Abstract
Grassland degradation not only results in soil degradation and severe decreases in land productivity, but also can promote the emission of soil carbon and nitrogen compounds as greenhouse gases into the atmosphere. The primary objective of this study was to characterize the impact of grassland degradation on carbon and nitrogen budgets in Inner Mongolia, China. We investigated the changes of total carbon, organic carbon, inorganic carbon and total nitrogen that occur in a grassland ecosystem (including vegetation and top 30 cm soil layer) in the course of grassland degradation. Total carbon stored in the grassland ecosystem was reduced by up to 14%, depending on the severity of the degradation. Total nitrogen storage was reduced by almost 10% under severe degradation, but was slightly increased at light and intermediate degradation, indicating that grazing exclusion would not lead to an increase in nitrogen storage in the ecosystem. Over 98% of the total carbon and nitrogen stored in the grassland ecosystem was bound in the soil which provides the dominant and most stable carbon and nitrogen pool in the ecosystem. Most of the soil carbon and nitrogen storage was present in soil water-stable aggregates and was released as soil water-stable aggregates break in the course of grassland degradation. In conclusion, the carbon sequestration capacity of the vegetation decreased significantly, and substantial proportions of soil carbon and nitrogen were lost in the course of grassland degradation, resulting in unbalanced carbon and nitrogen budgets. Strategies to restore degraded grassland must be designed to increase the carbon and nitrogen storage potential of grassland ecosystems.
Introduction
In recent decades, extensive work has been conducted to improve our understanding of global carbon and nitrogen reserves and to quantify the pools and fluxes that constitute the cycles of these elements. Since the amount of C stored in soil is approximately twice that in the atmosphere (Schimel, Citation1995), the accumulation of C in the terrestrial biosphere could partially offset the effect of anthropogenic carbon dioxide (CO2) emissions on the atmospheric CO2 level (Houghton et al., Citation1999). Understanding the store and loss of C and N not only helps us understand how ecosystems will respond to natural and anthropogenic disturbances, but also quantify the influence of changing rates of C and N cycling and storage on global climate change.
Grasslands in some regions may serve as important global C sinks. The annual sink is approximately 0.5 Pg C for tropical grasslands and depends mainly on the baseline soil organic C level and annual precipitation (Davidson et al., Citation1995). Grasslands account for 40% of the total land area in China. However, by the end of the 20th century, 90% of the grassland in Inner Mongolia was degraded as a consequence of a rapid expansion in livestock numbers and economic reforms initiated in the 1980s (Chen & Wang, Citation2000). Degradation is one of the most serious environmental and socioeconomic problems in many arid and semiarid regions of the world (Gomes et al., Citation2003). For these reasons the effects of grassland degradation on soil C and N contents has become a concern in recent years (Dale & Peter, Citation2001; Breuer et al., Citation2006).
Existing research on soil C and N in relation to degradation suggests that C and N storage in soil has been significantly affected by changes in land use, grazing intensity and various ecosystem management strategies (Jones & Donnelly, Citation2004; Billings, Citation2006; Elmore & Asner, Citation2006; Liao et al., Citation2006). However, there are few published studies on the effects of grassland degradation on C and N storage and loss. Only a small number of reports focused on changing C and N in soils, while the accumulation of C and N in grassland communities was neglected. We investigated C and N storage and loss in vegetation and soil along with the grassland degradation process. The objective of this paper is to quantify the sink-source relations of C and N in the context of grassland degradation, and clarify the driving mechanism of C and N loss during degradation.
