Abstract
Background
Thoracic endovascular aortic repair (TEVAR) involving landing zone 2 can require extra-anatomic debranching (SR-TEVAR) to ensure left subclavian artery perfusion, resulting in increased costs. A single-branch device (Thoracic Branch Endoprosthesis [TBE], WL Gore, Flagstaff, AZ) provides a total endovascular solution. Comparative cost analysis of patients undergoing zone 2 TEVAR requiring left subclavian artery preservation with TBE versus SR-TEVAR is presented.
Methods
A single-center retrospective cost analysis was performed for aortic diseases requiring a zone 2 landing zone (TBE vs. SR-TEVAR) from 2014 to 2019. Facility charges were collected from the universal billing form UB-04 (form CMS 1450).
Results
Twenty-four patients were included in each arm. There were no significant differences in the overall mean procedural charges between the two groups: TBE, $209,736 ($57,761) vs. SR-TEVAR $209,025 ($93,943), P = 0.94. TBE resulted in reduced operating room charges ($36,849 [$8750] vs. $48,073 [$10,825], P = 0.02) and reduced intensive care unit and telemetry room charges, which did not reach statistical significance (P = 0.23 and 0.12, respectively). Device/implant charges were the primary cost driver in both groups. Charges associated with TBE were significantly higher: $105,525 ($36,137) vs. $51,605 ($31,326), P > 0.01.
Conclusions
TBE had similar overall procedural charges despite higher device/implant-related expenses and reduced facility resource utilization (lower operating room, intensive care unit, telemetry, and pharmacy charges).
Historically, procedural intervention for thoracic aortic disease has centered around open repair.Citation1,Citation2 With the endovascular revolution at the turn of the millennium, thoracic endovascular aortic repair (TEVAR) has been increasingly utilized for this indication.Citation3–6 Numerous prospective studies have shown that TEVAR has been associated with a substantial reduction in perioperative and long-term morbidity and mortality when compared to open intervention.Citation7,Citation8 Consequent to these favorable data, Society of Vascular Surgery guidelines have been modified to expand the role of the procedure to those with intact and ruptured descending thoracic aortic aneurysms, complicated type B aortic dissections, and other descending thoracic aortic pathologies, to include traumatic aortic transections, penetrating aortic ulcers, and intramural hematomas.Citation9
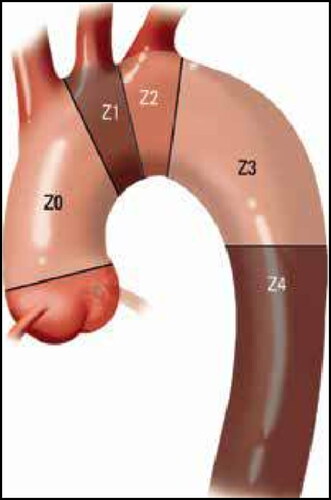
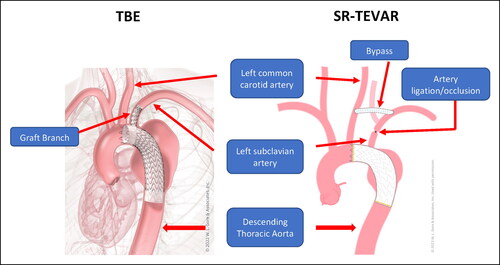
Although the benefits of TEVAR are well described in the literature, its role in the management of aortic pathology involving Ishimaru landing zone 2 () has been the subject of recent debate.Citation10–12 This is particularly pertinent since endovascular solutions to thoracic disease in this region can require extra-anatomic revascularization in a concurrent or staged fashion with subclavian artery revascularization followed by TEVAR (SR-TEVAR) to ensure left subclavian artery (LSA) perfusion and to help reduce the risk of stroke and spinal cord ischemia.Citation13–16 The WL Gore TAG Thoracic Branch Endoprosthesis (TBE; WL Gore, Flagstaff, AZ) is a novel single-branch stent graft system currently involved in a multicenter clinical trial. TBE enables a fully endovascular approach to maintain LSA perfusion in patients requiring zone 2 repair. Recent studies by Dake et al and Patel et al have supported the feasibility of TBE, highlighting appropriate rates of perioperative and 1-year branch vessel patency with minimal clinically significant type 1 C endoleaksCitation17,Citation18 ().
Figure 2. Anatomic description of thoracic branched endograft (TBE) vs SR-TEVAR (thoracic endovascular aortic repair with extra-anatomic debranching of the left subclavian artery). Anatomic illustrations courtesy of WL Gore & Associates.
