
The VAS rating (from 0 to 10) was first described by Vassiliou et al. to describe the overall difficulty faced during an operation due to the perplexity of the procedures involved in uterus removal, suturing, and knotting, among others. At the end of each operation, the surgeons were asked to assign a VAS score, with 1 being the easiest and 10 being the most difficult to operate [Citation11]. The total operative time was defined as the time from skin incision to skin closure, and the total time taken for the removal of the specimen was defined as the time between the loading of the fibroid into the specimen bag and the complete removal of the umbilical incision. The intraoperative blood loss was calculated by gauze and suction and irrigation plus. Intraoperative complications during surgery included injuries to the bladder, ureter, vasculature, and intestine, whereas postoperative complications consisted of anemia, fever, and ileus. Fever was considered present when two consecutive body temperature measurements, taken at least 6 h apart, exceeded 38 °C. The VAS rating system from 0 to 10, with 0 experiencing no pain and 10 being the most painful, was used to score the patients’ postoperative pain levels 1, 6, and 24 h postoperatively. Blood samples were taken from the patients within 24 h of surgery and hemoglobin changes were measured as the difference between the pre-and postoperative hemoglobin levels. The number of days spent in hospital was calculated as the time between the day of surgery and the time of discharge.
Statistical analysis
Statistical analysis was performed using Statistical Package for Social Science (SPSS) 22.0 (SPSS Inc., Chicago, IL, USA). Continuous data was expressed as mean ± standard deviation (SD), and Student’s t-test was used to compare the differences. Categorical variables were expressed as number and percentage, and the differences were assessed using chi-square test or Fisher’s exact test. P-values < 0.05 were considered statistically significant.
Results
Baseline characteristics
Eighty patients who underwent myomectomy were enrolled (n = 40 per group). The demographic characteristics of the two groups of patients are shown in . The final histopathologic diagnosis for each patient was benign leiomyoma without malignant tumor. The two groups of patients had no statistical differences with respect to age, BMI, parity, or indications for operation. The average age of the patients in the SILS + 1-M and LESS-M groups was 37.3 ± 6.7 years and 38.5 ± 6.2 years, respectively. The patients had no significant history of gynecologic surgery, including cesarean and adnexal operations. The patients in both groups were matched for the number and size of myomas, the maximum diameter of the myomas, and the location of the largest myoma. The mean diameter of the myomas in the SILS + 1-M and LESS-M groups was 79.2 ± 24.5 and 77.6 ± 28.7 mm, respectively. The number of myomas removed from patients in the SILS + 1-M and LESS-M groups was not significantly different (2.2 ± 1.1 and 2.4 ± 1.3, respectively). Moreover, there was no significant difference in the location of the largest myoma in the two groups.
Table 1. Baseline characteristics of the two groups
Surgical outcomes
The perioperative parameters for all patients are summarized in . The total operative time in the SILS + 1-M group was significantly shorter than that in the LESS-M group (83.5 ± 14.2 vs. 108.2 ± 18.1 min, p = 0.001). According to the VAS scores, the operation was more difficult in the LESS-M group than that in the SILS + 1-M group (6.1 ± 1.8 vs. 4.5 ± 1.1, p = 0.001). The specimen extraction time was not significantly different between the two groups (6.6 ± 1.8 vs. 6.9 ± 2.0). In the LESS-M group, insertion trocars were added in three cases owing to the difficulty in suturing the uterine incision. The EBL of the SILS + 1-M group was 113.4 ± 46.5 ml, which was significantly less than the 211.4 ± 60.3 ml that was lost in the LESS-M group (p = 0.001).
Table 2. Surgical outcomes of the two groups
The change in the hemoglobin level on postoperative day 1 was greater in the LESS-M group (18.2 ± 6.0) than in the SILS + 1-M group (13.1 ± 7.6) (p = 0.001). Although the number of patients with postoperative fever was less in the SILS + 1-M group (2.2% vs. 10%), there was no statistically significant difference between the two groups. The groups had similar postoperative pain scores and duration of hospital stay (3.1 ± 1.4 vs. 3.0 ± 1.3 days for SILS + 1-M and LESS-M, respectively). During surgery, no serious operative complications were recorded in either group.