Materials and methods
Study site
The study was conducted in 2007 and 2008 at a site located in Xiwu Banner near Xilinhot (44°28′51.82″–44°29′7.87″N, 117°59′5.17″–117°59′48.23″E) in the eastern part of Inner Mongolia, China, which has a temperate continental semi-arid monsoon climate on an average elevation of 1130 m. Annual mean precipitation is 350 mm, the annual mean temperature is 1°C. The annual frost-free period is approximately 106 days and the average annual wind speed is 4.3 m s−1. The soil on the location is of chestnut type, i.e. Calcic Kastanozems. The vegetation of the region predominantly consists of grassland plants such as C. pediform (15.96%, relative biomass), S. centauroides (15.43%), A. sibiricum (7.45%), L. chinensis (6.47%), C. squarosa (6.04%), S. officinalis (5.83%), S. baicalensis (2.92%) and A. senescens (2.20%). The site has been under a system of low-input free-range grazing by livestock for over 30 years. In this system, livestock are turned loose from their holding pen in the village and their distribution is managed by a herder.
Experimental design
The design of grassland degradation process was modelled after a piosphere where the grassland degradation radiated in a diminishing response away from the centre which was the location of the herdsman settlement. Following the classification of the degree of grassland degradation by Wang (Citation2008), quadrats were set up in plots of severe degradation (SD), moderate degradation (MD), light degradation (LD), as well as in a control plot (CP) with grazing exclusion for 5 years ().
Figure 1. Experimental design map. In this map, sampling sites and quadrats are shown at their positions as determined using the Global Positioning System (GPS). The directions of the arrows represent the course of grassland degradation (from light to severe).
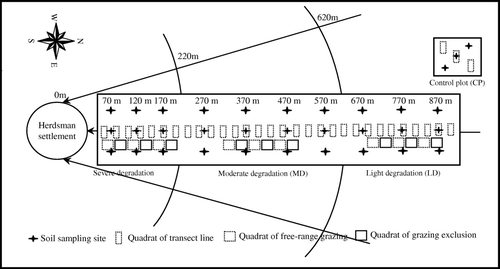
Along the piosphere gradient, soil samples were collected from three replicate transects radiating from the herdsman settlement to distances of approximately 870 m, covering sites from severe to light degradation. On each belt transect, soil samples from 0–5, 5–15 and 15–30 cm depth were collected in 50 m intervals in the SD plot, and at 100 m intervals in the MD and LD plots. Soil bulk density was determined for every soil sample. Soil water-stable aggregates (SWSA) in 0–15 cm depth were determined by a wet-sieving method from every soil sample site on the middle belt transect.
Aboveground plant sampling quadrats (20 cm×50 cm) were established at 20 m intervals along a transect line from the herdsman settlement to a distance of 900 m. Nine no-grazing plots (1×1 m), three for each degree of degradation, were established using cages to exclude cattle herded onto the rangeland in spring 2007 and 2008. In August of these years, aboveground plant samples inside and outside of the grazing exclosures were collected. The region inside the no-grazing cages was the grazing exclusion plot, and it was the free-range grazing region outside the cages. Three soil sampling sites and plant sampling quadrats were established in the control plot. For the geo-statistical analysis, each sample site was positioned precisely using GPS.
Sampling and analysis
Field sampling was conducted in mid-August of 2007 and 2008. The remaining biomass in each quadrat was clipped by species at the ground level. Because the standing crop of the steppe communities reaches its annual peak at the middle to end of August, the estimated community biomass approximated aboveground net primary production of the grassland ecosystem (Bai et al., Citation2004). Fine, fragmented and partially decomposed litter lying on the soil surface was not included and mixed with mineral soil since it could not be separated completely from mineral soil. Biomass samples were dried at 80°C for 48 h and weighed. After drying, material of the same species from many quadrats were combined and then divided into three replicates for analysis of total C and total N content by dry combustion of duplicate subsamples using a Sumigraph NC-80 Analyzer (Sumika Chemical Analysis Service, Co., Tokyo, Japan).
The soil was sampled from locations along the three transect lines within each degradation plot. Fifteen soil cores (3.5 cm diameter) were collected manually with a soil core sampler randomly placed at a sampling site. Air-dried soil samples, roots and litter lying on the soil surface were smashed and coarsely ground to pass through a 2 mm sieve. Soil bulk density was measured using the soil cores (100 cm3 volume) obtained from different soil layers, with three replicates for each site. Bulk density samples (hand-sampled cores) were oven-dried for 48 h at 105 °C (drying to constant weight), weighed, and bulk density was determined as the mass of dry soil per volume of field-moist soil. Dry sieving and wet sieving methods were used to assess the size distribution of the soil water-stable aggregate (SWSA; Kemper & Rosenau, Citation1986).
