Abstract
The SARS-CoV-2 virus caused a globally growing pandemic called coronavirus disease 2019 (COVID-19) that has disrupted social, political, and medical environments around the world. Nations are assessing ways to reopen businesses while trying to balance health care risks and economic fallouts. Strategies involving antibody testing have been proposed before phased reopening of the economy. Therefore, assessing the sensitivity and specificity of antibody tests for symptomatic and asymptomatic COVID-19 patients remains paramount to prevent COVID-19 outbreaks. The antibody tests for SARS-CoV-2 detect the presence of IgA, IgM, or IgG antibodies produced by B cells. There are four major types of antibody tests: rapid diagnostic tests, enzyme-linked immunosorbent assays, neutralization assays, and chemiluminescent immunoassays. Currently, there is no standard antibody test for detecting SARS-CoV-2 antibodies during or after exposure or infection. The antibody tests for SARS-CoV-2 have a low specificity within the first week of exposure and increase in the second and third weeks. The current data on antibody tests have several limitations in quality and the presence of bias. Specifically, many antibody tests have a high false-negative rate and a high risk of bias for participant selection, application of index tests, reference standard used, and flow and timing for antibody tests that may incorrectly report the accuracy of COVID-19 antibody tests. In this review, we summarize the current methods, sensitivity/specificity, and gaps in knowledge concerning COVID-19 antibody testing.
Target audience: All physicians
Learning objectives: After completing the article, the learner should be able to
1. Understand how COVID-19 antibody tests work and recognize their uses and limitations
2.Apply the guidelines for administering COVID-19 antibody tests
Faculty credentials/disclosure: Mr. Kopel is an MD/PhD student at Texas Tech University Health Sciences Center. Dr. Goyal was assistant program director in internal medicine at the Medical Center of Central Georgia and assistant professor of medicine at Mercer University; he is currently completing a gastroenterology fellowship at the Wright Center for Graduate Medical Education. Dr. Perisetti is a fellow in gastroenterology at the University of Arkansas for Medical Sciences. The authors and planner have no conflicts of interest to disclose.
Accreditation: The A. Webb Roberts Center for Continuing Medical Education of Baylor Scott & White Health is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.
Designation: The A. Webb Roberts Center for Continuing Medical Education of Baylor Scott & White Health designates this journal CME activity for a maximum of 1.0 AMA PRA Category 1 CreditTM. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
ABIM MOC: Successful completion of this CME activity, which includes participation in the evaluation component, enables the participant to earn up to 1.0 Medical Knowledge points in the American Board of Medicine’s (ABIM) Maintenance of Certification (MOC) program. The CME activity provider will submit participant completion information to ACCME for the purpose of granting ABIM MOC credit.
Process: To complete this CME activity, read the entire article and then go to https://ce.bswhealth.com/Proceedings2020. You will register for the course, pay any relevant fee, take the quiz, complete the evaluation, and claim your CME credit. For more information about CME credit, email [email protected].
Expiration date: January 1, 2023.
The severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) has caused a global pandemic known as coronavirus disease 2019 (COVID-19). Despite increasing SARS-CoV-2 infections globally, there is increased social and political pressure to reopen economic activity and bring “normalcy” to people’s lives. Therefore, health care authorities have begun to encourage the use of antibody testings to prevent the spread and evaluate the presence of immunity for SARS-CoV-2 infection.Citation1,Citation2 However, a lack of a rigorous antibody test with high specificity and sensitivity has remained a challenge.Citation1,Citation2 Furthermore, the prevalence of COVID-19 antibodies, the sensitivity and specificity of the antibody test, and antibody titers that confer immunity remain open areas of investigation.Citation1,Citation2 These uncertainties have important social implications concerning restriction of work, travel, or social gatherings based on COVID-19 antibody status to reduce exposure to vulnerable populations.
Currently, the US Food and Drug Administration has given emergency use authorizations to commercial test manufacturers for COVID-19 antibody tests.Citation2 These tests are required to be assessed for sensitivity and specificity before their use in clinical practice.Citation2 However, the method for assessing the performance of COVID-19 antibody tests varies between manufacturers due to the type of clinical samples used.Citation2 In most cases, antibody tests are assessed to an index or reference test to determine their accuracy.Citation2 The Food and Drug Administration has allowed manufacturers to establish the accuracy of COVID-19 antibody tests using reverse-transcriptase–polymerase chain reaction (RT-PCR) testing from symptomatic COVID-19 patients.Citation2 However, the nasopharyngeal swabs used for RT-PCR can overestimate the sensitivity and specificity of the antibody tests.Citation2 Therefore, assessing the sensitivity and specificity of antibody tests in both symptomatic and asymptomatic COVID-19 patients poses a paramount challenge for managing the outbreak.
A comprehensive literature review was performed from January 1, 2020, to August 1, 2020, from the PubMed, Scopus, Web of Science, and Embase databases using the keyword search phrases “COVID-19,” “SARS-CoV-2,” and “antibody tests.” In this review, we summarize the availability, sensitivity, specificity, and gaps in current knowledge concerning COVID-19 antibody tests used worldwide ().