Discussion
Since its first description in 1994, laparoscopic myomectomy has been regularly performed to treat patients diagnosed with uterine myomas who wish to preserve their fertility. Although laparoscopic myomectomy was initially considered as a safe and effective alternative to abdominal myomectomy [Citation12], the warning given by the FDA had drastically decreased the application of traditional laparoscopic myomectomy [Citation8]. To avoid the potential exacerbation of an undiagnosed uterine sarcoma caused by the application of power morcellation, some gynecologists perform a transvaginal extraction or laparotomy, which involves creating a closed environment, through a specially designed isolation bag, for myoma retrieval [Citation13,Citation14]. Unless ruptured, the isolation bag will shield the spillage of fragments created by the morcellation. Unfortunately, transvaginal extraction of myoma or laparotomy not only aggravates the physical trauma experienced by the patients, but may also increase the risk of isolation bag rupture. To summarize these issues, in a clinical setting, a gynecologist must not only consider the risks of power morcellation, including the spread of an undiagnosed uterine sarcoma, but also take into account the advantages offered by minimally invasive surgery. Such surgeries have the potential to reduce morbidity and postoperative pain, and mostly demand shorter hospitalizations, often leading to an earlier return to work compared to patients who undergo laparotomy [Citation15].
In 1969, Clifford Wheeless first reported transumbilical single-incision laparoscopic salpingectomy. Since then, LESS is widely used in gynecologic oncology. Compared to traditional multiport laparoscopic surgery, many observational studies have reported far better benefits for performing LESS to treat benign and malignant gynecological tumors [Citation16,Citation17]. However, LESS is not without challenges, including the need to overcome the physical difficulties of operating in a small and single channel. In addition, not to undermine the possible collision of the surgeons’ hands and instruments while operating, but LESS also makes it relatively difficult to perform laparoscopic suturing and knotting at the end of surgery. Furthermore, all operations need to be completed by a single surgeon, necessitating a longer learning curve, in which a surgeon needs to operate on at least 40 patients to significantly reduce their total operation time [Citation18,Citation19]. Despite this, LESS-M also has its share of advantages, particularly the fact that specimens are removed through the umbilical incision, which avoids power morcellation, thus reducing the risk of spreading of fibroids [Citation20]. Although SILS + 1 is an emerging technique for the treatment of gastrointestinal malignancy [Citation21,Citation22], there is limited research on its use in gynecological tumors. SILS + 1-M reduces the technical requirements for an extended learning curve to perform LESS myomectomy on patients, and is also a better choice for surgeons who have previously attempted this type of surgery. Here, we achieved these goals by shortening the umbilical incision and making an additional 5-mm incision in the left lower abdomen. Although both the LESS-M group and the SILS + 1-M group had similar advantages in terms of acquiring specimens through the umbilical incision, the clinical outcomes of the SILS + 1-M group were associated with a significant decrease in the overall surgical time when compared to LESS-M.
Currently, clinicians use misoprostol and vasopressin to effectively reduce blood loss during laparoscopic myomectomy [Citation23,Citation24]. Consistent with the results projected in Song T’s report [Citation20], we also conclude that it is easier for the surgeon to perform a SILS + 1-M that adds an auxiliary hole for better operative opportunities. The total operative time in the SILS + 1-M group was reduced owing to avoidance of time-consuming collisions between single-site channel instruments and better and faster suturing and knotting, which resulted in reduced intraoperative bleeding. Han’s research results showed that the operation time of the LESS-M group was significantly prolonged compared to that of traditional laparoscopic surgery group [Citation25]. However, Kim’s research results showed that the operation time of the LESS-M group was not significantly reduced compared to that of the traditional laparoscopic surgery group [Citation26]. The main reason for the difference is likely to be related to the experience and initial learning curve of the surgeons. Initial operations could be performed in a more skilled way by overcoming the previously mentioned operational difficulties.
The results of several studies and some spontaneous cases of uterine rupture after laparoscopic myomectomy strongly suggest correlations between the suturing technique and uterine rupture in patients during future pregnancies. Therefore, in the future, a better suturing technique is essential for women with fertility requirements [Citation27,Citation28]. The SILS + 1-M offers better and faster suturing, as well as, consequently, lowered risk of uterine rupture during future pregnancy. Similar studies with longer follow-up are necessary to answer further questions relating to pregnancy outcomes after SILS + 1-M and LESS-M.
Another potential advantage of placing an auxiliary hole in the left lower abdomen during SILS + 1-M is that it could be used to place a pelvic drainage tube for an average of 48 h after the operation. The mean drainage fluid volume is approximately 305 ± 89.4 ml, which, if not drained, would increase the probability of fever and adhesions after surgery. Although SILS + 1-M adds an auxiliary hole, the umbilical incision is smaller than that made in LESS (1.5–1.7 cm vs. 2.0–2.5 cm, respectively). Moreover, the damage to the navel is less, and it is more cosmetic.
One obvious benefit of LESS-M is that the excised uterine myomas are contained in a specimen retrieval endopouch that can be removed quickly and easily through the additional umbilical incision. Other on-going research in this area includes studies conducted on the in-bag powered morcellation technique used during single-port-assisted laparoscopic myomectomy [Citation29]. Regarding this particular area of research, the safe extraction of enucleated myomas has become a major issue that requires further consideration. Although the umbilical incision was reduced in the SILS + 1-M group, it did not affect the removal of fibroids, and there was no significant difference in the time of removal of specimen between the two groups.
