Abstract
Background: Hyperglycemia usually impairs wound healing by dysregulating the inflammatory response and angiogenesis. This study aimed to examine the synergistic effect of dapagliflozin and Zamzam water (ZW) on the healing of diabetic wounds and to explore their anti-inflammatory and proangiogenic effects.
Materials and methods: A full-thickness excisional wound was made on the backs of all groups after two weeks of diabetes induction. Forty rats were divided into five groups, with eight rats per group; Group 1: Control non-diabetic rats; Group II: Untreated diabetic rats; Group III: Diabetic rats drinking ZW; Group IV: Diabetic rats receiving an oral dose of 1 mg/kg dapagliflozin; and Group V: Received both dapagliflozin and ZW. The healing of diabetic wounds was assessed by measuring wound closure, oxidative stress markers, immunohistochemical staining of NF-βB, VEGF, CD34, CD45, Ki-67, and eNOS, gene expression of MMP-9, TGF-β1, EGF-b1, FGF, and Col1A1, protein levels of TNFα, IL-1β, IL6, Ang II, and HIF-1α by ELISA assay, and histological examination with H & E and Masson’s trichrome. Combined treatment with dapagliflozin and ZW significantly (p < 0.05) enhanced the wound closure and antioxidant enzyme level, with apparent histological improvement, and shortened the inflammatory stage of the diabetic wound by decreasing the level of inflammatory markers NF-κB, TNF-α, IL-1β, IL6, and CD45. Therefore, it improved angiogenesis markers VEGF, CD34, eNOS, EGF-β1, FGF, Ang II, and HIF-1α, increasing Ki-67 cellular proliferation. Moreover, it enhanced the remodeling stage by increasing MMP-2, TGF-β1, and Col1A1 levels compared to diabetic rats.
Introduction
Skin integrity is crucial for protection against pathogens. Diabetic foot ulcers, a common form of chronic skin ulcer, affect diabetic patients with diabetic neuropathy, peripheral arterial diseases, and disturbed blood sugar levels, threatening the lives of patients with diseases such as sepsis, cellulitis, and osteomyelitis [Citation1]. Inflammation of the affected skin segment with neutrophils and macrophage infiltration is considered one of the essential early steps of wound healing. It prevents wound superinfection in DM-aggravated inflammation, resulting from massive infiltration of inflammatory cytokines. Moreover, it postpones skin wound re-epithelization, re-organization of the extracellular matrix, and wound closure [Citation2, Citation3]. Wound infiltration with abundant neutrophils and macrophages leads to the overproduction of reactive oxygen species and further oxidative insult with inflammatory cellular impairment of fibroblasts and keratinocytes, which is essential for re-epithelization and extracellular matrix development [Citation4]. Furthermore, diabetes mellitus negatively impacts the proangiogenic effect by inhibiting the activity of eNOS [Citation5], and EPC mobilization [Citation6]. Therefore, angiogenesis impairment is associated with the upregulation of HIF and VEGF, which play crucial roles in the delayed proliferative phase of wound healing [Citation7]. Unfortunately, the available treatments for diabetic foot ulcers are considered unsatisfactory, as most therapies are composed of stem cells and growth factors, which are costly, and their long-term efficacy has not been predicted [Citation8].
Dapagliflozin was the first innovative drug to block the SGLT-2 family and inhibit renal glucose reabsorption. The effect of this drug on lowering blood glucose levels was confirmed by several clinical trials, which showed a downgrade in fasting and postprandial sugar levels and a decrease in HbA1c [Citation9]. Besides its antidiabetic properties, it exhibits a cytoprotective effect as it decreases blood pressure [Citation10], lowers body weight [Citation11], and decreases resistance to insulin [Citation12]. Recently, several studies have revealed cardiometabolic advantages concerning its anti-inflammatory properties [Citation13]. Recent studies have shown that short-term medication with canagliflozin, an SGLT-2, enhances the migration of EPC by increasing the expression of eNOS, suggesting its role in the processes of healing and repair [Citation14]. Additionally, a recent study reported that dapagliflozin enhanced the restoration of endothelial lining following carotid artery injury in STZ-induced diabetic mice [Citation15].
Millions of Muslims use ZW throughout the world. ZW is located in the holy mosque of Makkah. The analysis of ZW revealed its alkaline properties. Natural alkaline water contains several elements, such as zinc, magnesium, and selenium, which aid in producing the antioxidant enzymes glutathione peroxidase, superoxide dismutase, and catalase [Citation16]. In addition, ZW decreases blood glucose levels in diabetic rats [Citation17]. Prior work has stated that the intake of ZW for two months decreased HbA1c levels in patients with diabetes. Moreover, it increased the antioxidant activity of gentamicin-induced stress in an experimental model [Citation18, Citation19].
Our study aimed to examine the consequences of the concomitant use of ZW with the oral SGL2 inhibitor, dapagliflozin, on wound healing in an experimental model of diabetes and to explore its mechanistic effect.
Materials and methods
Animals
Forty-three-month-old male albino rats were purchased from the National Research Center of Giza, Egypt. Our study rats weighed 250–300 g and were housed in separate polycarbonate cages with four rats in each cage at a controlled temperature of 22 - 24 °C and a 12-h day/night cycle with standard water and pellets ad libitum. The Research Ethics Committee, Faculty of Veterinary Medicine, Mansoura University approved the research protocol with code number VM.R.23.01.41. Before starting the study for two weeks, the rats were housed to adjust to the experiment’s environment.
Induction of diabetes
The rats’ body weights and blood sugar levels were assessed at the commencement of the study. Diabetes was induced in rats in groups II to V by intraperitoneal injection of a single dose (60 mg/kg) of STZ from Sigma-Aldrich Corporation (St. Louis, MO, USA). Blood was drawn from the tail vein of rats 72 h after STZ administration, and fasting blood sugar levels were determined using a glucometer (ACCU Check; Roche, Basel, Switzerland). Rats with blood glucose levels greater than 250 mg/dl were considered diabetic and included in the study; those with lower levels were excluded [Citation20].
Induction of an excisional skin wound
Excisional wounds on the skin of all rats were induced according to the method described by De Masi et al. [Citation21] Two weeks after the induction of diabetes, rats were anesthetized with ketamine (50 mg/kg). Subsequently, the hair on the back was shaved. Excisional wounds were made in all layers of the skin using a scalpel blade, reaching the underlying muscle layer. The damaged area was left open. Buprenorphine was administered intraperitoneally at 0.05 mg/kg every 8–12 h to reduce postoperative pain [Citation22]. The wound, away from the reach of the rats, was considered high and extended from the back to the back of the neck.