The C and N concentrations in the soil and soil water-stable aggregate size fractions were determined by dry combustion of duplicate subsamples using the Sumigraph NC-80 Analyzer. Since the studied soil was calcareous, inorganic carbon had to be removed prior to analysis of soil organic carbon. The soil samples were pre-treated with 1 M hydrochloric acid (HCL) until fizzing stopped, washed with deionized water, air-dried and ground again (Gal et al., Citation2007). Then, the organic carbon concentration was determined using the Sumigraph NC-80 Analyzer. The soil inorganic carbon concentration was calculated as the difference between total carbon and organic carbon.
Statistical and data analysis
The soil total C and N storage down to 30 cm depth was calculated as follows:
Results
Carbon and nitrogen in the vegetation
Grassland degradation was accompanied by a succession of the vegetation which resulted in a changed pattern of C and N contents and storage in the species found in the four plots (). C and N storage decreased with increasing degradation for the total original dominant and companion plant species. Among the other 16 plant species analysed, C and N storage decreased with progressing degradation in seven (A. asphodeloides, I. dichotoma, H. minor, A. stenanthina, A. sieversiana, C. glaucum and L. bicolo), showed no obvious differences in two (L. leontopodioides and P. divaricatum) and tended to increase in seven (M. ruthenica, S. tschilliensis, S. scordifolia, B. bicaule, C. aristatum, P. tancetifolir and S. chamaejasme L.).
Table I. Carbon and nitrogen storage of different species on different types of degraded grassland.
The total C and N storage in the plant communities followed the general pattern seen in individual plant species. As shown in , C and N storage in the aboveground biomass declined as grassland degradation progressed. In the free-range grazing areas, C storage in the aboveground biomass were 527.57, 503.52 and 202.08 kg ha−1 in the LD, MD, and SD plots, respectively, accounting for negligible amounts (<2%) of the total C and N storage in the grassland ecosystem (including soil and vegetation). C losses amounted to 339.09, 363.14 and 664.58 kg ha−1 relative to 866.66 kg ha−1 in the CP, equaling 39.13%, 41.9% and 76.68% of the C storage in the CP. C storage also decreased by 33.23%, 39.47% and 50.78%, respectively, in the no-grazing plots of the three degradation plots compared with the CP, suggesting that grassland degradation decreased the potential of C sequestration in the community even in the absence of grazing. However, grazing tended to aggravate the decrease of C sequestration capacity, particularly on severely degraded grassland ().
Figure 2. Carbon and nitrogen storage in the aboveground biomass with increasing degrees of grassland degradation. a, Carbon storage in the course of grassland degradation. b, Carbon storage on the different types of degraded grassland. c, Nitrogen storage in the course of grassland degradation. d, Nitrogen storage on the different types of degraded grassland. Data shown are means±SEM (n = 3). Values followed by a different letter indicate that the means are significantly different (p <0.05) between the treatment plots (CP, LD, MD, SD) within two grazing types (Grazing exclusion and Free range grazing). CP, control plot; LD, light degradation; MD, moderate degradation; SD, severe degradation.
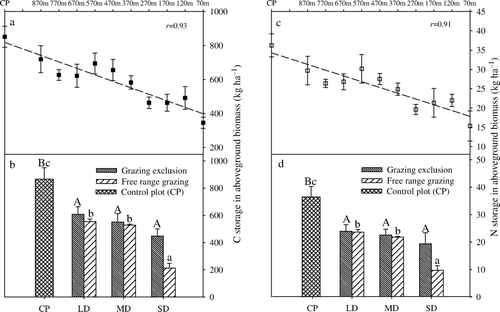
N storage in aboveground biomass varied remarkably between the degradation plots (). In free-range grazing areas, 22.57, 20.83 and 9.21 kg N ha−1 were found in the LD, MD and SD plot, respectively, accounting for less than 1% of N storage in the grassland ecosystem. These values corresponded to N losses of 13.82, 15.56 and 27.19 kg ha−1 relative to the 36.39 kg ha−1 in the CP. In the grazing exclusion plots, N storage slightly declined from LD to SD, and 57.56% of the N detected in the CP was lost on average in the degraded plot.