DIAGNOSTIC METHOD FOR DETECTING COVID-19 USING RT-PCR
Currently, the standard method for collecting specimens for COVID-19 testing involves nasopharyngeal swabs consisting of synthetic fiber swabs with a plastic shaft.Citation3,Citation4 As the name suggests, the swab is inserted into the nasopharyngeal space in the posterior pharynx and tonsillar area. Oligonucleotide primers are selected to detect the spike protein of SARS-CoV-2.Citation3,Citation4 Repeat testing can be performed in positive patients to monitor the progression of COVID-19 if symptoms persist.Citation3,Citation4 However, health care staff risk exposure to SARS-CoV-2, as nasopharyngeal swabs can induce sneezing, coughing, or gagging during the sample collection.Citation3,Citation5 Therefore, several clinics have adopted measures to self-collect oropharyngeal swabs, sputum, and turbinate swab samples.Citation6 This method has helped reduce exposure without compromising the sensitivity of the RT-PCR analysis but can be associated with a false-negative sample due to incomplete sample collection.Citation5 Many centers have also faced shortages in the supplies and personnel to adequately sample and monitor the progression of COVID-19.Citation7 Furthermore, RT-PCR requires expensive equipment and trained technicians at certified laboratories, long times to generate results, and risks of false-negatives in patients with low viral loads. Therefore, antibody tests have been used to aid in monitoring COVID-19 infections.
HUMORAL IMMUNE RESPONSE TO SARS-COV-2
Antibody tests detect the presence of antibodies produced by B cells toward a specific pathogen.Citation8 The activation and differentiation of B cells into antibody-secreting plasma cells are initiated through antigen-presenting cells (e.g., dendritic cells, macrophages, helper T cells). The body produces two major types of antibodies, immunoglobulin M (IgM) and immunoglobulin G (IgG), in response to an infection.Citation8 The IgM antibodies are produced soon after infection, while the IgG antibodies are produced later to maintain the body’s immune system to the same infection.Citation8,Citation9 The third type of immunoglobulin, known as IgA, is found on mucous membranes and aids the innate immune response.Citation9–11 Current clinical reports show that antibodies against SARS-CoV-2 viral particles develop between 6 and 10 days after infection, with peak IgM antibody levels at 12 days, and persist for 35 days. In contrast, the IgG antibodies peak around 17 days and persist for up to 49 days.Citation9–11 Serological or antibody tests detect immunoglobulins produced in the presence of antigens from SARS-CoV-2.
ANTIBODY TESTS FOR DETECTING COVID-19 ANTIBODIES
The antibody tests are a rapid method for detecting antigens (the spike, membrane, or nucleocapsid proteins) or antibodies for COVID-19 using lateral flow assays.Citation3 As shown in , there are four major types of antibody tests: rapid diagnostic tests (RDT), enzyme-linked immunosorbent assays (ELISA), neutralization assays, and chemiluminescent immunoassays.Citation12 Current World Health Organization guidelines recommend obtaining a blood sample during the first week of illness and then 3 to 4 weeks later to measure SARS-CoV-2 antibodies.Citation13 Within 5 days of infection, the IgM positive rate increased from 50% to 81%, whereas the IgG positive rate increased from 81% to 100% in COVID-19 patients.Citation13 Unlike the nasopharyngeal RT-PCR tests, the antibody tests allow for better collection of epidemiological data, determination of the immune status of asymptomatic individuals, and screening of previous exposure.Citation3 Currently, there is no accepted standard antibody test by which to compare the sensitivity or specificity for the SARS-CoV-2.Citation1 The Food and Drug Administration has required clinics and industries to estimate the analytic performance of any antibody test using a material containing SARS-CoV-2 at sufficient concentration to be detected.