Experimental groups
The experiment utilized 40 rats, which were divided into five groups of eight rats each. Group I (control group = 8 rats) comprised normal rats that received tap water and 0.5 ml of normal saline by gastric gavage. Thirty-two rats were identified as diabetic based on their blood sugar levels exceeding 250 mg/dl. These rats were then categorized into the remaining groups. Group II (diabetic) consisted of diabetic rats given tap water and 0.5 ml of normal saline through gastric gavage over four weeks. Group III (diabetic rats receiving ZW) comprised diabetic rats treated with ZW at 100 ml/cage dosage [Citation23]. Group IV (diabetic received dapagliflozin) included diabetic rats that received dapagliflozin orally at 1 mg/kg dissolved in 0.5 ml of saline daily during the entire experiment [Citation24]. Group V (diabetic received ZW + dapagliflozin) included diabetic rats that received combined ZW and dapagliflozin according to the previously mentioned doses. The excised wounds were covered daily with sterile gauze. Damage was measured and photographed on days 0, 3, 7, and 14 after wound induction. The rats were sacrificed on day 14 via cervical dislocation, and the wound areas were dissected. Part of the wound was immersed in 10% formalin for further histopathological and immunohistochemical examinations, and the rest of the tissue was stored at −80 °C for further biochemical and molecular analyses.
Evaluation of wound closure
Wound closure was assessed from day 0 to day 14 after an excisional wound injury. The unhealed area of the wound was measured using a traditional millimeter-graded ruler. The percentage of wound closure was calculated according to Wilson’s formula, which is as follows: The percentage of wound closure is equal to the diameter of the wound on day zero minus the wound diameter on day 14 divided by the wound diameter at day zero multiplied by 100 [Citation25].
Biochemical analysis
Using a mortar and pestle, a section of the excised skin wound (40 - 80 mg) was homogenized in a cold solution containing one milliliter of EDTA and 50 nM potassium phosphate (pH 7.5). Subsequently, the supernatant was collected and kept at 20 °C. The enzymes, SOD, GPX, lipid by MDA, and CAT, were measured using commercial kits according to the manufacturer’s instructions (Biodiagnostic, Cairo, Egypt). The proteins IL-1β, IL6, Ang II, TNF-α, and HIF-1α were assessed by ELISA kits (Cat# BMS630TEN), (Cat# EELR0015), (Cat# EK1574), (Cat# EELR2856), and (Cat# MBS2506922), respectively.
Histological examination
After dissecting the wounded area, the tissues were fixed in 10% formalin for 24 h. The tissue was dehydrated in ascending ethanol concentrations using an automated tissue-processing machine, followed by three stations of xylene as a clearing agent, and finally impregnated in paraffin. A section of paraffin blocks with a thickness of 5 μm was prepared. After dewaxing, the cells were stained with routine hematoxylin and eosin (H & E) and Manson’s trichrome [Citation26].
Immunohistochemical assessment of anti-NF-κB, anti-VEGF, anti-CD34, anti-CD45, anti-Ki-67, and anti-eNOS expression
Immunohistochemical analysis was performed according to the steps established by Eid et al. [Citation27]. The paraffin block containing wound tissue was cut into 5 μm sections, deparaffinized with xylene, and rehydrated with descending grades of ethanol. The antigen was retrieved by boiling for 10 min in 0.1 M citrate buffer. After that, 0.3% hydrogen peroxide was applied; then incubated with primary antibodies of anti-NF-κB (Cat# A3108; 1:50), anti-eNOS (Cat# PA3-031A; 1:250), anti-VEGF-A (Cat# GB14165; 1:500), anti-CD34 (Cat# MS-363-P0 and -P1; 1:200), anti-CD45 (Cat# Mob040; 1:200), and anti-Ki-67 (Cat# RMAP004; 1:100) overnight at 4 °C. The tissue was incubated with peroxidase (Dako C, Germany) before the secondary goat anti-rabbit antibody (Cat# K-205587) was applied for 30 min at room temperature. Finally, the slides were covered with Mayer’s hematoxylin solution. An expert pathologist from the Department of Pathology examined the slides to assess immunohistochemical positivity using a high-power X40 lens. Eight fields from three rats in each group were photographed using a digital camera (Leica EC3, Leica Company, Wetzlar, Germany) connected to an Alecia microscope (DM 500, Germany). Immune strength was automatically assessed using ImageJ software (Bethesda 150 MD, USA). The photographs were converted to eight bits, and the color was adjusted to gray before processing.
Quantitative real-time PCR (qRT-PCR)
Total RNA was extracted from the tissue homogenates of various groups using a direct-Zol RNA Mini-prep Plus (Cat# R2070; ZYMO Corporation, California, USA). A Beckman–daul spectrophotometer was used for quantitative and qualitative analyses. The Superscript IV one-step real-time PCR Kit (Cat# 1254200; Thermo Fisher Scientific, USA) was used to determine the reverse transcriptase activity and perform PCR on the extracted RNA. Thermal profiling was carried out using a 96-well Step-One instrument (Applied Biosystems, California, USA) as follows: reverse transcription for 10 min at 45 degrees Celsius, inactivation for 2 min at 98 degrees Celsius, and initial denaturation for 40 cycles of 10 s at 98 degrees Celsius, 10 s at 55 degrees Celsius, and 30 s at 72 degrees Celsius. Real-time PCR findings of housekeeping and target genes from real-time PCR are expressed as the cycle threshold. To reduce the variation in expression, the mean critical threshold expression value of the target gene and the housekeeping gene GAPDH were calculated using the 2-ΔΔCt technique. provides the primer sequences for the genes encoding the MMP-2, COL1A1, TGF-β1, EGF-β1, FGF, and GAPDH.
Table 1. Primer sequence of the examined genes.
Statistical analysis
The data are presented as the mean and standard deviation. ANOVA and Tukey’s post-hoc tests were used for comparisons. GraphPad Prism (version 8.0; GraphPad, La Jolla, California, USA) was used for all analyses. Statistical significance was set at a p-value of 0.05.