The effective hospitalization period and VAS scores were similar between the two groups at 1, 6, and 24 h. In addition, the incidences of intraoperative and postoperative complications were not significantly different between the two groups, with no serious overall complications observed in either group. Although previous literature has reported that some cases are converted to laparotomy, none of the patients included in our study were transferred to laparotomy.
This study has several limitations, largely owing to its retrospective nature. First, as the number of samples depends on their complete record, the sample size is small. Secondly, the decision on which treatment to pursue was made based on professional judgment of the surgeon and the choice of the patients, which inevitably leads to selection bias. Thirdly, the follow-up period is too short to observe the postoperative influence. In the future, it will be necessary to perform a multicenter, prospective, large-sample study with a long-term follow-up.
Conclusion
In conclusion, the use of SILS + 1-M to treat myomectomy can effectively reduce postoperative pain, accelerate postoperative recovery, and improve incision appearance and patient satisfaction, and is worthy of promotion. SILS + 1-M is a feasible and relatively easy option for patients with myomas who have a uterine-sparing requirement, and, compared to LESS-M, is especially suitable for surgeons in the initial learning curve stage of their careers.
Author contributions
Conception and design of the study: Gaoli Niu, Yanhong Zhai. Study search: Hua Zhao. Interpretation of the retrieved studies: Hong Wang Drafting the article: Gaoli Niu, Yanhong Zhai. Revising the article: Lingli Zhao. All authors contributed to the article and approved the submitted version.
Consent for publication
Not applicable.
Ethical approval
The study was approved by the Research Ethics Board of the First Affiliated Hospital of Henan Polytechnic University [KY2021-04-025].
Data availability statement
The data used to support the findings of this study are available from the corresponding author upon request.
Disclosure statement
All authors have no conflicts of interest or financial ties to disclose.
Additional information
Funding
References
- Drayer SM, Catherino WH. Prevalence, morbidity, and current medical management of uterine leiomyomas. Int J Gynaecol Obstet. 2015;131(2):1–7. doi:10.1016/j.ijgo.2015.04.051.
- Donnez J, Dolmans MM. Uterine fibroid management: from the present to the future. Hum Reprod Update. 2016;22(6):665–686. doi:10.1093/humupd/dmw023.
- He Z, Xie C. Laparoendoscopic single-port subtotal hysterectomy: An innovative V-shaped resection of the uterine body. Asian J Surg. 2022;45(5):1196–1197. doi:10.1016/j.asjsur.2022.01.106.
- Nelson AL, Ritchie JJ. Severe anemia from heavy menstrual bleeding requires heightened attention. Am J Obstetrics Gynecol. 2015;213(1):97.e91–97.e96. doi:10.1016/j.ajog.2015.04.023.
- Rakotomahenina H, Rajaonarison J, Wong L, Brun JL. Myomectomy: Technique and current indications. Minerva Ginecol. 2017;69(4):357–369. doi:10.23736/s0026-4784.17.04073-4.
- Gauthier T, Lacorre A, Legendre G, et al. Should we perform subtotal hysterectomy associated with sacral colpopexy for genital prolapse to prevent the risk of endometrial cancer? Prog Urol. 2021;31(7):439–443. doi:10.1016/j.purol.2021.03.006.
- Parker WH, Kaunitz AM, Pritts EA, et al. U.S. Food and Drug Administration’s guidance regarding morcellation of leiomyomas: Well-intentioned, but is it harmful for women? Obstet Gynecol. 2016;127(1):18–22. doi:10.1097/aog.0000000000001157.
- Glaser LM, Friedman J, Tsai S, Chaudhari A, Milad M. Laparoscopic myomectomy and morcellation: A review of techniques, outcomes, and practice guidelines. Best Pract Res Clin Obstet Gynaecol. 2018;46:99–112. doi:10.1016/j.bpobgyn.2017.09.012.
- Boruta DM. Laparoendoscopic single-site surgery in gynecologic oncology: An update. Gynecol Oncol. 2016;141(3):616–623. doi:10.1016/j.ygyno.2016.03.014.
- Gao Y, Liu Y, Ma X, Wei L, Chen W, Song L. The incidence and risk factors of peripherally inserted central catheter-related infection among cancer patients. Ther Clin Risk Manag. 2015;11:863–871. doi:10.2147/TCRM.S83776.
- Zhao J, Sun J, Li D, Xu WJ. Laparoscopic versus open reduction of idiopathic intussusception in children: an updated institutional experience. BMC Pediatr. 2022;22(1):44. doi:10.1186/s12887-022-03112-9.
- Dubuisson JB, O’Leary T, Feki A, Bouquet DEJJ, Dubuisson J. Laparoscopic myomectomy. Minerva Ginecol. 2016;68(3):345–351.