Table 1. SARS-CoV-2 antibody test types*
SOUTH AMERICAN ANTIBODY STUDIES
A meta-analysis of antibody tests in Brazil examined 16 commercially available antibody tests registered in the Brazilian Health Regulatory Agency.Citation14 It examined the pooled sensitivity and specificity for detecting SARS-CoV-2 IgM/IgG antibodies and naso/oropharyngeal swabs.Citation14 The pooled sensitivity and specificity were 82% and 97%, respectively, for the IgM antibodies. For IgG antibodies, the pooled sensitivity and specificity were 97% and 98%, respectively.Citation14 For the naso/oropharyngeal swabs, the pooled sensitivity and specificity were 97% and 99%, respectively. Although the IgG antibodies and naso/oropharyngeal swabs had high sensitivity and specificity, the results are limited by a high rate of false-negative results from tests that detect SARS-CoV-2 IgM antibodies and a lack of studies validating the antibody results. Furthermore, many of the studies used small sample sizes (n ≤ 20); only half of the antibody tests included more than 150 samples. The lack of large samples in the antibody studies reduces the reliability of applying these antibodies to larger sample sizes.Citation14
AUSTRIAN ANTIBODY STUDIES
Several reports in Austria examined the accuracy of commercially available antibody tests.Citation15–17 One study assessed the sensitivity and specificity of four commercial ELISA and two RDT tests in 77 COVID-19 patients compared with the nasopharyngeal RT-PCR.Citation17 The study found that sensitivities for the ELISA and RDT antibody tests were around 80% within the first week of infection. The specificities for the ELISA tests within 2 weeks were 83% (IgA), 98% (IgG), and 97% (IgM and total antibody).Citation17 However, the study had a low sample size for comparing the antibody tests and did not include neutralization assays or chemiluminescent immunoassays in their analysis.Citation17 The results supported previous reports that the sensitivities and specificities of the antibody tests increased weeks after a patient was exposed or developed symptoms associated with COVID-19.Citation17
Another study examined the sensitivity and specificity of the EDI ELISA for detecting SARS-CoV-2 IgM and IgG antibodies in 64 RT-PCR samples of confirmed COVID-19 patients with serial blood samples at different time points.Citation15 The study found that the sensitivity of the ELISA test was 5.9% for IgM and 2.9% for IgG 5 days after symptom onset.Citation15 Between 5 and 10 days, the sensitivity of the ELISA test increased to 37.1% for IgM and 37.1% for IgG.Citation15 After 10 to 15 days, the sensitivity of the ELISA test increased to 76.4% for IgM and 82.4% for IgG. After 3 weeks, the sensitivity increased to 94.4% for IgM and 100% for IgG.Citation15 A recent Austrian study also compared the effectiveness of the fully automated Elecsys Anti-SARS-CoV-2 immunoassay with the EDI ELISA for the detection of SARS-CoV-2 antibodies in human plasma.Citation16 The study examined 64 SARS-CoV-2 RT-PCR samples along with blood samples from 200 healthy blood donors and 256 intensive care unit patients collected before the COVID-19 outbreak.Citation16 The authors found that the peak sensitivities for the Elecsys assay, EDI IgM ELISA, and EDI IgG ELISA were 100%, 94%, and 100%, respectively, between 15 and 22 days.Citation16 These studies suggest that antibody tests should only be administered 3 weeks after a patient has developed symptoms and tested positive for COVID-19.
FRENCH ANTIBODY STUDIES
A French study evaluated the performance of six RDT and three ELISA antibody tests for diagnosis of COVID-19 in 34 hospitalized patients positive for SARS-CoV-2 RNA.Citation18 Within 7 days of symptom onset, both the RDT and ELISA tests were able to detect SARS-CoV-2 in 50% of the samples.Citation18 After 2 weeks, the average sensitivity of the RDT and ELISA antibody tests was 80% to 100%.Citation18 Specifically, >90% of hospitalized COVID-19 patients tested positive for SARS-CoV-2 using the iSIA and Accu-Tell POC tests and the I.D.Vet IgG ELISA assays.Citation18 Three antibody tests, EUROIMMUN IgG and IgA, UNscience, and Zhuhai Livzon, had sensitivities below 90%.Citation18 A subsequent French study that examined four RDT tests also showed a sensitivity of 60% to 80% after 10 days and 100% at 2 weeks.Citation19 Another French study assessed the performance of three SARS-CoV-2 antibody test kits (Abbott Architect SARS-CoV-2 CLIA IgG, Euroimmun Anti-SARS-CoV-2 ELISA IgG/IgA assays, and LFIA NG-Test IgG-IgM COVID-19) using 293 samples from COVID-19 patients.Citation20 The study found that all three SARS-CoV-2 antibody tests had a sensitivity around 80%, which increased after 2 weeks in COVID-19–positive patients.Citation20 Overall, the best method for detecting SARS-CoV-2 antibodies was detecting IgG after 14 days of symptom onset, which was independent of the antibody test used.