Results
Effect of combined treatment with ZW and dapagliflozin on diabetic wound healing
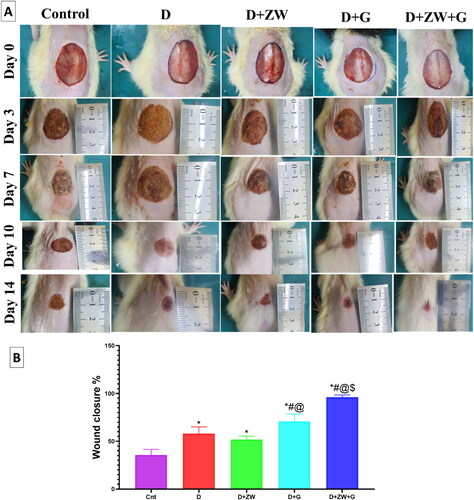
As shown in , combined treatment with ZW + dapagliflozin resulted in faster closure of the diabetic wound starting on day 10 and reaching a maximum on day 14 compared to the diabetic group. On day 14, the combined treatment significantly increased wound contraction by 96.3% compared to other experimental groups: control group, 42.25%; diabetic group, 58.1%; diabetes + ZW group, 52.5%; and diabetes + dapagliflozin group, 70.8% (). These results indicate that combined treatment dramatically affects the progression of diabetic wound closure.
Figure 1. A: Wound healing in diabetic rats from the five experimental groups at days zero, three, seven, ten, and fourteen; B: Percentage of wound healing on day 1. The mean (n = 8) ± standard deviation was used to express the data. * vs. Control group; # vs. Diabetic group; @ vs. Diabetic + ZW group, and $ vs. Diabetic + dapagliflozin group.
Histological effect of combined treatment with ZW and dapagliflozin in a diabetic wound
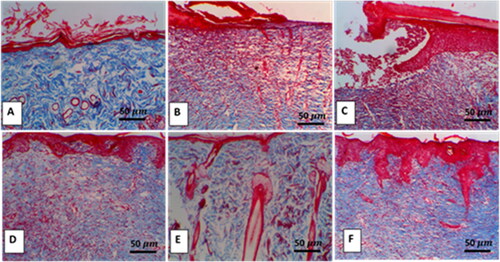
H & E examination of the diabetic wound showed extensive ulceration in the epidermis, which was replaced by infiltration of dead neutrophils admixed with numerous erythrocytes, forming a cellular crust with eosinophilic necrotic cellular and karyorrhectic debris. In contrast, the dermis revealed scant, vertically arranged granulation tissue around the blood capillaries, with multifocal hemorrhage aggregations admixed with mild numbers of cellular infiltrates. The adjacent epithelium was hyperplastic with acanthosis and spongiosis (). On the contrary, the combined treatment of diabetic rats with ZW and dapagliflozin resulted in significant histological improvement of wounds, mild epidermal thickening with fibroblast proliferation, collagen deposition, and enhanced new vascular formation (). Increased collagen deposition in the combination treatment group was confirmed using Masson’s trichrome staining ().
Figure 2. Representative photomicrograph of skin from different treatment groups. A: Control skins show a normal histological appearance of the epidermis and dermis in low-power and inset images. B: Skins of the diabetic group show extensive ulceration with numerous neutrophilic infiltrations and scanty granulation tissue (thick arrow), inset, adjacent epidermis showing marked neutrophilic aggregates (arrowhead) invading and surrounding the hyperplastic epithelium. C: the diabetic group shows cellular crust (thick arrow), and the dermal layers show hemorrhage admixed with a mild number of cellular infiltrates (thin arrow), inset, the adjacent epithelium is hyperplastic with acanthosis and spongiosis (arrowhead). D: the diabetic + ZW group typically arranged complete epidermal layers (line) with dermal ectatic blood capillary (arrowhead) and few inflammatory cells, focal edema (star), and dense granulation tissue. E: the diabetes + dapagliflozin group showed a complete epidermis with extensive epidermal hyperplasia (thick arrow) with thickened dermal collagen deposition and diminished blood vessels (thin arrow), inset, the hyperplastic epidermal cells showed few vacuolar degenerations (arrowhead). F: the diabetic + ZW + dapagliflozin group shows mild epidermal thickening (line) with fibroblast proliferation, collagen deposition, and enhanced newly vascular formation, inset, fibroblast infiltrations, and increased blood vessels. Image magnification = 400X, bar = 50 µm.
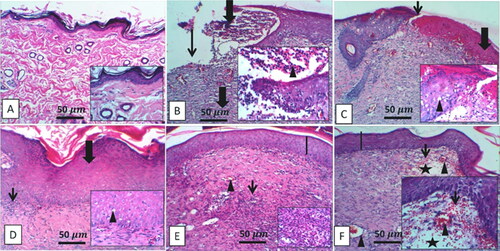
Figure 3. Representative Masson trichrome staining shows collagen deposition in wound tissue of different experimental groups. A: Deep blue coloration of collagen deposition in the control group. B and C: Sparse, thin, disorderly collagen deposition in diabetic groups. D and E: High collagen deposition in diabetic + ZW and diabetic + dapagliflozin. F: Intense collagen matrix deposition in the diabetic + ZW + dapagliflozin group. Image magnification = 400X, bar = 50 µm.
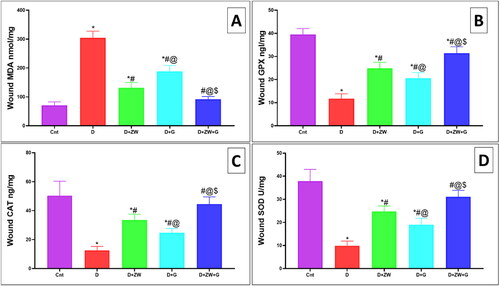
Impact of ZW with dapagliflozin on diabetic wound-induced oxidative stress
The level of oxidative stress markers in the diabetic wound showed a significantly (p < 0.05) high level of lipid peroxidation marker MDA by 304 ± 23.16 () with lower level of the activity of antioxidant enzymes GPX, CAT, and SOD by 11.75 ± 2.12, 12.5 ± 2.82, and 9.8 ± 2.03, respectively (D) in comparison to control rats. On the other hand, the Diabetic + ZW + dapagliflozin group significantly improved (p < 0.05) the oxidative insult in the diabetic wound with low level of MDA by 92 ± 9.24 and high value of the GPX, CAT, and SOD by 31.38 ± 2.82, 44.5 ± 5.04, and 31.13 ± 2.8, respectively. Treating diabetic rats with ZW and dapagliflozin greatly improved diabetic wound-induced oxidative stress and enhanced wound closure.