- Boza A, Misirlioglu S, Taskiran C, Urman B. Contained power morcellation versus transvaginal extraction for retrieval of laparoscopically removed myomas: A comparison of perioperative outcomes. Surg Innov. 2019;26(1):72–76. doi:10.1177/1553350618790710.
- Zapardiel I, Boria F, Halaska MJ, De Santiago J. Laparoscopic power morcellation: Techniques to avoid tumoral spread. J Minim Invasive Gynecol. 2021;28(8):1442–1443. doi:10.1016/j.jmig.2020.09.012.
- Delgado-Sánchez E, Peay-Pinacho JA, Hernández Gutiérrez A, Álvarez Bernardi J, Zapardiel I. Role of single-site and mini-laparoscopy in gynecologic surgery. Minerva Obstet Gynecol. 2021;73(2):166–178. doi:10.23736/s2724-606x.20.04607-9.
- Hwang JH, Kim SR, Kim JH, Kim BW. Gasless single-port access laparoscopy using a J-shaped retractor in patients undergoing adnexal surgery. Surg Endosc. 2021;35(6):2457–2464. doi:10.1007/s00464-020-07654-w.
- Jiang L, Tong D, Li Y, Liu Q, Liu K. Application of single-port laparoscopic surgery in myomectomy. Front Oncol. 2021;11:722084. doi:10.3389/fonc.2021.722084.
- Kale A, Terzi H, Yavuz A, Kale E. Single-port access total laparoscopic hysterectomy with Korean-made OCTO Port: Turkish surgeons’ initial experience. J Obstet Gynaecol. 2016;36(1):114–118. doi:10.3109/01443615.2015.1041885.
- Gasparri ML, Mueller MD, Taghavi K, Papadia A. Conventional versus single port laparoscopy for the surgical treatment of ectopic pregnancy: A meta-analysis. Gynecol Obstet Invest. 2018;83(4):329–337. doi:10.1159/000487944.
- Song T, Kim TJ, Lee SH, Kim TH, Kim WY. Laparoendoscopic single-site myomectomy compared with conventional laparoscopic myomectomy: a multicenter, randomized, controlled trial. Fertil Steril. 2015;104(5):1325–1331. doi:10.1016/j.fertnstert.2015.07.1137.
- Zhou W, Dong CZ, Zang YF, et al. Initial experience of single-incision plus one port left-side approach totally laparoscopic distal gastrectomy with uncut Roux-en-Y reconstruction. World J Gastroenterol. 2020;26(31):4669–4679. doi:10.3748/wjg.v26.i31.4669.
- Ahn HS, Chang MS, Han DS. Comparing the surgical outcomes of dual-port laparoscopic distal gastrectomy and three-port laparoscopic distal gastrectomy for gastric cancer. Ann Surg Treat Res. 2021;100(1):18–24. doi:10.4174/astr.2021.100.1.18.
- Protopapas A, Kathopoulis N, Chatzipapas I, et al. Misoprostol vs vasopressin as a single hemostatic agent in laparoscopic myomectomy: Comparable, or just better than nothing? J Obstet Gynaecol Res. 2020;46(11):2356–2365. doi:10.1111/jog.14465.
- Protopapas A, Giannoulis G, Chatzipapas I, et al. Vasopressin during laparoscopic myomectomy: Does it really extend its limits? J Minim Invasive Gynecol. 2019;26(3):441–449. doi:10.1016/j.jmig.2018.05.011.
- Han CM, Lee CL, Su H, Wu PJ, Wang CJ, Yen CF. Single-port laparoscopic myomectomy: initial operative experience and comparative outcome. Arch Gynecol Obstet. 2013;287(2):295–300. doi:10.1007/s00404-012-2562-5.
- Kim SK, Lee JH, Lee JR, Suh CS, Kim SH. Laparoendoscopic single-site myomectomy versus conventional laparoscopic myomectomy: a comparison of surgical outcomes. J Minim Invasive Gynecol. 2014;21(5):775–781. doi:10.1016/j.jmig.2014.03.002.
- Chao AS, Chang YL, Yang LY, et al. Laparoscopic uterine surgery as a risk factor for uterine rupture during pregnancy. PLoS ONE. 2018;13(5):e0197307. doi:10.1371/journal.pone.0197307.
- Tian YC, Long TF, Dai YM. Pregnancy outcomes following different surgical approaches of myomectomy. J Obstet Gynaecol Res. 2015;41(3):350–357. doi:10.1111/jog.12532.
- Won YB, Lee HJ, Eoh KJ, et al. In-bag power morcellation technique in single-port laparoscopic myomectomy. Obstet Gynecol Sci. 2018;61(2):267–273. doi:10.5468/ogs.2018.61.2.267.