Carbon and nitrogen in the soil
Soil total and organic C storage in the top 0–30 cm soil depth decreased with increasing grassland degradation, but the inorganic C storage increased in the most severe stage of degradation (a). The spatial pattern of soil total C exhibited a fairly homogeneous increase along the grassland degradation processed, and roughly 85% of the area showed values between 6 and 7.5 kg m−2 (b). Soil organic C is an important element of the soil C pool, and accounted for 84.63% to 91.25% of the total C storage in the soil. The amounts of total soil C storage were 7.29, 6.83 and 6.59 kg m−2 in the LD, MD and SD plots, respectively, accounting for over 98% of the total C storage in the grassland ecosystem (including soil and vegetation), and these values were lowered by 0.30, 0.76 and 1.01 kg m−2 with respect to the 7.59 kg m−2 found in the CP.
Figure 3. Carbon and nitrogen storage in the soil. a, Soil carbon storage in the grassland degradation process. b, Spatial distribution of soil total carbon storage. c, Soil carbon storage on the different types of degraded grassland. d, Soil nitrogen storage in the grassland degradation process. e, Spatial distribution of soil total nitrogen storage. f, Soil nitrogen storage on the different types of degraded grassland. Data shown are means±SEM (n = 3). Values followed by a different letter indicate that the means are significantly different (p <0.05) between the treatment plots (CP, LD, MD, SD). CP, control plot; LD, light degradation; MD, moderate degradation; SD, severe degradation.
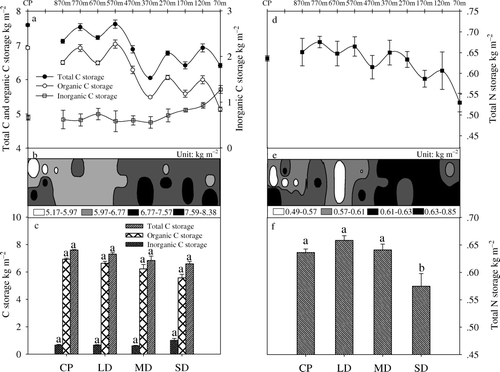
N storage in the soil varied strongly along the grassland degradation processed. The amounts measured were 0.63, 0.66, 0.64 and 0.57 kg m−2 in the CP, LD, MD and SD plot, respectively, accounting for over 99% of the total N stored in the grassland ecosystem. Soil N storage decreased from light to severe degradation, but the value detected in CP was lower than those in MD and LD. Only 9.69% of N was lost in SD compared with CP, which indicated that grazing exclusion for five years would not lead to an increase in N storage in the ecosystem. The spatial distribution of soil N storage indicated that about 36% of the area had N storage above that in the CP.
Carbon and nitrogen in SWSA
Grassland degradation significantly affected the size distribution of soil water-stable aggregates (SWSA) in 0–15 cm depth (). The percentage of SWSA in the ≥0.5 mm size fraction decreased with increasing degradation, whereas the proportion of SWSA in the < 0.5 mm fraction increased. C and N storage in the SWSA size fractions varied remarkably with the degree of grassland degradation (). C storages in SWSA were 3.64, 3.76, 3.13 and 2.57 kg m−2 in CP, LD, MD and the SD plot, respectively, accounting for 86.19, 85.44, 83.23 and 71.23% of the total C storage in the top 15 cm of soil. Similarly, N storage in SWSA were 0.31, 0.29, 0.24 and 0.23 kg m−2, accounting for 82.30, 70.09, 68.34 and 66.50% of the total N storage in the top soil.