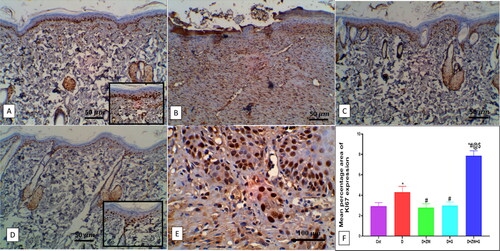
Dapagliflozin with ZW mitigates diabetic wound inflammation
The excised wound of diabetic rats showed a significant (p < 0.05) high level of the immunoexpression of the nuclear inflammatory transcription factor NF-κB by 8.92 ± 0.68 (), with a subsequent low level in the protein level of pro-inflammatory cytokines IL-1β, IL6, TNFα by 1042 ± 131.1, 984.8 ± 104.1, and 95.75 ± 20.08, respectively (C). In this context, compared to control rats, the number of dermal and epidermal CD45 immunostained positive cells increased by 7.58 ± 1.11 (). On the contrary, combined administration of ZW and dapagliflozin significantly (p < 0.05) improved the inflammatory response in diabetic wounds by lowering the immunoexpression of dermal and epidermal NF-κB and CD45 ( and ) by 0.91 ± 0.06 and 1.37 ± 0.36 with a low level in IL-1β, IL6, and TNF-α by 248.5 ± 37.2, 293.1 ± 25.94, and 26.88 ± 3.27, respectively in comparison with the diabetic group. The co-administration of ZW and dapagliflozin exhibited robust anti-inflammatory effects in diabetic wounds, shortening the initial inflammatory response and improving wound closure.
Figure 5. Representative photomicrograph of NF-κB expression in skin section of different groups. A: the control group shows no expression. B: the diabetic group shows high expression in epidermal and dermal cells, inset, high expression in the cytoplasm of invading polymorphonuclear and mononuclear cells. C and D: the Diabetic + ZW and diabetic + dapagliflozin groups show few expressions in epidermal and dermal cells. E: the diabetic + ZW + dapagliflozin group shows mild expression in epidermal and dermal cells. F: Histogram shows the percentage of skin that was immunostained with NF-κB. * vs. Control group; # vs. Diabetic group; @ vs. Diabetic + ZW group, and $ vs. Diabetic + dapagliflozin group. Image magnification = 400X, bar = 50 µm.
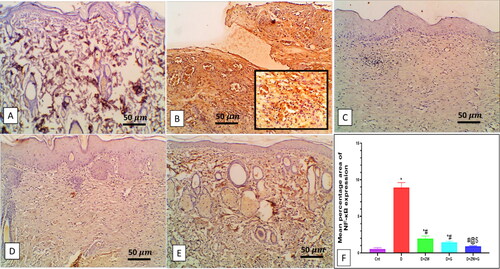
Figure 6. Impact of the combined effect of ZW and dapagliflozin on the protein level of A: IL-1β; B: IL6; C: TNF-α; D: Ang II; and E: HIF-1α by elisa assay. * vs. Control group; # vs. Diabetic group; @ vs. Diabetic + ZW group, and $ vs. Diabetic + dapagliflozin group.
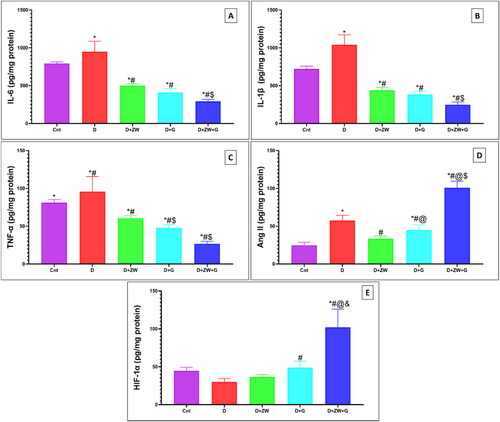
Figure 7. Representative photomicrograph of CD45 immunoexpression in skin section of different groups. A: the control group shows no expression of CD45-positive cells in lower and higher power images (inset images). B: the diabetic group shows high expression in the dermal capillary, Intravascular blood cells, and dermal cellular infiltrates. C) The diabetic + ZW group shows few epidermal and dermal expression, inset, few positive immunostained epidermal and subepidermal endothelial cells. D and E: the diabetic + dapagliflozin and diabetic + ZW + dapagliflozin groups show faint expression in dermal tissue. F: Histogram showing the percentage of skin that was immunostained with CD45. * vs. Control group; # vs. Diabetic group; @ vs. Diabetic + ZW group, and $ vs. Diabetic + dapagliflozin group. Image magnification = 400X, bar = 50 µm.
Dapagliflozin with ZW enhances regeneration and cellular proliferation
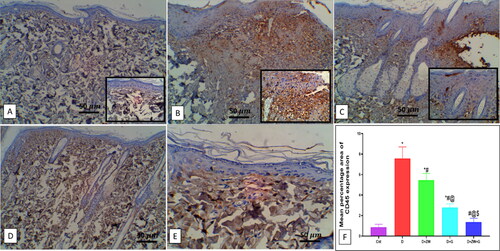
In comparison to treated rats, diabetic wounds showed a significant (p < 0.05) low value in the mRNA of transcription factors TGF-β1, MMP-2, and Col1A1 by 0.98 ± 0.13, 0.56 ± 0.18, and 0.44 ± 0.08, respectively (C), and immunostained Ki-67 positive proliferating cells by 4.30 ± 0.57 (). On the contrary, treatment of diabetic rats with ZW and dapagliflozin has significantly improved (p < 0.05) tissue regeneration and cellular proliferation showing a higher value in the gene expression of TGF-β1, MMP9, and Col1A1 by 2.82 ± 0.19, 3.01 ± 0.20, and 2.64 ± 0.14, respectively. In addition, the number of Ki-67-immunostained proliferating cells increased by 7.87 ± 0.48 (). This finding indicated that the co-administration of ZW and dapagliflozin improved diabetic wound regeneration and cellular proliferation.
Figure 8. Effect of ZW and dapagliflozin on the gene expression of A: TGF-β1; B: MMP-2; C: Col1A1; D: EGF-β1; and E: FGF. * vs. Control group; # vs. Diabetic group; @ vs. Diabetic + ZW group, and $ vs. Diabetic + dapagliflozin group.
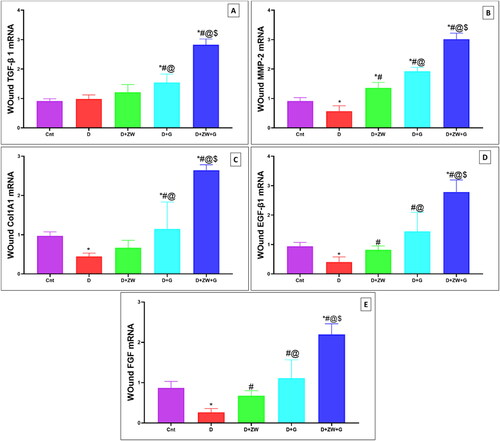
Figure 9. Representative photomicrograph of Ki-67 immunoexpression in skin section of different experimental groups. A: the control group shows no Ki-67 immunostained positive cells. C: the Diabetic + ZW and D: the diabetic + dapagliflozin groups show the same expression level of Ki-67 with high nuclear staining of epidermal cells. B: the diabetic group shows a moderate expression of Ki-67 in epidermal and dermal cells, inset, positive nuclear staining of epidermal, endothelial, and fibroblast cells. E: the diabetic + ZW + dapagliflozin group shows a strong nuclear expression of Ki-67 in epidermal and dermal cells. F: Histogram presented the percentage of skin immunostained with Ki 67. * vs. Control group; # vs. Diabetic group; @ vs. Diabetic + ZW group, and $ vs. Diabetic + dapagliflozin group. Image magnification = 400X, bar = 50 µm (A, B, C, and D), bar= 100 µm (E).