GERMAN ANTIBODY STUDIES
A German study examined four automated immunoassays—Abbott Architect i2000, Roche Cobas e411 analyzer, LIAISON XL platform, and VIRCLIA automation system—compared with two ELISA assays—Euroimmun SARS-CoV-2 IgG and Virotech SARS-CoV-2 IgG ELISA—for their sensitivity to detect SARS-CoV-2.Citation21 The VIRCLIA automation system had the highest sensitivity of 89%. The other antibody tests had sensitivities that ranged from 66.7% to 77.8%.Citation21 A subsequent German study using samples from 26 COVID-19 patients found sensitivities for the Roche, Euroimmun, and Epitope of 92.3%, 96.2%–100%, and 100%, respectively; specificities were 100%, 100%, and 84%–86%, respectively.Citation22 These immunoassays were able to identify 84% to 96% of COVID-19–negative samples and 92.3% to 95.2% of COVID-19–positive samples.Citation22
FINNISH ANTIBODY STUDIES
A Finnish study assessed the performance of six commercial immunoassays (Abbott Architect SARS-COV-2 IgG, Diasorin Liaison SARS-COV-2 S1/S2 IgG, Euroimmun SARS-COV-2 IgG, Euroimmun SARS-COV-2 IgA, Acro Biotech 2019-nCoV IgG/IgM, and Xiamen Biotime Biotechnology SARS-COV-2 IgG/IgM).Citation23 The study included 70 serum samples from COVID-19 patients and 81 control patients.Citation23 The sensitivity for the assays ranged from 68.3% to 97.5%, and the specificity ranged from 43.8% to 81.3%. The variability is believed to be related to the specific protein or antibodies used to detect SARS-CoV-2.Citation23 Specifically, the authors suggested that improvements to antigenic features of the SARS-CoV-2 antibody tests and test workflow would improve sensitivity and specificity of the screening methods.Citation23
BELGIAN ANTIBODY STUDIES
A Belgian group studied the effectiveness of five SARS-CoV-2 antibody tests, including the Maglumi 2019-n-CoV IgG and IgM assays, Euroimmun AntiSARS-CoV-2 IgG and IgA assays, and three lateral flow rapid tests.Citation24 The study found Maglumi IgG/IgM and Euroimmun IgG/IgA test sensitivities of 84.4% and 64.3%, respectively.Citation24 The sensitivity of the three lateral flow assays ranged from 91% and 94% 2 weeks after symptom onset for COVID-19.Citation24
ITALIAN ANTIBODY STUDIES
An Italian study compared the Abbott Architect SARS-CoV-2 IgG immunoassay to an indirect immunofluorescence assay and a neutralization assay from COVID-19 samples collected at different times from symptom onset.Citation25 The sensitivities of the Abbott Architect SARS-CoV-2 IgG immunoassay at 7, 14, and >14 days were 8.3%, 61.9%, and 100%, respectively. In contrast, the indirect immunofluorescence assay had sensitivities of 58.3%, 85.7%, and 100% at 7, 14, and >14 days, respectively.Citation25 The study suggests that indirect immunofluorescence assays may be better for detecting SARS-CoV-2 antigens or antibodies at the early stages of infection compared to the Abbott Architect SARS-CoV-2 IgG immunoassay. An Italian study also assessed the sensitivity and specificity of the LIAISON SARS-CoV-2 S1/S2 IgG assay using 1500 clinical samples. The study found that the sensitivity of the assay at 5 and 15 days was 91.3% and 95.7%, respectively.Citation26
SPANISH ANTIBODY STUDIES
A Spanish study compared the sensitivity and specificity of a commercial IgG and IgA ELISA (Euroimmun) with three lateral flow immunoassays (Hangzhou Alltest Biotech, Wuhan UNscience Biotechnology, and Guangzhou Wondfo Biotech) from 109 COVID-19 patients.Citation27 The Euroimmun SARS-CoV-2 test had a sensitivity and specificity of 100% and 80.6%, respectively. The sensitivities of the lateral flow immunoassays ranged from 81.2% to 100%; the specificities ranged from 80.6% to 100%.Citation27 Physicians are encouraged to utilize lateral flow immunoassays with the highest sensitivity possible. A subsequent Spanish study evaluated a lateral flow immunoassay AllTest COVID-19 IgG/IgM using serum samples from 100 SARS-CoV-2-negative patients and 90 patients with SARS-CoV-2 confirmed with nasopharyngeal RT-PCR.Citation28 The study found that the AllTest COVID-19 IgG/IgM had a sensitivity of 64.4% among COVID-19 patients at symptom onset. After 2 weeks, the sensitivity of the antibody test increased to 88%.Citation28
CHINESE ANTIBODY STUDIES
A recent longitudinal study from the Renmin Hospital of Wuhan University studied antibody tests’ detection of antibodies against the SARS-CoV-2 envelope protein E and nucleocapsid protein N antigens from 112 COVID-19 patients over a month.Citation29 The study found that 52% of the COVID-19 patients were positive for both IgM and IgG, 6% were negative for both antibodies, 1% were positive for only IgM, and 41% were positive for only IgG. The IgM antibodies appeared within a week of symptom onset and lasted for a month before gradually decreasing. In contrast, the IgG antibody formed 10 days after exposure to SARS-CoV-2 and remained high after 2 months of exposure. However, this study only looked at SARS-CoV-2 antibodies against envelope protein E and nucleocapsid protein N antigens.Citation29 The study did not compare whether a similar trend was found with other antibody kits detecting antibodies against SARS-CoV-2 spike protein. Furthermore, the study did not assess the sensitivity or specificity of the antibody test kits. Along with the small sample size, it remains suspect whether a similar trend would be validated using other antibody tests. Another Chinese study tested the sensitivity and specificity of colloidal gold immunochromatography assay for SARS-CoV-2–specific IgM/IgG in 150 patients with COVID-19 pneumonia.Citation30 Using the RT-PCR results for comparison, the study found that the colloidal gold immunochromatography assays had a sensitivity of 71.1% and a specificity of 96.2%.Citation30 The sensitivity increased to 95.2% for patients with symptoms lasting >14 days.