Figure 4. Soil water-stable aggregate size distribution and C and N storage in the top soil (0–15 cm). a, Size distribution of soil water-stable aggregates on the different types of degraded grassland. b, C storage in the soil water-stable aggregate size fractions. c, N storage in soil water-stable aggregate size fractions. d, Proportion of large and small soil water-stable aggregates (≥0.5 mm and < 0.5 mm) in the course of grassland degradation. e, C storage in large and small soil water-stable aggregates (≥0.5 mm and < 0.5 mm). f, N storage in large and small soil water-stable aggregates (≥0.5 mm and < 0.5 mm). CP, control plot; LD, light degradation; MD, moderate degradation; SD, severe degradation.
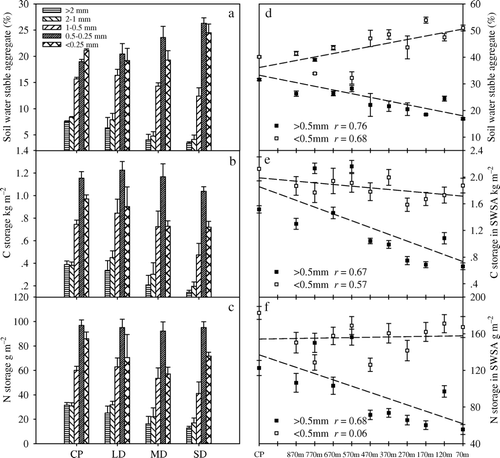
C and N are stored mainly in SWSA of the 0.5–0.25 mm size fraction. With increasing grassland degradation, C and N storage in the ≥0.5 mm size fraction decreased significantly. C and N storage in the < 0.5 mm size fraction were expected to increase because the percentage of SWSA in this fraction was increasing (d), but this was not the case. Therefore, the soil seemed to lose C and N as SWSAs broke down in the course of grassland degradation.
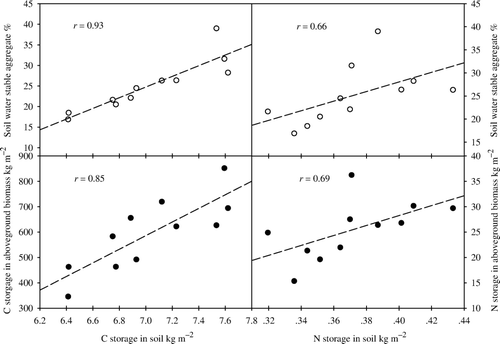
Relationships between C and N in the vegetation and soil, and the SWSA size distribution
As shown in , the correlation between C storage in the soil and C storage in the aboveground biomass was positive and significant (r =0.85, n = 11). There also was a strong and significant correlation (r =0.93, n = 11) between C storage in the soil and the proportion of large SWSA (≥0.5 mm). Similarly, positive correlations were observed between N storage in the soil and in the aboveground biomass (r =0.69, n = 11), and between soil N storage and the proportion of large SWSA (≥0.5 mm). These results indicated that accumulation of C in the aboveground biomass might affect soil C storage, and that the stability of C and N in the soil is related to the proportion of large SWSA. Our findings confirmed that grassland degradation results in soil aggregate breaking, accompanied by losses of soil C and N.
Discussion
Grassland degradation influenced the dynamics of C and N in grassland ecosystems by changing the grassland production capacity, as well as the soil physical and chemical properties. In fact, grassland utilization (e.g. grazing, mowing and land-use changes), natural disturbances (e.g. fire and drought) and natural conditions (e.g. soil types, stand, and altitudinal gradient) can create a mosaic of grassland types and affect the C and N storage of grassland ecosystems (He et al., Citation2008). Despite these caveats, estimating the storage and loss of C and N can help to characterize the effects of grassland degradation in northern China, and to understand the significance of increasing the C and N storage capacity through the management of degraded grassland.