ZW and dapagliflozin promotes proangiogenic effect and angiogenesis in diabetic wound
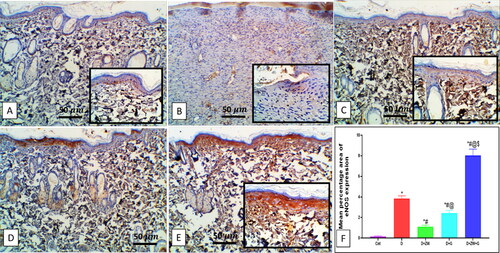
Compared to the diabetic group, concomitant treatment of diabetic rats with ZW and dapagliflozin significantly (p < 0.05) higher the protein level of the proangiogenic factor Ang II by 101 ± 8.56 (), and EGF-1b and FGF by 2.87 ± 0.40 and 2.19 ± 0.26 at the level of mRNA expression (). Furthermore, it enhanced angiogenesis response in regenerated tissue by a significant (p < 0.05) high level of HIF-1α protein and immunostaining of CD34, VEGF, and eNOS in vascular endothelial and fibroblast cells by 102.1 ± 23.82, 7.28 ± 0.45, 5.12 ± 0.52, and 8.02 ± 0.62, respectively (). These results indicated that the co-administration of ZW and dapagliflozin improved angiogenesis in diabetic wound healing compared to that in diabetic rats.
Figure 10. Representative photomicrograph of CD34 immunoexpression in skin section of different treatment groups. A: the control group shows no expression of CD34. B: the diabetic group shows a moderate expression of CD34 in dermal tissue, and the Insets show moderate cytoplasmic and nuclear expression in invading inflammatory and capillary endothelial cells. C: the diabetic + ZW group shows minimal focal expression in the cytoplasm of superficial epidermal cells. D: the diabetic + dapagliflozin group shows a mild expression of CD34 with little epidermal expression and mild expression in subepidermal cellular infiltrate and vascular endothelial cells. E: the diabetic + ZW + dapagliflozin group shows high expression in epidermal cells, dermal endothelial cells, inset, moderate expression in endothelial cells with few expressions in inflammatory cells. F: Histogram showing the percentage of skin that was immunostained with CD34. * vs. Control group; # vs. Diabetic group; @ vs. Diabetic + ZW group, and $ vs. Diabetic + dapagliflozin group. Image magnification = 400X, bar = 50 µm.
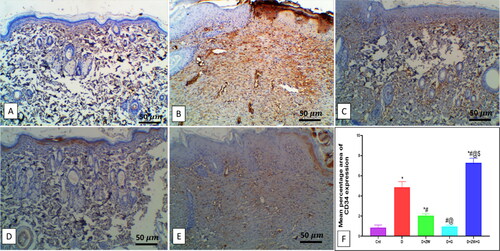
Figure 11. Representative immunohistochemical Micrograph of VEGF expression in skin sections of different groups. A: the control group shows a faint expression of VEGF in the dermal blood vessel. B: the diabetic group shows mild expression in dermal blood vessels. C: the diabetic + ZW group shows minimal expression in dermal endothelial and fibroblastic cells; see inset. D: the diabetic + dapagliflozin group shows few expressions in dermal vessels and fibroblasts. E: the diabetic + ZW + dapagliflozin group shows a high expression of VEGF in vascular endothelial and fibroblast cells; see inset. F: Histogram of the percentage of skin that was immunostained with VEGF. * vs. Control group; # vs. Diabetic group; @ vs. Diabetic + ZW group, and $ vs. Diabetic + dapagliflozin group. Image magnification = 400X, bar = 50 µm.
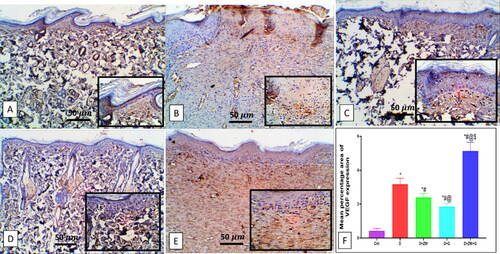
Figure 12. Representative photomicrograph of eNOS immunoexpression in the skin sections of different experimental groups. A: No expression in the control group. B: the diabetic group shows moderate expressions in dermal vascular endothelium and invading cells. C and D: the diabetic + ZW and diabetic + dapagliflozin groups show few expressions in dermal vascular endothelium and invading cells. E: the diabetic + ZW + dapagliflozin group shows high expression in epidermal cells, dermal vascular endothelial blood vessels, blood cells, invading cells, insert, high expression in the cytoplasm of invading polymorphonuclear and mononuclear cells, vascular endothelial cells. Faint to mild expression in the sebaceous gland and invading Mono- and polymorphonuclear cells. F: Histogram represented the percentage of skin that was immunostained with eNOS. * vs. Control group; # vs. Diabetic group; @ vs. Diabetic + ZW group, and $ vs. Diabetic + dapagliflozin group. Image magnification = 400X, bar = 50 µm.
Discussion
Diabetic wound healing is considered one of the most common clinical complications if not managed [Citation28]. Experimental excisional wounds are an ideal model for examining the stages of wound healing, such as the inflammatory, proliferative, and regenerative stages [Citation29]. Diabetes impairs all phases of wound healing, as it prolongs the inflammatory process at the wound edges [Citation30], and impairs angiogenesis, which delays the healing process and induces ulcer formation [Citation31]. Previous studies have reported that diabetes retards wound healing by prolonging the inflammatory process, impairing angiogenesis and wound remodeling [Citation32]. Currently, there is a lack of information on the pharmacological effects of oral hypoglycemic drugs, SGL2 inhibitors, and alkaline ZW on the healing of diabetic wounds. In this regard, the synergistic effect of dapagliflozin, a novel oral hypoglycemic drug with the most potent antioxidant, alkaline water, on diabetic wounds and its impact on inflammatory and proangiogenic responses was examined.