While several studies have evaluated the role of TBE in the management of zone 2 aortic pathology, there is a paucity of literature examining its economic burden on payers and healthcare systems. The purpose of this study was to perform a single-center cost analysis of patients undergoing TEVAR requiring zone 2 revascularization, comparing TBE with SR-TEVAR.
METHODS
The study was approved by the Baylor Scott and White Research Institute institutional review board under protocol 014-209. The study was a single-center retrospective data review comparing costs of the two treatment strategies (TBE vs. SR-TEVAR) for aortic diseases that require a zone 2 proximal landing zone. The patients who received TBE were enrolled exclusively through a prospectively consented, IRB-approved trial that has been reported previously;Citation19 however, cost analysis was not prespecified. Other patients during the same study timeframe continued to receive standard-of-care therapy, which included SR-TEVAR. Patients were enrolled in this arm either because their primary surgeon was not an investigator on the TBE trial (n = 3) or because patient anatomy was unsuitable for the TBE trial or device (n = 21). As there was no additional physical risk to patients nor study-specific interventions, and data were deidentified, consent for this retrospective cost-analysis study was waived by the IRB.
Facility-based charges for inpatient hospitalizations associated with these two procedures were collected from the universal billing form UB-04 (form CMS 1450) from our institution. This form contains all facility-based charges including, but not limited to, operating room (OR), intensive care unit (ICU), telemetry or critical care unit, and pharmacy expenses. OR and hospital-related charges were aggregated in the SR-TEVAR group to facilitate direct comparison of the cohorts. Additionally, device-related costs reported included all OR implants and devices used in the respective procedures.
Data were collected between 2014 and 2019 during the timeframe of TBE study enrollment at our institution. Twenty-four patients underwent treatment with TBE during this period and were included in the analysis. Patients were chosen for the SR-TEVAR arm if they did not meet the anatomic constraints required for enrollment in the TBE study; those in this arm included all patients in this timeframe who underwent SR-TEVAR. Twenty-eight patients were identified meeting these requirements; however, four patients did not have all charge data available, leaving 24 patients available for inclusion in this arm. Clinical outcomes are briefly reported in this study and have been previously outlined in published results.Citation19 Results and cost analysis were compared using Student’s t tests in SPSS software.
Implantation of the TBE device has been previously outlined.Citation18 For the SR-TEVAR procedure, open subclavian revascularization is done through a standard transverse left cervical incision. Bypass or transposition is performed based on patient anatomy and physician preference. SR-TEVAR is performed in conjunction with the TEVAR or in a delayed fashion as soon as the day following the revascularization procedure or during a separate admission, at surgeon discretion.
RESULTS
Twenty-four patients were studied in each arm. In the SR-TEVAR cohort, carotid subclavian bypass was performed in 16 patients (66.7%) while subclavian carotid transposition was performed in 8 patients (33.3%). One patient in the TBE arm experienced a complication related to partial left common carotid artery coverage, which required repeat operation for placement of an antegrade common carotid artery stent placement as well as stent extension in the subclavian artery for a type I C endoleak. Events leading to longer hospital stay and potentially increased costs (spinal cord ischemia, respiratory failure, urinary tract infection, etc.) were included in the data analysis for overall cost comparison. There was no significant difference in the overall mean procedural charges between the two groups: TBE, $209,735 ($57,761) vs. SR-TEVAR $209,025 ($93,943), P = 0.94. The TBE procedure resulted in significantly reduced OR charges: $36,849 ($8750) vs. $48,073 ($10,825), P = 0.02. Use of the TBE device was associated with a reduced ICU charge and telemetry room charge for time usage, which did not reach statistical significance (P = 0.23 and 0.12, respectively). Pharmaceutical usage and charges for the entire hospitalization were also significantly reduced in the TBE arm: $9451 ($15,932) vs. SR-TEVAR $23,668 ($35,831), P = 0.04. The device/implant charge was the primary cost driver in both groups, with the charge associated with the TBE group being significantly higher: $105,525 ($36,137) vs. $51,605 ($31,326), P < 0.01 ().