In this study, C storage in a grassland ecosystem (including soil and aboveground biomass C) were found to be 7.68, 7.34, 6.88 and 6.61 kg m−2 in CP, LD, MD and the SD plot, respectively. C losses were 0.33, 0.80 and 1.07 kg m−2 in LD, MD and the SD plot, respectively, relative to the value determined for the CP plot. These losses amounted to 4.55–16.13% of the C stored in the grassland ecosystem. N storage in the grassland ecosystem (including soil and aboveground biomass N) varied from 0.57 kg m−2 to 0.66 kg m−2, depending on the degree of grassland degradation. N storage of the CP was lower than in MD and LD, and 9.90% of the N storage in the CP plot was lost in SD. This result indicated that grassland after 5 years of grazing exclusion is a very weak N source and that N storage was decreasing, which is in agreement with a previous report (He et al., Citation2008). One plausible explanation may be that an increase in aboveground biomass leads to greater competition for resources including nutrients and water, and that the increased nutrient demand causes a net decrease of soil N. The absence of large grazing animals would likely result in more soil N mineralization, and the increased accumulation of fresh litter and partially decomposed organic materials might keep precipitation from permeating the mineral soil when the natural disturbances – including large animal grazing and fire – are excluded.
The C and N stored in the aboveground biomass were quite low (<2%) and accounted for negligible fractions of the total C and N stored in the ecosystem. However, the aboveground biomass may serve as a globally important C sink. By the end of the 20th century, 90% of the grassland of Inner Mongolia (China) had become degraded as a consequence of the rapid expansion (Chen & Wang, Citation2000). As a consequence, vegetation communities and grassland productivity changed significantly (Huang et al., Citation2007), which resulted in decreases of the C and N sink capacity. In our study, C losses in the aboveground biomass relative to the CP were 339.09, 363.14 and 664.58 kg ha−1 in LD, MD and SD, respectively, which was in agreement with the published estimate of less than 720 kg m−2 (He et al., Citation2008). Of these losses, 33%, 55% and 77% were attributable to overgrazing.
The C and N stored in the soil accounted for over 98% of the total C and N storage in the ecosystem. In this study, the C stored in the soil decreased from 7.59 kg m−2 to 6.59 kg m−2 with increasing grassland degradation; notably, these figures were lower than the global mean value of 10.6 kg m−2 (Post et al., Citation1982). Zhou et al. (Citation2007) reported that the soil C storage in the agro-pastoral ecotone of Duolun county of Inner Mongolia ranged from 8 kg m−2 to 10 kg m−2. This study also indicated that the amount of C and N stored in soil water-stable aggregates accounted for 86.19–71.23% and 82.30–66.50% of the total storage of C and N in the soil, respectively. Soil C and N is mainly found in soil water-stable aggregates of 0.5–0.25 mm size, and the losses of soil C and N observed in degraded grassland were paralleled by a breaking up of the soil water-stable aggregates. C losses in the soil amounted to 0.30, 0.76 and 1.01 kg m−2 in LD, MD and SD, respectively, but degradation-related N losses were detected only in SD (0.04 kg m−2) in this study. Zhao et al. (Citation2009) reported C losses of 0.71–1.57 kg m−2, depending on the severity of degradation; the corresponding losses of N were 0.06–0.16 kg m−2. The discrepancies between these values and our results can be explained by differences in the vegetation, soil type, and soil depth, because total soil C and N contents depend more strongly on soil type than on the type of land-use (Tan et al., Citation2004; Breuer et al., Citation2006). In 1980, the soil C and N storage in the grassland ecosystem characterized in the present study had been determined to be 13.6 kg m−2 and 1.3 kg m−2, respectively (Wang & Cai, Citation1988). Compared with these earlier data, soil C storage had decreased by 49.92%, 48.10% and 44.60% in the SD, MD and LD plots, respectively. Similarly, soil N storage was lower by 56.15%, 50.77% and 49.23%, respectively, in our study. Evidently, a substantial amount of C and N has been lost over the last two decades at the study site. Some of this C and N may have been emitted into the atmosphere, but greater proportions probably have been lost due to wind erosion (Hu et al., Citation2005). In total, grassland degradation has reduced the sequestration potential and led to C and N losses by erosion and oxidation, instead of the C sequestration that is desirable.