TAIWANESE AND SINGAPOREAN ANTIBODY STUDIES
A Taiwanese study examined the accuracy of four RDT SARS-CoV-2 tests (Alltest 2019-nCoV IgG/IgM Rapid Test, Dynamiker 2019-nCoV IgG/IgM Rapid Test, ASK COVID-19 IgG/IgM Rapid Test, and Wondfo SARS-CoV-2 Antibody Test) in 16 patients with COVID-19 confirmed with RT-PCR and 58 COVID-19–negative patients over 3 months.Citation31 The average sensitivity and specificity for detecting SARS-CoV-2 antibodies after 3 weeks of contracting COVID-19 was approximately 100% for both IgG and IgM.Citation31 Although the sensitivity and specificity were high, the sample size for this study was low compared to other studies. This result was confirmed in a Singaporean study examining the performance of the Abbott Architect SARS-CoV-2 IgG assay in COVID-19 patients.Citation32 The study included 177 positive COVID-19 patients and 163 control patients. The specificity of the Abbott Architect SARS-CoV-2 IgG assay was 100%.Citation32 In contrast, the sensitivity of the Abbott Architect SARS-CoV-2 IgG assay was estimated to be 8.6% at 6 days, 43.6% at 7 to 14 days, 84% at 14 to 20 days, and 84.4% at 21 days.Citation32
summarizes the individual country studies.
Table 2. Sensitivity and specificity of different antibody tests conducted globally*
COCHRANE META-ANALYSIS OF ANTIBODY STUDIES
A recent analysis conducted by Cochrane assessed the diagnostic accuracy of antibody tests to determine whether a person presenting with a current or previous COVID-19 infection would be detected using commercially available antibody test kits in different seroprevalence surveys.Citation33 The study included antibody tests, such as ELISA, chemiluminescence immunoassays, and lateral flow assays, from 57 publications (38 from Asia, 15 from Europe, 1 from the USA, and 1 from China) containing 15,976 samples, of which 8526 were confirmed COVID-19 cases.Citation33 The reference standards for comparing these antibody tests were RT-PCR tests and clinical diagnosis based on established guidelines. After removing studies with biases (e.g., lack of blinding), the Cochrane review included 19 studies on hospitalized COVID-19 patients and antibody tests. None of the studies exclusively examined the effectiveness of antibody tests in asymptomatic participants.
Over two-thirds of the studies diagnosed COVID-19 based exclusively on RT-PCR results alone, which leaves the potential for false-negative RT-PCR results.Citation33 Pooled results for IgG, IgM, IgA, total antibodies, and IgG/IgM were found to have low sensitivities in the first week of symptoms (30.1%) but rose in the second (72.2%) and third (91.4%) weeks following exposure.Citation33 Little data exist to evaluate the precision of COVID-19 antibody tests after a month.Citation33 Assuming a prevalence of 5% for COVID-19 in a national screening survey, current antibody tests would have a false-positive rate of 21% and a false-negative rate of 0.4%. If the antibody tests were applied in a high-risk setting, such as in health care workers, with a prevalence set at 50%, the COVID-19 antibody tests would have a lower false-positive rate (2%) but higher false-negative rate (8%). Therefore, the high false-positive rates for population studies or high false-positive rates in high-risk settings may inflate COVID-19 immunity levels in the community or unnecessarily expose patients to health care workers who are harboring the virus.
WORLDWIDE TESTING
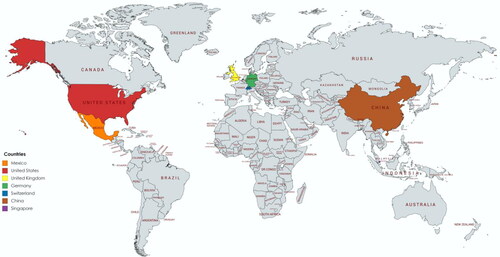
As shown in , Mexico, the United States, the United Kingdom, Germany, Switzerland, China, and Singapore have developed commercially available SARS-CoV-2 antibody tests.Citation12 Many of the commercial antibody kits did not report the time at which the sample was measured to detect the SARS-CoV-2 antibodies against the spike protein or nucleocapsid protein. Currently, no standard SARS-CoV-2 antibody kit has been adopted in the US or abroad. Although antibody tests are generally cheaper than other laboratory tests, resource-scarce nations or those without insurance may be less willing to ask for a SARS-CoV-2 antibody. Furthermore, the lack of rigorous comparison between the SARS-CoV-2 antibody kits in their sensitivity, specificity, cost, and standardization has introduced uncertainty into the accuracy and reliability of the kits to monitor SARS-CoV-2. Further complicating matters is the long turnaround times for different SARS-CoV-2 antibody kits. A greater intentional initiative is needed to help standardize and improve the accuracy of antibody kits to detect SARS-CoV-2 virus.