Table 1. Cost comparison of TBE vs SR-TEVAR*
DISCUSSION
In this retrospective, single-center study of patients undergoing zone 2 TEVAR with LSA revascularization vs a TBE procedure, we found that although TBE had higher overall device/implant-related expenses (P < 0.01) than SR-TEVAR, it had similar overall procedural charges (P = 0.94), despite the cost of the device. Of note, device cost as reported includes all devices used in the procedure performed. TBE was associated with reduced facility resource utilization, which was primarily due to lower incurred OR (P < 0.02), ICU (P = 0.23), telemetry room (P = 0.12), and pharmacy (P < 0.04) charges. We hypothesize that the lower costs associated with the TBE device demonstrated here are related to a shorter length of stay for the single TBE procedure versus the SR-TEVAR combined/staged procedure. To our knowledge, this is one of the first studies to directly compare the economic impact of these two procedures in a real-world setting.
The utility of the analysis rests in its ability to provide more granular information about cost drivers so that physician leaders, hospital administration, and product manufacturers can focus their attention on value-added activities.Citation20,Citation21 Our results may be particularly valuable to healthcare decision makers and systems exploring the true cost of treating thoracic aortic disease using the current investigational single-branched device. Similar to other cost studies examining endovascular delivery of stent grafts, TBE was associated with higher implant-related expenses than its open surgical counterpart.Citation22,Citation23 However, one could argue that given the novelty of TBE, the cost of this procedural modality may decrease over time with widespread market penetration and saturation. It is also possible that this timeline may be accelerated if blanket approval from the US Food and Drug Administration (FDA) is obtained, manufacturer profit margins are optimized, and economies of scale are achieved with implant production meeting market demand. This trend has certainly been observed in several European countries where medical device companies, policy makers, and national healthcare systems have synergized to balance technological adoption and device affordability.Citation24
Further procedural and device component refinement with widespread adoption of TBE may yield a reduction in critical cost drivers such as a possible decrease in length of overall stay and a reduction in OR, facility, and pharmacy costs. We have already observed a statistically significant reduction in these variables and hypothesize that this may be due to several factors. The minimally invasive nature of the index procedure results in a decreased physiologic burden placed upon the patient, thus requiring minimal perioperative pharmacologic assistance. Additionally, there may be a reduction of postoperative complications such as chyle leak, neck hematoma, and hemo/pneumothorax that may otherwise increase treatment costs; however, clinical outcome data will need to confirm this advantage. A postoperative complication to bear in mind with TBE is type I C endoleak, which has been shown to be clinically insignificant and self-resolving in studies by Dake et al and Patel et al.Citation17,Citation18 We also anticipate that OR costs may experience further declines with reductions in operator learning curves. This has certainly been observed with other endovascular solutions utilized in the field of cardiovascular surgery.Citation25,Citation26
Since gaining FDA approval for use of the TBE device and after all procedures in our study were completed, the Centers for Medicare and Medicaid Services (CMS) granted an increase in reimbursement when the TBE device is used based on a New Technology Additional Payment (NTAP) designation. This designation, when billed with the appropriate coding and modifiers by the performing facility, provides an additional payment to the facility to assist in covering the cost of the specific device (in this case TBE) that is felt to carry a clinical advantage but has a higher cost than the traditional procedural device. The NTAP granted by CMS to the TBE device is an additional $27,807 (https://public-inspection.federalregister.gov/2022-16472.pdf, p. 484-92). If this additional payment is extrapolated to the data presented herein, use of the TBE device would be less expensive than the traditional approach, although likely not statistically significant.
Several limitations are inherent to the design of this study. First, our analysis compared the direct costs of TBE versus SR-TEVAR and hence we were unable to account for all potential sources of indirect costs, such as lost workdays of patients and family members during postprocedural recovery. Our study should not be interpreted as an indiscriminate recommendation for TBE application in all patients undergoing TEVAR requiring LSA revascularization, since costing studies cannot substitute for sound clinical judgment. Therefore, each patient’s individual circumstances and anatomy must be taken into consideration before tailoring therapeutic intervention. Second, we did not perform a true cost-effectiveness analysis utilizing Markov microsimulation models to determine quality-adjusted life years and incremental cost-effectiveness ratios. In this regard, it is critical to remain cognizant of the primary objective of this study, which provides a preliminary determination of the optimal modality for this indication from an economic perspective. We intend on deriving and disseminating this data in future studies. Third, although we have included the average national reimbursement for TBE and SR-TEVAR based on CMS diagnosis-related group coding with and without comorbidities for these procedures (), we are unable to add specific payment data for our own institution due to proprietary restrictions. Payment for specific diagnosis-related group codes may vary by region, and our data, although useful, may not be translated to all regions of the United States. Patients included in the SR-TEVAR cohort in this review did not meet anatomic requirements for treatment in the TBE group, resulting in subsequent treatment specifically in the SR-TEVAR group. Although this anatomic difference and subsequent treatment exists, the authors do not feel it resulted in any significant difference in outcomes for the purpose of the current cost review study.