In conclusion, our results suggest that in the course of the grassland degradation at the study site, the C sequestration capacity of the vegetation has been decreased, and that significant portions of the soil C and N have been lost which created a self-perpetuating cycle. To increase the C and N storage potential in this and similar grassland ecosystems, rehabilitation strategies to overcome grassland degradation must be designed. Since the Kyoto Protocol opened the possibility of using the biosphere as a carbon sink (Olsson & Ardo, Citation2006), the restoration of degraded rangeland through improved land management schemes should become part of C sequestration programmes (Abril & Bucher, Citation2001; Derner & Schuman, Citation2007). Grassland degradation in Inner Mongolia recently has prompted the local as well as the Chinese government to officially restrict or ban livestock grazing in the region. As a consequence, grazing exclusion and mowing have become the encouraged land-use types for grasslands. Different management practices that enhance or reduce C and N storage in the grasslands of the region have significant implications for the global C and N budgets (He et al., Citation2008), because the grasslands of northern China represent a significant portion of the Eurasian continent (Ojima et al., Citation1993). With regard to C storage, the grasslands with the highest potential for C sequestration are those that have been depleted by poor management strategies in the past (Jones & Donnelly, Citation2004). Based on our results, we conclude that the restoration of degraded grasslands in northern China bears a great potential for increasing the C and N storage in the region.
Acknowledgements
Funding for this study was provided by the National Basic Research Program of China (No. 2007CB106800), and the National Natural Science Foundation of China (30590382, 30860060). Grassland Carbon Fixation, Greenhouse Gas Reduction and Demonstration in China (2008BAD95B03) supported by MOST.
References
- Abril , A. and Bucher , E. H. 2001 . Overgrazing and soil carbon dynamics in the western Chaco of Argentina . Applied Soil Ecology , 16 : 243 – 249 .
- Bai , Y. F. , Han , X. G. , Wu , J. G. , Chen , Z. Z. and Li , L. H. 2004 . Ecosystem stability and compensatory effects in the Inner Mongolia grassland . Nature , 431 ( 9 ) : 181 – 183 .
- Billings , S. A. 2006 . Soil organic matter dynamics and land use change at a grassland/forest ecotone . Soil Biology & Biochemistry , 38 : 2934 – 2943 .
- Breuer , L. , Huisman , J. A. , Keller , T. and Frede , H. G. 2006 . Impact of a conversion from cropland to grassland on C and N storage and related soil properties: analysis of a 60-year chronosequence . Geoderma , 133 : 6 – 18 .
- Chen , Z. Z. and Wang , S. P. 2000 . Typical steppe ecosystem of China , Beijing : Chinese Science Press .
- Dale , W. J. and Peter , S. C. 2001 . Effects of forest management on soil C and N storage: meta analysis . Forest Ecology and Management , 140 : 227 – 238 .
- Davidson , E. A. , Nepstad , D. C. , Klink , C. and Trumbore , S. 1995 . Pasture soils as carbon sink . Nature , 376 : 472 – 473 .
- Derner , J. D. and Schuman , G. E. 2007 . Carbon sequestration and rangelands: a synthesis of land management and precipitation effects . Journal of Soil and Water Conservation , 62 : 77 – 85 .
- Elmore , A. J. and Asner , G. P. 2006 . Effects of grazing intensity on soil carbon stocks following deforestation of a Hawaiian dry tropical forest . Global Change Biology , 12 : 1761 – 1772 .
- Gal , A. , Vyn , T. J. , Micheli , E. , Kladivko , E. J. and McFee , W. W. 2007 . Soil carbon and nitrogen accumulation with long-term no-till versus moldboard plowing overestimated with tilled-zone sampling depths . Soil & Tillage Research , 96 : 42 – 51 .