The inflammatory response is related to the progression of diabetes mellitus, as there is a clear relationship between elevated blood sugar levels, oxidative stress, and inflammation in diabetic wounds [Citation33]. In our study, combined treatment with dapagliflozin and ZW accelerated diabetic wound healing by accelerating wound contracture in diabetic rats, starting on day 10 and reaching a maximum on day 14. These findings align with a study by Moni et al. [Citation34] that examined the protective effect of ZW on normal wound healing. Furthermore, dapagliflozin-loaded exosomes significantly improve diabetic wounds [Citation35]. Likewise, the combined treatment synergistically enhanced the histological structure of diabetic damage by completely healing the epidermal layer with dermal neovascularization, a few infiltrations of inflammatory cells, fibroblast proliferation, and increased collagen deposition, as confirmed by Masson’s trichrome staining. Collagen is an essential extracellular component that supports skin integrity [Citation36]. This idea supports observing granulation tissue production and collagen deposition by H & E and trichrome staining. The study conducted by Zhang et al. [Citation35] reported that dapagliflozin-loaded exosomes improved the healing of diabetic wounds by the formation of epidermal and dermal tissue with an increase in collagen Mason trichrome staining, which is consistent with our findings.
Diabetes mellitus alters the immunological response, leading to marked inflammatory reactions and the overproduction of reactive oxygen species [Citation37, Citation38]. A previous study reported a marked increase in the pro-inflammatory cytokines in wound injury [Citation39], especially IL-1β, IL6, and TNF-α, which produce a chemotactic effect on leukocytes, exacerbating the inflammatory phase [Citation39]. In the present study, synergistic treatment with oral dapagliflozin and ZW resulted in two necessary biological actions. First, they abrogated diabetic wound oxidative insult by significantly increasing the activity of SOD, CAT, and GPX antioxidant enzymes, with a notable decrease in MDA. Secondly, they shorten the inflammatory process by decreasing the immunoexpression of transcription factor NF-κB and CD45-positive inflammatory leukocytes, with a consequent decrease in the protein level of IL-1β, IL6, and TNF-α inflammatory cytokines, compared to the diabetic group.
Our results agree with those of Taha et al. [Citation40], who reported that ZW halts cyclosporine-induced renal oxidative stress, and those of Zaibi et al. [Citation41], who reported the antioxidant effect of dapagliflozin on human renal proximal tubular epithelial cells. Furthermore, the studies conducted by Moni et al. [Citation34] and Nandhidha and Punnagai [Citation42] documented the anti-inflammatory effect of ZW and dapagliflozin on normal wounds. The synergistic effect of the combined treatment shortened the inflammatory process and accelerated the proliferative phase owing to its antioxidant power, with subsequent anti-inflammatory effects on diabetic wounds. The proliferative phase is marked by enhanced cellular proliferation, as evidenced by a significant increase in Ki-67 expression in fibroblasts, basal epidermal cells, and keratinocytes [Citation43]. In the present study, combined oral administration of 1 mg/kg of dapagliflozin with ZW significantly elevated Ki-67 immunoexpression in the epidermal and dermal layers of wound sections, comparable to those in diabetic groups. This indicates that the synergistic action between the two lines of treatment markedly improved the proliferative phase.
Angiogenesis plays a crucial role in wound healing [Citation44]. Diabetes is associated with defects in angiogenesis, which further impair wound healing. A previous study by Hamed et al. [Citation45] reported that reduced VEGF levels delayed wound healing after 12 days post-excisional wound injury in an experimental model. In our study, combined treatment significantly enhanced angiogenic markers by increasing the immunoexpression of CD34, eNOS, and VEGF-A and the gene expression of proangiogenic growth factors EGF-β1 and FGF, which are considered beneficial for vascular endothelial cellular growth.
Furthermore, it elevated the protein levels of Ang II and HIF-1α, which are mandatory in angiogenesis and wound healing. This finding is supported by the research of Zhang et al. [Citation35], who stated that dapagliflozin-loaded exosomes improved diabetic wound angiogenesis by upregulating the HIF-1α/VEGF-A pathway. In addition, the work performed by Moni et al. [Citation34] reported that ZW plays a fundamental role in angiogenesis during the healing of normal wounds, which can be explained by its trace element content, including zinc, which is supposed to impact angiogenesis positively.
The remodeling process is the final stage of wound healing and is characterized by a balance between the synthesis and degradation of collagen, leading to wound closure [Citation46]. The TGF-β1 multifactorial anti-inflammatory cytokine is essential in collagen formation, angiogenesis, fibroblast proliferation, and extracellular matrix remodeling [Citation47]. Previous studies have established the positive impact of TGF-β1 and MMP-2 activity on collagen deposition [Citation48].
Based on the above evidence, the findings of our study revealed that oral ingestion of dapagliflozin and ZW significantly increased the gene expression of TGF-β1, Col1A1, and MMP-2 compared to that in the diabetic group, thereby increasing collagen formation. This result supports our finding that the combined treatment accelerated the closure of the diabetic wound, which started on day ten and was completed on day 14. Despite these findings, there are some limitations to our experimental study. Firstly the small number of experimental rats resulted in limited statistical power by focusing our study on young male rats, so in the future, the effect of combined treatment on rats of different ages and both genders will be examined. Secondly, the study was designed on the hypoglycemic dose of dapagliflozin only, so in future studies, multiple doses of dapagliflozin with ZW will be investigated to evaluate the most potent dose in wound healing speed. Overall, this combination showed powerful healing power regarding their ant-inflammatory and proangiogenic effects, which can be applied to humans in the future.
Conclusions
The combined treatment with dapagliflozin and Zamzam water shortens the inflammatory stage by enhancing the level of antioxidant enzymes and suppressing the NF-κB transcription factor. This leads to decreased pro-inflammatory cytokines TNFα, IL-1β, and IL6 and the number of CD45 cells. Moreover, it improved cell proliferation by increasing Ki-67 expression and enhancing angiogenesis by upregulating CD34, eNOS, VEGF-A, EGF-β1, FGF, AngII, and HIF-1α. Furthermore, it improved the remodeling process by upgrading TGF-β1 and Col1A1. In conclusion, this combined treatment offers a novel approach for treating patients with diabetes who suffer from wound injuries, surgical interventions, or diabetic foot ulcers, effectively protecting them from gangrenous complications.