Table 3. Commercially available COVID-19 antibody diagnostic tests by continent*
As shown in , the European Commission, the Johns Hopkins Center for Health Security, the Centers for Disease Control and Prevention (CDC), the American Association for Clinical Chemistry, and the Infectious Diseases Society of America have released recommendations for administering SARS-CoV-2 antibody tests.Citation8,Citation34–37 The CDC recommends that physicians and health care organizations adopt three approaches for choosing and optimizing antibody tests. First, in populations with >5% prevalence of COVID-19, clinicians should choose antibody tests with high specificity (e.g., 99.5%).Citation35 Second, physicians are encouraged to administer SARS-CoV-2 antibody tests for any person who previously had or was exposed to COVID-19.Citation35,Citation36 Third, physicians are encouraged to use orthogonal testing algorithms that administer SARS-CoV-2 antibody tests after a patient has tested positive for COVID-19.Citation34,Citation35 Currently, the CDC has no evidence to demonstrate the performance advantage of assays, whether the antibody kits test for IgG, IgM, and IgG or total antibody.Citation35,Citation37 Although IgA antibodies are used in SARS-CoV-2 antibody kits, the CDC discourages physicians from using IgA antibodies for determining immune status to SARS-CoV-2 due to the dynamics of IgA detection in patient samples.Citation35,Citation36
Table 4. Society recommendations for SARS-CoV-2 antibody testing*
The CDC also encourages physicians to use antibody tests only for diagnostic purposes. The antibody tests should not be used to determine the immune status of COVID-19 patients until further clinical studies have established the presence, durability, and duration of immunity to COVID-19.Citation35 Furthermore, the CDC discourages use of SARS-CoV-2 antibody kits to make decisions on whether a patient can return to the workplace, congregate in large gatherings, or live in close communities.Citation35 A similar recommendation was given by the European Commission, which also emphasized the limited information that SARS-CoV-2 antibody tests can provide toward assessing an individual’s immune status to SARS-CoV-2.Citation34 Despite the current limitations of SARS-CoV-2 antibody kits, the European Commission has encouraged administering SARS-CoV-2 antibody kits for large-scale epidemiological population surveys to assess the immune status of patients and guide deescalation strategies.Citation34 The American Association for Clinical Chemistry and the Infectious Diseases Society of America also made similar recommendations.Citation36,Citation37
Overall, antibody tests are ineffective at diagnosing COVID-19 after the first week of symptom onset and should only be used 2 weeks after symptom onset. The overall duration of IgG, IgM, and IgA antibodies after COVID-19 infection remains unknown. Therefore, it is uncertain whether antibody tests may be effective for detecting previous COVID-19 in the general population. Most antibody tests have been evaluated using hospitalized patients with higher COVID-19 antibody titers. It is unclear whether the same antibody tests can be applied to asymptomatic patients or patients with a mild case of COVID-19 with lower antibody titers. Variability in administering antibody tests related to onset of COVID-19 symptoms has also produced uncertainty about the sensitivity of the antibody tests.Citation33 Further studies are needed to compare antibody tests to COVID-19 results from RT-PCR for nonexposed individuals and those with asymptomatic, mild, and severe COVID-19.
LIMITATIONS
The applicability of antibody tests is also limited for many reasons. Most studies assessing the detection capability of antibody tests used small samples, and methods for reporting the results for COVID-19 infections were inconsistent.Citation33 For example, some studies did not report patients with COVID-19 who had false-negative results or included data from patients without COVID-19 before the current pandemic.Citation8,Citation34–37 Furthermore, most patients were hospitalized with advanced COVID-19 infections, and studies excluded those with mild or asymptomatic cases. In addition, more than half of the antibody tests were developed at different institutions and are not available for purchase. Therefore, it is difficult to assess or replicate the validity and reliability of many tests currently being used to detect COVID-19 antibodies.Citation8,Citation34–37 This issue is further compounded by the large number of antibody studies from Asian countries that were preprints and did not undergo rigorous peer review before being published. Several of these studies also had a high risk of bias for participant selection, application of index tests, reference standard used, and flow and timing for antibody tests that may incorrectly report the accuracy of COVID-19 antibody tests.
Lastly, the association between IgA levels and COVID-19 infection severity remains unknown. IgA levels appear to fluctuate in COVID-19 patients most likely due to the shift in the innate to adaptive immune response. It is unknown whether serum IgA levels may correlate with either the severity or immune status of COVID-19 patients after infection. These combined factors make assessing the overall reliability of antibody tests an open area of investigation.
CONCLUSION
As the COVID-19 pandemic continues, accurate antibody tests are essential for public health interventions to monitor the spread and assess the level of immunity within a given population. Currently, the accuracy of COVID-19 antibody tests is primarily limited by the timing of test administration. In general, antibody tests should only be used at least 2 weeks after the initial symptoms or exposure to COVID-19 in patients who did not receive an RT-PCR test or had negative RT-PCR results for COVID-19. However, it remains unknown whether antibody tests can be applied accurately months after exposure to COVID-19. Furthermore, the duration of antibodies against COVID-19 remains an area of active investigation that is important in developing public health measures. Despite the limitations with current COVID-19 antibody tests, further research and improved methodology will improve the applicability of these tests for monitoring the progression of COVID-19 in the months and years to come.
- Weinstein MC, Freedberg KA, Hyle EP, Paltiel AD. Waiting for certainty on Covid-19 antibody tests—at what cost? N Engl J Med. 2020;383(6):e37. doi:https://doi.org/10.1056/NEJMp2017739.