Table 2. National average facility DRG payment for TBE and SR-TEVAR*
Finally, this study was subject to type II error and a risk of generalizability bias given the relatively small number of cases at our institution in either study arm. Higher-powered multicenter studies are required in the future to understand national variations in claims and to confirm our findings. Irrespective of the limitations mentioned above, this is one of the first studies in the literature to directly compare the real-world cost of an investigational single-branch device versus SR-TEVAR in patients undergoing zone 2 TEVAR.
In conclusion, although the TBE procedure had similar overall procedural charges despite higher device/implant-related expenses than with standard extra-anatomic revascularization, its application was also associated with reduced facility resource utilization. This was primarily due to lower OR, ICU, telemetry floor, and pharmacy charges. If clinical outcomes are shown to be equal or advantageous for TBE compared to SR-TEVAR, the faster recovery, possible shorter hospital stay, and lower utilization of resources with TBE should be considered when choosing a treatment strategy, given the overall costs are essentially equal. Future multicenter studies are required to understand national variations in claims tied to these two procedural modalities.
Disclosure statement/Funding
Dr. Gable is a consultant, on the speakers bureau, and receives research support from WL Gore; is a consultant and on the speakers bureau of Silk Road; and is a consultant, on the speakers bureau, and receives research support from Medtronic. Dr. Shutze is on the speakers bureau and receives research support from both Medtronic and Terumo. Dr. Brinkman is a consultant, on the speakers bureau, and receives research support from Medtronic; and on the speakers bureau and receives research support from Terumo and WL Gore. The other authors have no potential conflicts to disclose.
Additional information
Funding
- Mitchell MB, Rutherford RB, Krupski WC. Thoracic and Thoracoabdominal Aortic Aneurysms: Evaluation and Decision Making. 8th ed. Philadelphia, PA: Saunders, Elsevier; 2013:2084.
- Safi HJ, Miller CC 3rd, Estrera AL, et al. Staged repair of extensive aortic aneurysms: long-term experience with the elephant trunk technique. Ann Surg. 2004;240(4):677–685. doi:10.1097/01.sla.0000140756.30517.1b.
- Bavaria JE, Appoo JJ, Makaroun MS, Verter J, Yu Z-F, Mitchell RS. Endovascular stent grafting versus open surgical repair of descending thoracic aortic aneurysms in low-risk patients: a multicenter comparative trial. J Thorac Cardiovasc Surg. 2007;133(2):369–377. doi:10.1016/j.jtcvs.2006.07.040.
- Dake MD, Miller DC, Semba CP, Mitchell RS, Walker PJ, Liddell RP. Transluminal placement of endovascular stent-grafts for the treatment of descending thoracic aortic aneurysms. N Engl J Med. 1994;331(26):1729–1734. doi:10.1056/NEJM199412293312601.
- Scali ST, Goodney PP, Walsh DB, et al. National trends and regional variation of open and endovascular repair of thoracic and thoracoabdominal aneurysms in contemporary practice. J Vasc Surg. 2011;53(6):1499–1505. doi:10.1016/j.jvs.2011.02.014.
- Patel HJ, Williams DM, Drews JD, et al. A 20-year experience with thoracic endovascular aortic repair. Ann Surg. 2014;260(4):691–696. discussion 696-697. doi:10.1097/SLA.0000000000000930.
- Makaroun MS, Dillavou ED, Kee ST, et al. Endovascular treatment of thoracic aortic aneurysms: results of the phase II multicenter trial of the GORE TAG thoracic endoprosthesis. J Vasc Surg. 2005;41(1):1–9. doi:10.1016/j.jvs.2004.10.046.
- Patterson B, Holt P, Nienaber C, Cambria R, Fairman R, Thompson M. Aortic pathology determines midterm outcome after endovascular repair of the thoracic aorta: report from the Medtronic thoracic endovascular registry (MOTHER) database. Circulation. 2013;127(1):24–32. doi:10.1161/CIRCULATIONAHA.112.110056.
- Upchurch GR Jr, Escobar GA, Azizzadeh A, et al. Society for Vascular Surgery clinical practice guidelines of thoracic endovascular aortic repair for descending thoracic aortic aneurysms. J Vasc Surg. 2021;73(1S):55S–83S. doi:10.1016/j.jvs.2020.05.076.
- Kawatou M, Minakata K, Sakamoto K, et al. Comparison of endovascular repair with branched stent graft and open repair for aortic arch aneurysm. Interact Cardiovasc Thorac Surg. 2017;25(2):246–253. doi:10.1093/icvts/ivx111.