| List of abbreviations | ||
| eNOS | = | endothelial Nitric Oxide Synthetase |
| EPC | = | Endothelial Progenitor Cell |
| HIF | = | Hypoxia-Inducible Factor |
| VEGF | = | Vascular Endothelial Growth Factor |
| SGLT-2 | = | Sodium-Glucose Cotransporter-2 |
| HbA1c | = | glycosylated hemoglobin; |
| ZW | = | Zamzam water |
| STZ | = | streptozotocin; |
| SOD | = | Super oxide dismutase |
| GPX | = | Glutathione Peroxidase; |
| MDA | = | Malonaldehyde; |
| CAT | = | Catalase |
| IL-1β | = | Interleukin 1 Beta |
| IL6 | = | Interleukin 6; |
| Ang II | = | Angiotensin II |
| TNFα | = | Tumour necrosis factor alpha |
| HIF 1-α | = | Hypoxia-inducible factor 1-alpha |
| Nf-κB | = | Nuclear factor kappa B; |
| MMP-2 | = | Matrix metalloproteinases-2 |
| COL1A1 | = | Collagen, type I, alpha 1 chain; |
| TGF-β1 | = | Transforming growth factor beta 1; |
| EGF-β1 | = | Epidermal Growth Factor |
| FGF | = | Fibroblast growth factors |
| GAPDH | = | glyceraldehyde 3-phosphatase and glyceraldehyde3. Phosphate dehydrogenase |
Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
The data that support this research will be shared upon reasonable request to the corresponding authors.
References
- Lau HC, Kim A. Pharmaceutical perspectives of impaired wound healing in diabetic foot ulcer. J Pharm Investig. 2016;46(5):1–13. doi:10.1007/s40005-016-0268-6.
- Gushiken LFS, Beserra FP, Bastos JK, Jackson CJ, Pellizzon CH. Cutaneous wound healing: An update from physiopathology to current therapies. Life (Basel). 2021;11(7):665. doi:10.3390/life11070665.
- Nosenko MA, Ambaryan SG, Drutskaya MS. Proinflammatory cytokines and skin wound healing in mice. Mol Biol (Mosk). 2019;53(5):741–754. doi:10.1134/S0026898419050136.
- Deng L, Du C, Song P, et al. The role of oxidative stress and antioxidants in diabetic wound healing. Oxid Med Cell Longev. 2021;2021:8852759–8852711. doi:10.1155/2021/8852759.
- Du XL, Edelstein D, Dimmeler S, Ju Q, Sui C, Brownlee M. Hyperglycemia inhibits endothelial nitric oxide synthase activity by posttranslational modification at the Akt site. J Clin Invest. 2001;108(9):1341–1348. doi:10.1172/JCI11235.
- Kränkel N, Adams V, Linke A, et al. Hyperglycemia reduces survival and impairs function of circulating blood-derived progenitor cells. Arterioscler Thromb Vasc Biol. 2005;25(4):698–703. doi:10.1161/01.ATV.0000156401.04325.8f.
- Costa PZ, Soares R. Neovascularization in diabetes and its complications. Unraveling the angiogenic paradox. Life Sci. 2013;92(22):1037–1045. doi:10.1016/j.lfs.2013.04.001.
- Emanuelli T, Burgeiro A, Carvalho E. Effects of insulin on the skin: Possible healing benefits for diabetic foot ulcers. Arch Dermatol Res. 2016;308(10):677–694. Epub 2016 Sep 21. doi:10.1007/s00403-016-1686-z.
- Ji L, Ma J, Li H, et al. Dapagliflozin as monotherapy in drug-naive Asian patients with type 2 diabetes mellitus: A randomized, blinded, prospective phase III study. Clin Ther. 2014;36(1):84–100.e9. doi:10.1016/j.clinthera.2013.11.002.
- Kohan DE, Fioretto P, Tang W, List JF. Long-term study of patients with type 2 diabetes and moderate renal impairment shows that dapagliflozin reduces weight and blood pressure but does not improve glycemic control. Kidney Int. 2014;85(4):962–971. doi:10.1038/ki.2013.356.
- Bolinder J, Ljunggren Ö, Kullberg J, et al. Effects of dapagliflozin on body weight, total fat mass, and regional adipose tissue distribution in patients with type 2 diabetes mellitus with inadequate glycemic control on metformin. J Clin Endocrinol Metab. 2012;97(3):1020–1031. doi:10.1210/jc.2011-2260.
- Merovci A, Solis-Herrera C, Daniele G, et al. Dapagliflozin improves muscle insulin sensitivity but enhances endogenous glucose production. J Clin Invest. 2014;124(2):509–514. doi:10.1172/JCI70704.
- Tahara A, Kurosaki E, Yokono M, et al. Effects of SGLT2 selective inhibitor ipragliflozin on hyperglycemia, hyperlipidemia, hepatic steatosis, oxidative stress, inflammation, and obesity in type 2 diabetic mice. Eur J Pharmacol. 2013;715(1–3):246–255. doi:10.1016/j.ejphar.2013.05.014.
- Nandula SR, Kundu N, Awal HB, et al. Role of Canagliflozin on function of CD34 + ve endothelial progenitor cells (EPC) in patients with type 2 diabetes. Cardiovasc Diabetol. 2021;20(1):44. doi:10.1186/s12933-021-01235-4.
- Albiero M, Tedesco S, Amendolagine FI, et al. Inhibition of SGLT2 rescues bone marrow cell traffic for vascular repair: role of glucose control and ketogenesis. Diabetes. 2021;70(8):1767–1779. doi:10.2337/db20-1045.
- Khalid N, Ahmad A, Khalid S, Ahmed A, Irfan M. Mineral composition and health functionality of Zamzam water: A review. Int J Food Prop. 2014;17(3):661–677. doi:10.1080/10942912.2012.660721.
- Jin D, Ryu SH, Kim HW, et al. Anti-diabetic effect of alkaline-reduced water on OLETF rats. Biosci Biotechnol Biochem. 2006;70(1):31–37. doi:10.1271/bbb.70.31.
- Bamosa A, Elnour A, Kaatabi H, Al Meheithif A, Aleissa K, Al-Almaie S. Zamzam water ameliorates oxidative stress and reduces hemoglobinA1c in Type 2 diabetic patients. J Diabetes Metab. 2013;04(03):4. doi:10.4172/2155-6156.1000249.
- Abdullah AM, Abdelsalam E, Abdullah B, Khaled A. Antioxidant effects of Zamzam water in normal rats and those under induced-oxidative stress. J Med Plants Res. 2012;6:5507–5512.
- Metwally MMM, Ebraheim LLM, Galal AAA. Potential therapeutic role of melatonin on STZ-induced diabetic central neuropathy: A biochemical, histopathological, immunohistochemical and ultrastructural study. Acta Histochem. 2018;120(8):828–836. doi:10.1016/j.acthis.2018.09.008.