- Woloshin S, Patel N, Kesselheim AS . False negative tests for SARS-CoV-2 infection—challenges and implications. N Engl J Med. 2020;383(6):e38. doi:https://doi.org/10.1056/NEJMp2015897.
- Sullivan PS, Sailey C, Guest JL, et al. Detection of SARS-CoV-2 RNA and antibodies in diverse samples: protocol to validate the sufficiency of provider-observed, home-collected blood, saliva, and oropharyngeal samples. JMIR Public Health Surveill. 2020;6(2):e19054. doi:https://doi.org/10.2196/19054.
- Tang Y-W, Schmitz JE, Persing DH, Stratton CW. Laboratory diagnosis of COVID-19: current issues and challenges. J Clin Microbiol. 2020;58(6):e00512. doi:https://doi.org/10.1128/JCM.00512-20.
- Tu Y-P, Jennings R, Hart B, et al. Swabs collected by patients or health care workers for SARS-CoV-2 testing. N Engl J Med. 2020;383(5):494–496. doi:https://doi.org/10.1056/NEJMc2016321.
- Pascarella G, Strumia A, Piliego C, et al. COVID-19 diagnosis and management: a comprehensive review. J Intern Med. 2020;288(2):192–206. doi:https://doi.org/10.1111/joim.13091.
- Zainol Rashid Z, Othman SN, Abdul Samat MN, Ali UK, Wong KK. Diagnostic performance of COVID-19 serology assays. Malays J Pathol. 2020;42(1):13–21.
- Gronvall G, Connell N, Kobokovich A, et al. Developing a National Strategy for Serology (Antibody Testing) in the United States. Baltimore, MD: The Johns Hopkins Center for Health Security; 2020.
- Ghaffari A, Meurant R, Ardakani A. COVID-19 serological tests: how well do they actually perform? Diagnostics. 2020;10(7):453. doi:https://doi.org/10.3390/diagnostics10070453.
- Azkur AK, Akdis M, Azkur D, et al. Immune response to SARS-CoV-2 and mechanisms of immunopathological changes in COVID-19. Allergy. 2020;75(7):1564–1581. doi:https://doi.org/10.1111/all.14364.
- Yu HQ, Sun BQ, Fang ZF, et al. Distinct features of SARS-CoV-2-specific IgA response in COVID-19 patients. Eur Respir J. 2020;56(2):2001526. doi:https://doi.org/10.1183/13993003.01526-2020.
- Kobokovich A, West R, Gronvall G. Serology-Based Tests for COVID-19. Baltimore, MD: The Johns Hopkins Center for Health; 2020. https://www.centerforhealthsecurity.org/resources/COVID-19/serology/Serology-based-tests-for-COVID-19.html.
- Yan Y, Chang L, Wang L. Laboratory testing of SARS-CoV, MERS-CoV, and SARS-CoV-2 (2019-nCoV): current status, challenges, and countermeasures. Rev Med Virol. 2020;30(3):e2106. doi:https://doi.org/10.1002/rmv.2106.
- Castro R, Luz PM, Wakimoto MD, Veloso VG, Grinsztejn B, Perazzo H. COVID-19: a meta-analysis of diagnostic test accuracy of commercial assays registered in Brazil. Braz J Infect Dis. 2020;24(2):180–187. doi:https://doi.org/10.1016/j.bjid.2020.04.003.
- Bundschuh C, Egger M, Wiesinger K, et al. Evaluation of the EDI enzyme linked immunosorbent assays for the detection of SARS-CoV-2 IgM and IgG antibodies in human plasma. Clin Chim Acta. 2020;509:79–82. doi:https://doi.org/10.1016/j.cca.2020.05.047.
- Egger M, Bundschuh C, Wiesinger K, et al. Comparison of the Elecsys Anti-SARS-CoV-2 immunoassay with the EDI enzyme linked immunosorbent assays for the detection of SARS-CoV-2 antibodies in human plasma. Clin Chim Acta. 2020;509:18–21. doi:https://doi.org/10.1016/j.cca.2020.05.049.
- Traugott M, Aberle SW, Aberle JH, et al. Performance of severe acute respiratory syndrome coronavirus 2 antibody assays in different stages of infection: comparison of commercial enzyme-linked immunosorbent assays and rapid tests. J Infect Dis. 2020;222(3):362–366. doi:https://doi.org/10.1093/infdis/jiaa305.
- Tuaillon E, Bolloré K, Pisoni A, et al. Detection of SARS-CoV-2 antibodies using commercial assays and seroconversion patterns in hospitalized patients. J Infect. 2020;81(2):e39–e45. doi:https://doi.org/10.1016/j.jinf.2020.05.077.
- Demey B, Daher N, François C, et al. Dynamic profile for the detection of anti-SARS-CoV-2 antibodies using four immunochromatographic assays. J Infect. 2020;81(2):e6–e10. doi:https://doi.org/10.1016/j.jinf.2020.04.033.
- Nicol T, Lefeuvre C, Serri O, et al. Assessment of SARS-CoV-2 serological tests for the diagnosis of COVID-19 through the evaluation of three immunoassays: two automated immunoassays (Euroimmun and Abbott) and one rapid lateral flow immunoassay (NG Biotech). J Clin Virol. 2020;129:104511. doi:https://doi.org/10.1016/j.jcv.2020.104511.