- Gomes , L. , Arrue , J. L. and Lopez , M. V. 2003 . Wind erosion in a semiarid area of Spain: the WELSONS project . Catena , 52 : 235 – 256 .
- He , N. , Yu , Q. , Wu , L. , Wang , Y. and Han , X. 2008 . Carbon and nitrogen store and storage potential as affected by land-use in a Leymus chinensis grassland of northern China . Soil Biology & Biochemistry , 40 : 2952 – 2959 .
- Houghton , R. A. , Hackler , J. L. and Lawrence , K. T. 1999 . The U.S. carbon budget: contributions from land-use change . Science , 285 : 574 – 578 .
- Hu , Y. F. , Liu , J. Y. , Zhuang , D. F. , Cao , H. X. , Yan , H. M. and Yang , F. T. 2005 . Distribution characteristics of 137Cs in wind-eroded soil profile and its use in estimating wind erosion modulus . Chinese Science Bulletin , 50 : 933 – 937 .
- Huang , D. , Wang , K. and Wu , W. L. 2007 . Dynamics of soil physical and chemical properties and vegetation succession characteristics during grassland desertification under sheep grazing in an agro-pastoral transition zone in Northern China . Journal of Arid Environments , 70 : 120 – 136 .
- Jones , M. B. and Donnelly , A. 2004 . Carbon sequestration in temperate grassland ecosystems and the influence of management, climate and elevated CO2 . New Phytologist , 164 : 423 – 439 .
- Kemper , W. D. and Rosenau , R. C. 1986 . “ Aggregates stability and size distribution ” . In Methods of soil analysis , Edited by: Klute , A. Society of Agronomy/Soil Science Society of America .
- Liao , J. D. , Boutton , T. W. and Jastrow , J. D. 2006 . Storage and dynamics of carbon and nitrogen in soil physical fractions following woody plant invasion of grassland . Soil Biology & Biochemistry , 38 : 3184 – 3196 .
- Ojima , D. S. , Dirks , B. O. M. , Glenn , E. P. , Owensby , C. E. and Scurlock , J. M. O. 1993 . Assessment of C budget for grasslands and dryland of the world . Water, Air, and Soil Pollution , 70 : 95 – 109 .
- Olsson , L. and Ardo , J. 2006 . Soil carbon sequestration in degraded semiarid agro-ecosystems – perils and potentials . Ambio , 31 : 471 – 477 .
- Post , W. M. , Emanuel , W. R. , Zinke , P. J. and Stangenberger , A. G. 1982 . Soil carbon pools and world life zones . Nature , 298 : 156 – 159 .
- SAS Institute Inc 2001 . SAS/Stat software: Changes and enhancements through release 8.1 Cary, NC : SAS Inst. Inc.
- Schimel , D. S. 1995 . Terrestrial ecosystems and the carbon cycle . Global Change Biology , 1 : 77 – 91 .
- Tan , Z. X. , Lal , R. , Smeck , N. E. and Calhoun , F. G. 2004 . Relationship between surface soil organic carbon pool and site variables . Geoderma , 121 : 187 – 195 .
- Wang , J. W. and Cai , Y. C. 1988 . “ Studies on genesis, types and characteristics of the soils of the Xilin River basin ” . In Research on grassland ecosystem , Edited by: Jiang , S. Beijing : Chinese Science Press .
- Wang , M. 2008 . Effect of grazing intensities on grassland ecosystem health in Leymus chinensis meadow steppe , College of Ecology and Environmental Science, Inner Mongolia Agricultural University .
- Zhao , H. , He , Y. , Zhou , R. , Su , Y. , Li , Y. and Drake , S. 2009 . Effects of desertification on soil organic C and N content in sandy farmland and grassland of Inner Mongolia . Catena , 77 : 187 – 191 .
- Zhou , Z. Y. , Sun , O. J. , Huang , J. H. , Li , L. H. and Han , X. G. 2007 . Soil carbon and nitrogen stores and storage potential as affected by land-use in an agro-pastoral ecotone of northern China . Biogeochemistry , 82 : 127 – 138 .