- João D, Masi EC, Campos AC, et al. The influence of growth factors on skin wound healing in rats. Braz J Otorhinolaryngol. 2016;82(5):512–521. doi:10.1016/j.bjorl.2015.09.011.
- Suckow MA, Gobbett TA, Peterson RG. Wound healing delay in the ZDSD rat. In Vivo. 2017;31(1):55–60. doi:10.21873/invivo.11025.
- Mraisel A, Abu Ali A. Protective effect of Zamzam water against kidneys damage induced in male rats: Immunohistochemistry evidence. J Biosci Appl Res. 2017;3(2):42–47. doi:10.21608/jbaar.2017.124882.
- Han S, Hagan DL, Taylor JR, et al. Dapagliflozin, a selective SGLT2 inhibitor, improves glucose homeostasis in normal and diabetic rats. Diabetes. 2008;57(6):1723–1729. doi:10.2337/db07-1472.
- Byrnes KR, Barna L, Chenault VM, et al. Photo biomodulation improves cutaneous wound healing in an animal model of type II diabetes. Photomed Laser Surg. 2004;22(4):281–290. doi:10.1089/pho.2004.22.281.
- Alturkistani HA, Tashkandi FM, Mohammedsaleh ZM. Histological stains: a literature review and case study. Glob J Health Sci. 2015;8(3):72–79. doi:10.5539/gjhs.v8n3p72.
- Eid BG, Alhakamy NA, Fahmy UA, et al. Melittin and diclofenac synergistically promote wound healing in a pathway involving TGF-β1. Pharmacol Res. 2022;175:105993. doi:10.1016/j.phrs.2021.105993.
- Sharp A, Clark J. Diabetes and its effects on wound healing. Nurs Stand. 2011;25(45):41–47. doi:10.7748/ns2011.07.25.45.41.c8626.
- Zhou J, Ni M, Liu X, Ren Z, Zheng Z. Curcumol promotes vascular endothelial growth factor (VEGF)-mediated diabetic wound healing in streptozotocin-induced hyperglycemic rats. Med Sci Monit. 2017;23:555–562. doi:10.12659/msm.902859.
- Park NY, Lim Y. Short term supplementation of dietary antioxidants selectively regulates the inflammatory responses during early cutaneous wound healing in diabetic mice. Nutr Metab (Lond). 2011;8(1):80. doi:10.1186/1743-7075-8-80.
- Bodnar RJ. Chemokine regulation of angiogenesis during wound healing. Adv Wound Care (New Rochelle). 2015;4(11):641–650. doi:10.1089/wound.2014.0594.
- Eming SA, Martin P, Tomic-Canic M. Wound repair and regeneration: Mechanisms, signaling, and translation. Sci Transl Med. 2014;6(265):265sr6. doi:10.1126/scitranslmed.3009337.
- Oguntibeju OO. Type 2 diabetes mellitus, oxidative stress and inflammation: Examining the links. Int J Physiol Pathophysiol Pharmacol. 2019;11(3):45–63.
- Moni SS, Sultan MH, Alshahrani S, et al. Physical characterization and wound healing properties of Zamzam water. Braz J Biol. 2022;82:e262815. doi:10.1590/1519-6984.262815.
- Zhang W, Wang L, Guo H, Chen L, Huang X. Dapagliflozin-loaded exosome mimetics facilitate diabetic wound healing by HIF-1α-mediated enhancement of angiogenesis. Adv Healthc Mater. 2023;12(7):e2202751. doi:10.1002/adhm.202202751.
- Miller EJ. Collagen types: Structure, distribution, and functions. In: Collagen. Boca Raton: CRC Press, 2018. p. 139–56.
- Fresta CG, Fidilio A, Caruso G, et al. A new human blood-retinal barrier model based on endothelial cells, pericytes, and astrocytes. Int J Mol Sci. 2020;21(5):1636. doi:10.3390/ijms21051636.
- Vijayakumar V, Samal SK, Mohanty S, Nayak SK. Recent advancements in biopolymer and metal nanoparticle-based materials in diabetic wound healing management. Int J Biol Macromol. 2019;122:137–148. doi:10.1016/j.ijbiomac.2018.10.120.
- Kany S, Vollrath JT, Relja B. Cytokines in inflammatory disease. Int J Mol Sci. 2019;20(23):6008. doi:10.3390/ijms20236008.
- Taha M, Elazab ST, Baokbah TAS, et al. Palliative role of Zamzam water against cyclosporine-induced nephrotoxicity through modulating autophagy and apoptosis crosstalk. Toxics. 2023;11(4):377. doi:10.3390/toxics11040377.
- Zaibi N, Li P, Xu SZ. Protective effects of dapagliflozin against oxidative stress-induced cell injury in human proximal tubular cells. PLoS One. 2021;16(2):e0247234. doi:10.1371/journal.pone.0247234.
- Nandhidha R1, Punnagai K. Evaluation of anti-inflammatory and wound healing potential of sodium glucose co-transporter2 (SGLT2) inhibitors. Res J Pharm Technol. 2022;15(10):4457–4462. doi:10.52711/0974-360X.2022.00747.
- Chen RF, Wang CT, Chen YH, et al. Hyaluronic acid-povidone-iodine compound facilitates diabetic wound healing in a streptozotocin-induced diabetes rodent model. Plast Reconstr Surg. 2019;143(5):1371–1382. doi:10.1097/PRS.0000000000005504.
- Veith AP, Henderson K, Spencer A, Sligar AD, Baker AB. Therapeutic strategies for enhancing angiogenesis in wound healing. Adv Drug Deliv Rev. 2019;146:97–125. doi:10.1016/j.addr.2018.09.010.
- Hamed S, Ullmann Y, Masoud M, Hellou E, Khamaysi Z, Teot L. Topical erythropoietin promotes wound repair in diabetic rats. J Invest Dermatol. 2010;130(1):287–294. doi:10.1038/jid.2009.219.
- Yao C, Markowicz M, Pallua N, Noah EM, Steffens G. The effect of cross-linking of collagen matrices on their angiogenic capability. Biomaterials. 2008;29(1):66–74. doi:10.1016/j.biomaterials.2007.08.049.
- Penn JW, Grobbelaar AO, Rolfe KJ. The role of the TGF-β family in wound healing, burns and scarring: A review. Int J Burns Trauma. 2012;2(1):18–28.
- Lechapt-Zalcman E, Prulière-Escabasse V, Advenier D, et al. Transforming growth factor-beta1 increases airway wound repair via MMP-2 upregulation: A new pathway for epithelial wound repair? Am J Physiol Lung Cell Mol Physiol. 2006;290(6):L1277–82. doi:10.1152/ajplung.00149.2005.