- Kohmer N, Westhaus S, Rühl C, Ciesek S, Rabenau HF. Brief clinical evaluation of six high-throughput SARS-CoV-2 IgG antibody assays. J Clin Virol. 2020;129:104480. doi:https://doi.org/10.1016/j.jcv.2020.104480.
- Haselmann V, Kittel M, Gerhards C, et al. Comparison of test performance of commercial anti-SARS-CoV-2 immunoassays in serum and plasma samples. Clin Chim Acta. 2020;510:73–78. doi:https://doi.org/10.1016/j.cca.2020.07.007.
- Jääskeläinen AJ, Kuivanen S, Kekäläinen E, et al. Performance of six SARS-CoV-2 immunoassays in comparison with microneutralisation. J Clin Virol. 2020;129:104512. doi:https://doi.org/10.1016/j.jcv.2020.104512.
- Montesinos I, Gruson D, Kabamba B, et al. Evaluation of two automated and three rapid lateral flow immunoassays for the detection of anti-SARS-CoV-2 antibodies. J Clin Virol. 2020;128:104413. doi:https://doi.org/10.1016/j.jcv.2020.104413.
- Meschi S, Colavita F, Bordi L, et al. Performance evaluation of Abbott ARCHITECT SARS-CoV-2 IgG immunoassay in comparison with indirect immunofluorescence and virus microneutralization test. J Clin Virol. 2020;129:104539. doi:https://doi.org/10.1016/j.jcv.2020.104539.
- Bonelli F, Sarasini A, Zierold C, et al. Clinical and analytical performance of an automated serological test that identifies S1/S2-neutralizing IgG in COVID-19 patients semiquantitatively. J Clin Microbiol. 2020;58(9):e01224. doi:https://doi.org/10.1128/JCM.01224-20.
- Serrano MM, Rodríguez DN, Palop NT, et al. Comparison of commercial lateral flow immunoassays and ELISA for SARS-CoV-2 antibody detection. J Clin Virol. 2020;129:104529. doi:https://doi.org/10.1016/j.jcv.2020.104529.
- Pérez-García F, Pérez-Tanoira R, Romanyk J, et al. Alltest rapid lateral flow immunoassays is reliable in diagnosing SARS-CoV-2 infection from 14 days after symptom onset: a prospective single-center study. J Clin Virol. 2020;129:104473. doi:https://doi.org/10.1016/j.jcv.2020.104473.
- Zhang G, Nie S, Zhang Z, Zhang Z. Longitudinal change of severe acute respiratory syndrome coronavirus 2 antibodies in patients with coronavirus disease 2019. J Infect Dis. 2020;222(2):183–188. doi:https://doi.org/10.1093/infdis/jiaa229.
- Shen B, Zheng Y, Zhang X, et al. Clinical evaluation of a rapid colloidal gold immunochromatography assay for SARS-Cov-2 IgM/IgG. Am J Transl Res. 2020;12(4):1348–1354.
- Wu JL, Tseng WP, Lin CH, et al. Four point-of-care lateral flow immunoassays for diagnosis of COVID-19 and for assessing dynamics of antibody responses to SARS-CoV-2. J Infect. 2020;81(3):435–442. doi:https://doi.org/10.1016/j.jinf.2020.06.023.
- Chew KL, Tan SS, Saw S, et al. Clinical evaluation of serological IgG antibody response on the Abbott Architect for established SARS-CoV-2 infection. Clin Microbiol Infect. 2020;26(9):1256.e9–1256-e11. doi:https://doi.org/10.1016/j.cmi.2020.05.036.
- Deeks JJ, Dinnes J, Takwoingi Y, et al.; Cochrane COVID-19 Diagnostic Test Accuracy Group. Antibody tests for identification of current and past infection with SARS-CoV-2. Cochrane Database Syst Rev. 2020;6:CD013652. doi:https://doi.org/10.1002/14651858.CD013652.
- European Commission. Guidelines on COVID-19 in Vitro Diagnostic Tests and Their Performance. April 15, 2020. https://ec.europa.eu/info/sites/info/files/testing_kits_communication.pdf.
- Centers for Disease Control and Prevention. Interim Guidelines for COVID-19 Antibody Testing in Clinical and Public Health Settings. Atlanta, GA: CDC; 2020. https://www.cdc.gov/coronavirus/2019-ncov/lab/resources/antibody-tests-guidelines.html.
- American Association for Clinical Chemistry. AACC Recommendations for SARS-CoV-2 Serology Testing. Washington, DC: AACC; 2020. https://www.aacc.org/science-and-practice/statements-on-covid-19-testing/aacc-recommendations-for-sars-cov-2-serology-testing.
- Infectious Diseases Society of America. IDSA COVID-19 Antibody Testing Primer. Arlington, VA: IDSA; 2020. https://www.idsociety.org/globalassets/idsa/public-health/covid-19/idsa-covid-19-antibody-testing-primer.pdf.