- Yoshitake A, Okamoto K, Yamazaki M, et al. Comparison of aortic arch repair using the endovascular technique, total arch replacement and staged surgery. Eur J Cardiothorac Surg. 2017;51(6):1142–1148. doi:10.1093/ejcts/ezx028.
- Maldonado TS, Dexter D, Rockman CB, et al. Left subclavian artery coverage during thoracic endovascular aortic aneurysm repair does not mandate revascularization. J Vasc Surg. 2013;57(1):116–124. doi:10.1016/j.jvs.2012.06.101.
- Matsumura JS, Rizvi AZ. Left subclavian artery revascularization: Society for Vascular Surgery practice guidelines. J Vasc Surg. 2010;52(4 Suppl):65S–70S. doi:10.1016/j.jvs.2010.07.003.
- Buth J, Harris PL, Hobo R, et al. Neurologic complications associated with endovascular repair of thoracic aortic pathology: incidence and risk factors. a study from the European Collaborators on Stent/Graft Techniques for Aortic Aneurysm Repair (EUROSTAR) registry. J Vasc Surg. 2007;46(6):1103–1110. discussion 11101111 doi:10.1016/j.jvs.2007.08.020.
- Rizvi AZ, Murad MH, Fairman RM, Erwin PJ, Montori VM. The effect of left subclavian artery coverage on morbidity and mortality in patients undergoing endovascular thoracic aortic interventions: a systematic review and meta-analysis. J Vasc Surg. 2009;50(5):1159–1169. doi:10.1016/j.jvs.2009.09.002.
- Lee TC, Andersen ND, Williams JB, Bhattacharya SD, McCann RL, Hughes GC. Results with a selective revascularization strategy for left subclavian artery coverage during thoracic endovascular aortic repair. Ann Thorac Surg. 2011;92(1):97–102. discussion 102-103. doi:10.1016/j.athoracsur.2011.03.089.
- Dake MD, Fischbein MP, Bavaria JE, et al. Evaluation of the Gore TAG thoracic branch endoprosthesis in the treatment of proximal descending thoracic aortic aneurysms. J Vasc Surg. 2021;74(5):1483–1490.e2. doi:10.1016/j.jvs.2021.04.025.
- Patel HJ, Dake MD, Bavaria JE, et al. Branched endovascular therapy of the distal aortic arch: preliminary results of the feasibility multicenter trial of the Gore thoracic branch endoprosthesis. Ann Thorac Surg. 2016;102(4):1190–1198. doi:10.1016/j.athoracsur.2016.03.091.
- Squiers JJ, DiMaio JM, Schaffer JM, et al. Surgical debranching versus branched endografting in zone 2 thoracic endovascular aortic repair. J Vasc Surg. 2022;75(6):1829–1836.e3. doi:10.1016/j.jvs.2021.12.068.
- Velmurugan MS, Nahar W. Factors determining the success or failure of ABC implementation. Cost Manage. 2010;5:35–46.
- Slapak-Iacobelli L, Wilde AH. Jr. A cost-benefit analysis for materials management information systems. Top Health Inf Manage. 1993;13(3):56–61.
- Stone DH, Horvath AJ, Goodney PP, et al. The financial implications of endovascular aneurysm repair in the cost containment era. J Vasc Surg. 2014;59(2):283–290.e1. doi:10.1016/j.jvs.2013.08.047.
- Arnaoutakis GJ, Hundt JA, Shah AS, Cameron DE, Black JH. 3rd. Comparative analysis of hospital costs of open and endovascular thoracic aortic repair. Vasc Endovasc Surg. 2011;45(1):39–45. doi:10.1177/1538574410380471.
- Schreyögg J, Bäumler M, Busse R. Balancing adoption and affordability of medical devices in Europe. Health Policy. 2009;92(2–3):218–224. doi:10.1016/j.healthpol.2009.03.016.
- Kalteis M, Benedikt P, Huber F, Haller F, Kastner M, Lugmayr H. Looking for a learning curve in EVAR based on the Zenith stent graft. Int J Angiol. 2012;21(4):223–228. doi:10.1055/s-0032-1331159.
- Forbes TL, Chu MW, Lawlor DK, DeRose G, Harris KA. Learning curve analysis of thoracic endovascular aortic repair in relation to credentialing guidelines. J Vasc Surg. 2007;46(2):218–222. doi:10.1016/j.jvs.2007.03.047.